Environmental Effects on Taxonomic Turnover in Soil Fauna across Multiple Forest Ecosystems in East Asia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Taxa and Environment Variables
2.3. Measurement of Order Turnover Rate
2.4. Data Analysis
3. Results
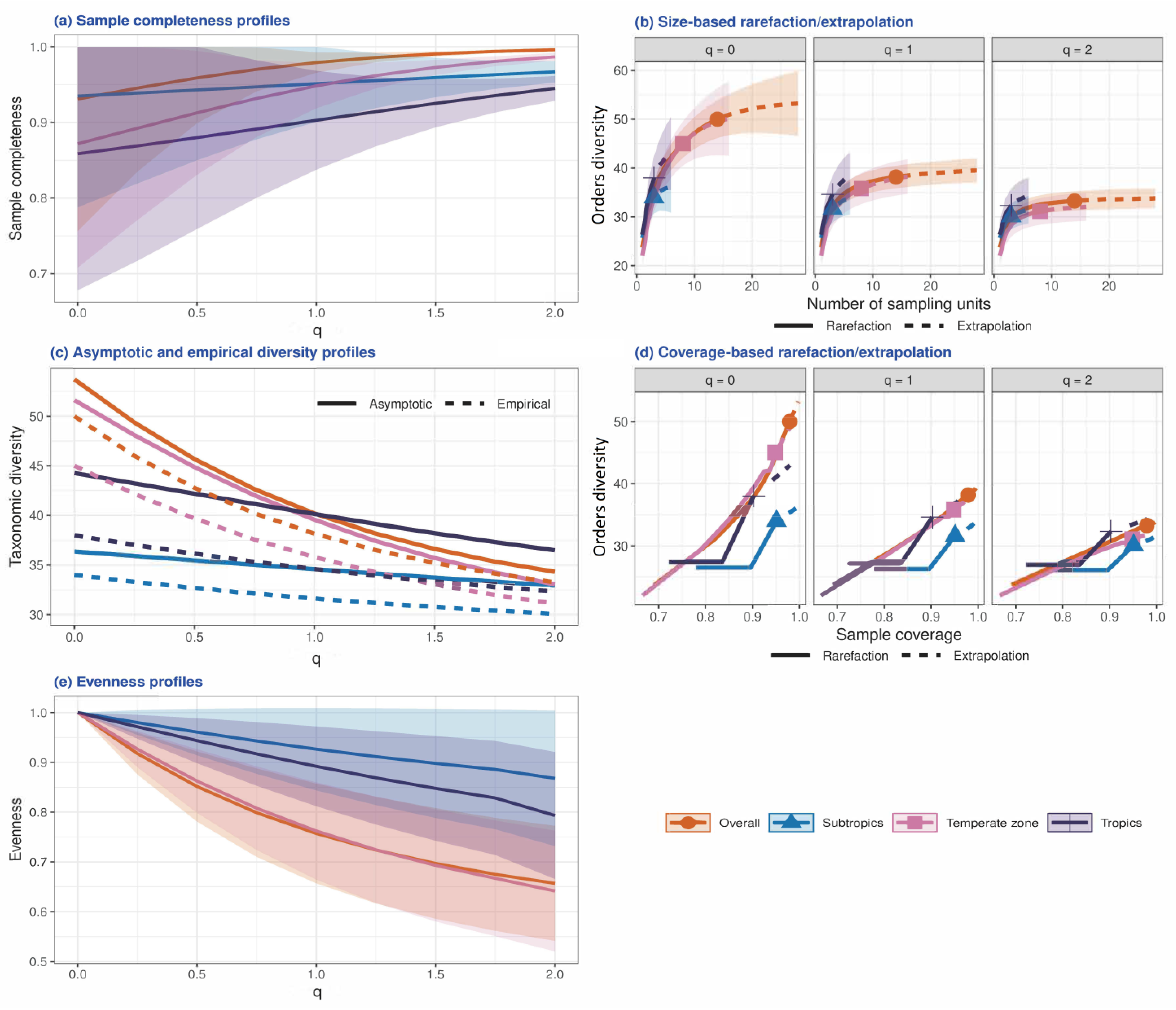
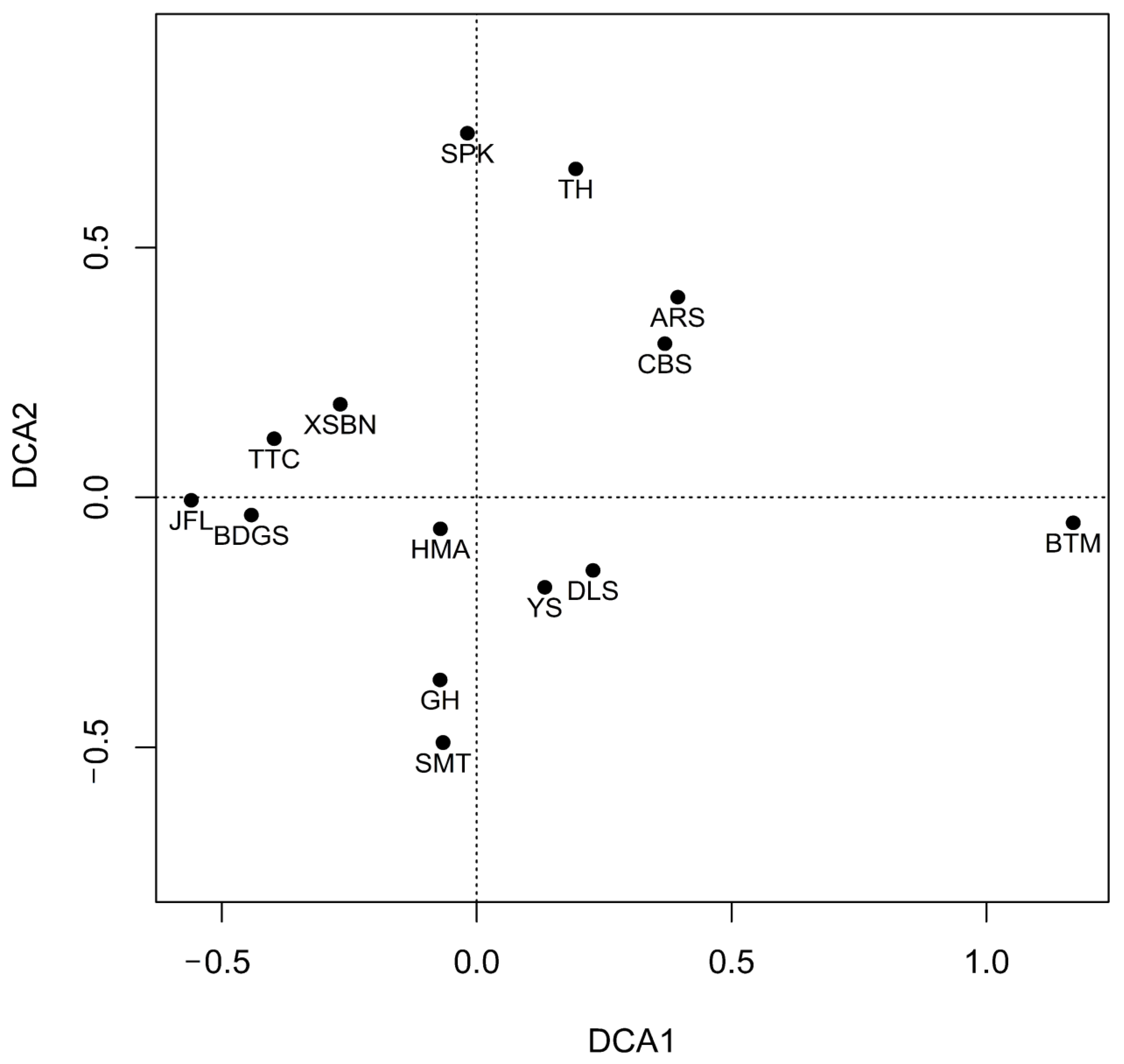
3.1. Structural Characteristics of Soil Fauna Communities
3.2. Patterns of Order Turnover
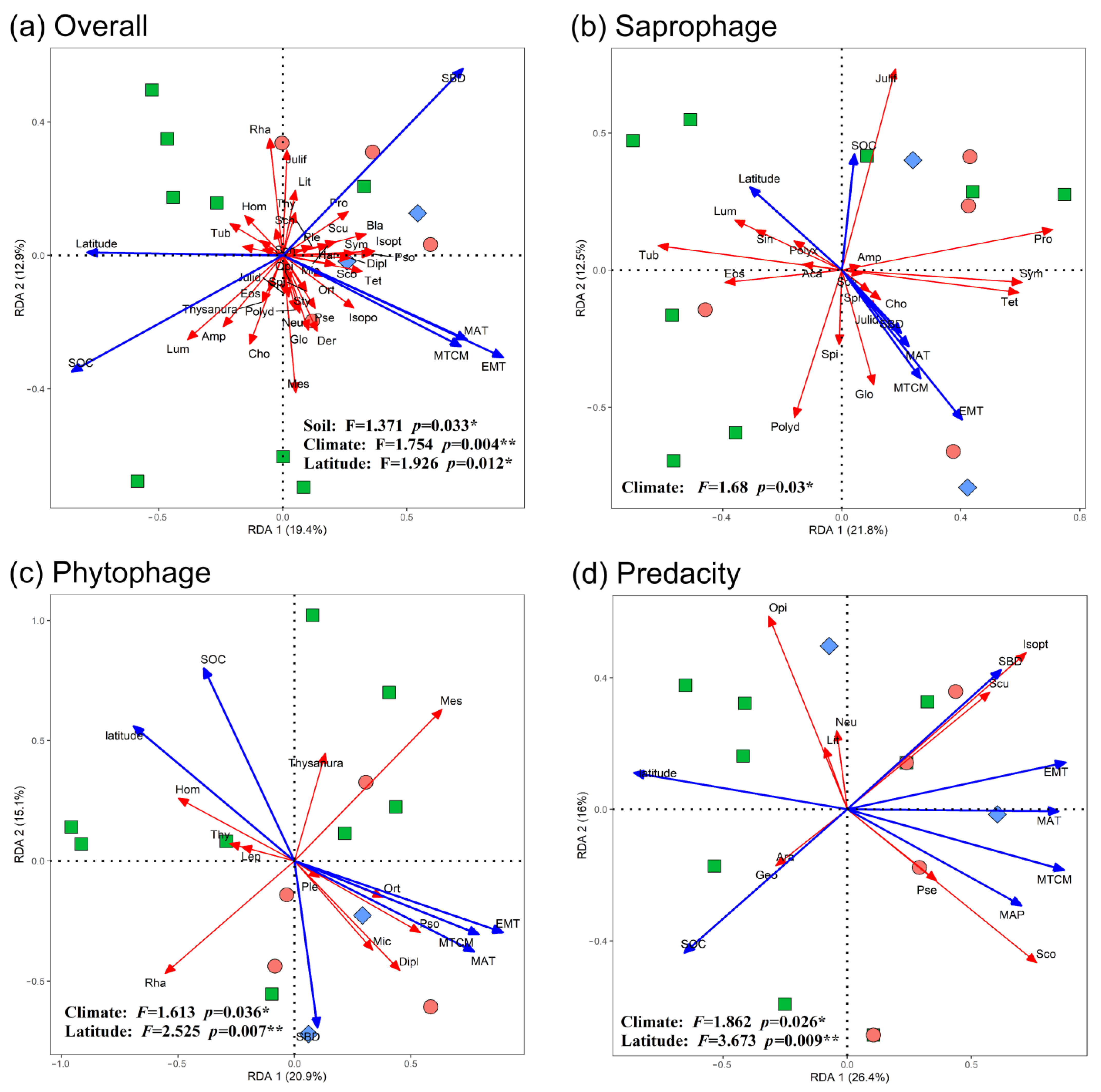
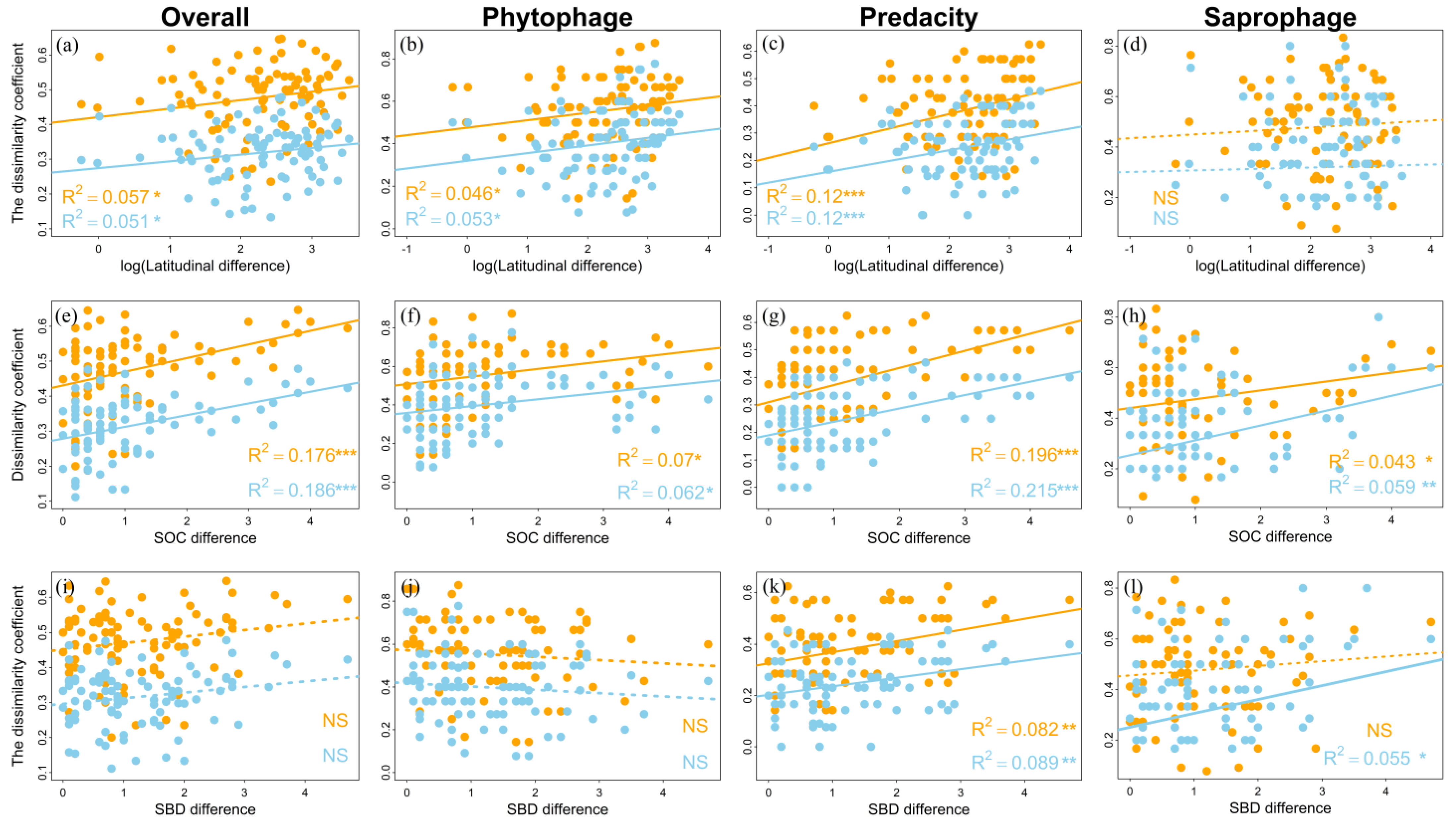
3.3. Determinants of Order Turnover
4. Discussion
4.1. Soil Fauna Community Distributions
4.2. Driving Forces of Soil Fauna Community Construction
4.3. Ecological Processes of Soil Fauna Community Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, Y.H.; Zhang, W.X.; Liu, S.J.; Wang, X.L.; Fu, S.L. Diversity and function of soil fauna. Acta Ecol. Sin. 2015, 35, 6614–6625. [Google Scholar]
- Wall, D.H.; Bardgett, R.D.; Behan-Pelletier, V.; Herrick, J.E.; Jones, T.H.; Six, J.; Strong, D.R.; Van Der Putten, W.H. Soil Ecology and Ecosystem Services; Oxford University Press: Oxford, UK, 2013; pp. 28–45. [Google Scholar]
- García-Palacios, P.; Maestre, F.T.; Kattge, J.; Wall, D.H. Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol. Lett. 2013, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Deyn, G.B.; Raaijmakers, C.E.; Zoomer, H.R.; Berg, M.P.; de Ruiter, P.C.; Verhoef, H.A.; Bezemer, T.M.; van der Putten, W.H. Soil invertebrate fauna enhances grassland succession and diversity. Nature 2003, 422, 711–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Chen, D.M.; Zhao, C.C. Functions of earthworm in ecosystem. Biodivers. Sci. 2007, 2, 142–153. [Google Scholar]
- Bardgett, R.D.; Wardle, D.A. Aboveground-Belowground Linkages: Biotic Interactions, Ecosystem Processes, and Global Change; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Bradford, M.A.; Jones, T.H.; Bardgett, R.D.; Black, H.I.J.; Boag, B.; Bonkowski, M.; Cook, R.; Eggers, T.; Gange, A.C.; Grayston, S.J.; et al. Impacts of soil faunal community composition on model grassland ecosystems. Science 2002, 298, 615–618. [Google Scholar] [CrossRef] [Green Version]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Cesarz, S.; Koller, R.; Worm, K.; Reich, P.B. Global change belowground: Impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Chang. Biol. 2012, 18, 435–447. [Google Scholar] [CrossRef]
- Johnston, A.S.A.; Sibly, R.M. The influence of soil communities on the temperature sensitivity of soil respiration. Nat. Ecol. Evol. 2012, 2, 1597–1602. [Google Scholar] [CrossRef]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218. [Google Scholar] [CrossRef] [Green Version]
- Johnston, A.S.A.; Sibly, R.M. Multiple environmental controls explain global patterns in soil animal communities. Oecologia 2020, 192, 1047–1056. [Google Scholar] [CrossRef] [Green Version]
- Crowther, T.W.; Hoogen, J.V.D.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, 772. [Google Scholar] [CrossRef]
- Zou, Y.; Werf, W.; Liu, Y.; Axmacher, J.C. Predictability of species diversity by family diversity across global terrestrial animal taxa. Glob. Ecol. Biogeogr. 2020, 29, 629–644. [Google Scholar] [CrossRef]
- Yin, X.; Song, B.; Dong, W.H.; Xin, W.D.; Wang, Y.Q. A review on the eco-geography of soil fauna in China. Acta Geogr. Sin. 2010, 65, 91–102. [Google Scholar] [CrossRef]
- Yang, X.D.; Zou, X.M. Soil fauna and leaf litter decomposition in tropical rain forest in Xishuanbanna, SW China: Effects of mesh size of litterbags. J. Plant Ecol. 2006, 30, 791–801. [Google Scholar]
- Ma, K.P. Measurement of biotic community diversity I α diversity (Part 1). Biodivers. Sci. 1994, 2, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Gaston, K.J. Global patterns in biodiversity. Nature 2020, 405, 220–227. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.; Cornell, H.V.; Comita, L.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Lafage, D.; Maugenest, S.; Bouzille, J.B.; Petillon, J. Disentangling the influence of local and landscape factors on alpha and beta diversities: Opposite response of plants and ground-dwelling arthropods in wet meadows. Ecol. Res. 2015, 30, 1025–1035. [Google Scholar] [CrossRef]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. The geographical structure of British bird distributions: Diversity, spatial turnover and scale. J. Anim. Ecol. 2001, 70, 966–979. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Guo, Y.; Liu, J.; Liu, J.; Adl, S.; Wu, D.; Lu, T. Contrasting beta diversity of spiders, carabids, and ants at local and regional scales in a black soil region, northeast China. Soil Ecol. Lett. 2021, 3, 103–114. [Google Scholar] [CrossRef]
- Kraft, N.J.; Comita, L.S.; Chase, J.M.; Sanders, N.J.; Swenson, N.G.; Crist, T.O.; Stegen, J.C.; Vellend, M.; Boyle, B.; Anderson, M.J.; et al. Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 2011, 333, 1755–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Yoshihara, Y. Local-scale disturbance by Siberian marmots has little influence on regional plant richness in a Mongolian grassland. Plant Ecol. 2013, 214, 29–34. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, Z.L.; Li, P.K.; Cao, R.F.; Jia, H.R.; Ye, Y.Z. Effects of Environment and Space on Species Turnover of Woody Plants across Multiple Forest Dynamic Plots in East Asia. Front. Plant Sci. 2016, 7, 1533. [Google Scholar] [CrossRef] [PubMed]
- Sreekar, R.; Koh, L.P.; Mammides, C.; Corlett, R.T.; Dayananda, S.; Goodale, U.M.; Kotagama, S.W.; Goodale, E. Drivers of bird beta diversity in the Western Ghats-Sri Lanka biodiversity hotspot are scale dependent: Roles of land use, climate, and distance. Oecologia 2020, 193, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Caruso, T.; La Diega, R.N.; Bernini, F. The effects of spatial scale on the assessment of soil fauna diversity: Data from the oribatid mite community of the Pelagian Islands (Sicilian Channel, southern Mediterranean). Acta Oecol. 2005, 28, 23–31. [Google Scholar] [CrossRef]
- Dunn, R.R.; Agosti, D.; Andersen, A.; Arnan, X.; Brühl, C.; Cerda, X.; Ellison, A.; Fisher, B.; Fitzpatrick, M.; Gibb, H.; et al. Climatic drivers of hemispheric asymmetry in global patterns of ant species richness. Ecol. Lett. 2009, 12, 324–333. [Google Scholar] [CrossRef]
- Medini, D.; Donati, C.; Tettelin, H.; Masignani, V.; Rappuoli, R. The microbial pan- genome. Curr. Opin. Genet. Dev. 2015, 15, 589–594. [Google Scholar] [CrossRef]
- Van Den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; De Goede, R.G.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.R.; Lin, Y.H.; Zhang, S.; Zhang, Y.X.; Li, G.X.; Ma, K.M. Shifting mechanisms of elevational diversity and biomass patterns in soil invertebrates at treeline. Soil Biol. Biochem. 2017, 113, 80–88. [Google Scholar] [CrossRef]
- Li, Z.; Heino, J.; Liu, Z.; Meng, X.; Chen, X.; Ge, Y.; Xie, Z. The drivers of multiple dimensions of stream macroinvertebrate beta diversity across a large montane landscape. Limnol. Ocean. 2020, 66, 226–236. [Google Scholar] [CrossRef]
- Li, Y.; Shipley, B.; Price, J.N.; Dantas, V.D.; Tamme, R.; Westoby, M.; Siefert, A.; Schamp, B.S.; Spasojevic, M.J.; Jung, V.; et al. Habitat filtering determines the functional niche occupancy of plant communities worldwide. J. Ecol. 2017, 106, 1001–1009. [Google Scholar] [CrossRef] [Green Version]
- Oliver, T.; Hill, J.K.; Thomas, C.D.; Brereton, T.; Roy, D.B. Changes in habitat specificity of species at their climatic range boundaries. Ecol. Lett. 2009, 12, 1091–1102. [Google Scholar] [CrossRef]
- John, R.; Dalling, J.W.; Harms, K.E.; Yavitt, J.B.; Stallard, R.F.; Mirabello, M.; Hubbell, S.P.; Valencia, R.; Navarrete, H.; Vallejo, M.; et al. Soil nutrients influence spatial distributions of tropical tree species. Proc. Natl. Acad. Sci. USA 2007, 104, 864–869. [Google Scholar] [CrossRef]
- Jia, H.R.; Chen, Y.; Yuan, Z.L.; Ye, Y.Z.; Huang, Q.C. Effects of environmental and spatial heterogeneity on tree community assembly in Baotianman National Nature Reserve, Henan, China. Pol. J. Ecol. 2015, 63, 175–183. [Google Scholar] [CrossRef]
- Escudero, A.; Valladares, F. Trait-based plant ecology: Moving towards a unifying species coexistence theory. Oecologia 2016, 180, 919–922. [Google Scholar] [CrossRef]
- Hubbell, C.S. A Unified Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Gao, M.X.; He, P.; Zhang, X.P.; Liu, D.; Wu, D.H. Relative roles of spatial factors, environmental filtering and biotic interactions in fine-scale structuring of a soil mite community. Soil Biol. Biochem. 2014, 79, 68–77. [Google Scholar] [CrossRef]
- Yin, W.Y.; Hu, S.H.; Shen, Y.F.; Ning, Y.Z.; Sun, X.D.; Wu, J.H.; Zhu, G.Y.; Zhang, M.Y.; Wang, M.; Chen, J.Y.; et al. Pictorical Keys to Soil Animals of China; Science Press: Beijing, China, 1998. [Google Scholar]
- Yin, W.Y. Soil Animals of China; Chinese Sciences Press: Beijing, China, 2000. [Google Scholar]
- Li, S.Q.; Lin, Y.C. Catalogue of Life China; Chinese Sciences Press: Beijing, China, 2016. [Google Scholar]
- Zhang, X.P.; Hou, W.L.; Chen, P. Soil animal guilds and their ecological distribution in the northeast of China. Chin. J. Appl. Environ. Biol. 2001, 7, 370–374. [Google Scholar]
- Huang, L.R.; Zhang, X.P. Community characteristic of Mid-micro soil animals in cold-temprate zone of the Daxing’an Mountains, China. Chin. J. Appl. Environ. Biol. 2008, 14, 388–393. [Google Scholar]
- Zhang, X.P.; Cao, H.C.; Feng, Z.K. Ecological and geographical study on small and middle soil animals in Daxing’anling Mountains, northeastern China. J. Beijing For. Univ. 2007, 29, 259–265. [Google Scholar]
- Nakamura, Y.; Fujikawa, T.; Yamauchi, K.; Tamura, H. Distribution and dynamics of some forest soil animals in Hokkaido. J. Jpn. For. Soc. 1970, 52, 80–88. [Google Scholar]
- Han, H.Y. Response of Soil Fauna to the Change of Environmental Factors in the Low-Mountain of the Changbai Mountains. Master’s Thesis, Northeast Normal University, Changchun, China, 2015. [Google Scholar]
- Xu, G.; Zhang, Y.; Zhang, S.; Ma, K. Effect of elevation on abundance distribution of different feeding groups in litter-dwelling soil fauna. Acta Pedol. Sin. 2017, 54, 237–244. [Google Scholar]
- Kwon, T.S.; Kim, Y.S.; Lee, S.W.; Park, Y.S. Changes of soil arthropod communities in temperate forests over 10 years (1998–2007). J. Asia Pac. Entomol. 2016, 19, 181–189. [Google Scholar] [CrossRef]
- Touyama, Y.; Nakagoshi, N. A comparison of soil arthropod fauna in coniferous plantations and secondary forests. Jpn. J. Ecol. 1994, 44, 21–31. [Google Scholar]
- Xu, S.B. Study on the Spatial-Temporal Dynamic of Meso-Micro Soil Fauna Communities and Its Influencing Factors in Baotianman Nature Reserve. Master’s Thesis, Henan University, Kaifeng, China, 2020. [Google Scholar]
- Yi, L. Influences of Secondary Succession of the Damaged Evergreen Broad-leaved Forest on Soil Animal Community in Tiantong Zhejiang Province. Ph.D. Thesis, East China Normal University, Shanghai, China, 2005. [Google Scholar]
- He, Z. Diversity of Soil Arthropod Diversity in Different Forest Types in South China. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2018. [Google Scholar]
- Li, Z.W.; Tong, X.L.; Zhang, W.Q.; Xie, G.Z.; Dai, K.Y. Diversity of soil invertebrate assemblages in the forest of Shimentai Nature Reserve, Guangdong Province. J. South China Agric. Univ. (Nat. Sci. Ed.) 2004, 25, 80–84. [Google Scholar]
- Chuan, C.Y.; Yuan, H.W.; Wang, Y.N. A Preliminary Study on Soil Animals of Ta-Ta-Chia Area. J. Exp. For. Natl. Taiwan Univ. 2003, 17, 239–245. [Google Scholar]
- Yang, X.D.; She, Y.P. The Character of Composition and Distribution on Soil Fauna under Tropical Forests of Xishuangbanna in Rainy Season. J. Northeast For. Univ. 1998, 26, 65–70. [Google Scholar]
- Xiong, Y. The Community Diversity of Soil Animals in the Tropical and Subtropical Forests and the Phylogeny of Collembola. Ph.D. Thesis, East China Normal University, Shanghai, China, 2005. [Google Scholar]
- Basset, Y.; Cizek, L.; Cuénoud, P.; Didham, R.K.; Guilhaumon, F.; Missa, O.; Novotny, V.; Ødegaard, F.; Roslin, T.; Schmidl, J.; et al. Arthropod diversity in a tropical forest. Science 2012, 338, 1481–1484. [Google Scholar] [CrossRef] [Green Version]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Sorensen, T.A. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content. K. Dan. Vidensk. Selsk. 1948, 5, 4–7. [Google Scholar]
- Jost, L. Partitioning diversity into independent alpha and beta components. Ecology 2007, 88, 2427–2439. [Google Scholar] [CrossRef] [Green Version]
- Chao, A.; Kubota, Y.; Zelený, D.; Chiu, C.; Li, C.; Kusumoto, B.; Yasuhara, M.; Thorn, S.; Wei, C.; Costello, M.J.; et al. Quantifying sample completeness and comparing diversities among assemblages. Ecol. Res. 2020, 35, 292–314. [Google Scholar] [CrossRef]
- Singh, D.; Slik, J.W.F.; Jeon, Y.-S.; Tomlinson, K.W.; Yang, X.; Wang, J.; Kerfahi, D.; Porazinska, D.L.; Adams, J.M. Tropical forest conversion to rubber plantation affects soil micro- & mesofaunal community & diversity. Sci. Rep. 2019, 9, 5893. [Google Scholar]
- Li, P.K.; Zhang, J.; Wang, S.L.; Zhang, P.P.; Chen, W.J.; Ding, S.Y.; Xi, J.J. Changes in the distribution preference of soil microbial communities during secondary succession in a temperate mountain forest. Front. Microbiol. 2022, 13, 923346. [Google Scholar] [CrossRef]
- Lososova, Z.; Chytry, M.; Comalova, S.; Kropac, Z.; Otypkova, Z.; Pysek, P.; Tichy, L. Weed vegetation of arable land in central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J. Cross-biom metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.H.; Ayres, E.; Bargett, R.D.; Wall, D.H.; Garey, J.R. Molecular study of worldwide distribution and diversity of soil animals. Proc. Natl. Acad. Sci. USA 2011, 108, 17720–17725. [Google Scholar] [CrossRef] [Green Version]
- Lubbers, I.M.; Berg, M.P.; de Deyn, G.B.; van der Putten, W.H.; van Groenigen, J.W. Soil fauna diversity increases CO2 but suppresses N2O emissions from soil. Glob. Chang. Biol. 2020, 26, 1886–1898. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.X.; Gao, M.X.; Liu, J.; Zaitsev, A.S.; Wu, D.H. Disentangling the drivers of ground-dwelling macro-arthropod metacommunity structure at two different spatial scales. Soil Biol. Biochem. 2018, 130, 55–62. [Google Scholar] [CrossRef]
- Song, D.; Pan, K.; Tariq, A.; Sun, F.; Li, Z.; Sun, X.; Zhang, L.; Olusanya, O.A.; Wu, X. Large-scale patterns of distribution and diversity of terrestrial nematodes. Appl. Soil Ecol. 2017, 114, 161–169. [Google Scholar] [CrossRef]
- Phillips, H.R.; Guerra, C.A.; Bartz, M.L.; Briones, M.J.; Brown, G.; Crowther, T.W.; Ferlian, O.; Gongalsky, K.B.; Van Den Hoogen, J.; Krebs, J.; et al. Global distribution of earthworm diversity. Science 2019, 366, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Convey, P. Antarctic Ecosystems. Encyclopedia of Biodiversity, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 179–188. [Google Scholar]
- Decaëns, T. Macroecological patterns in soil communities. Glob. Ecol. Biogeogr. 2010, 19, 287–302. [Google Scholar] [CrossRef]
- Petersen, H.; Luxton, M.A. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 1982, 39, 287–388. [Google Scholar] [CrossRef]
- Abrahamczyk, S.; Gottleuber, P.; Matauschek, C.; Kessler, M. Diversity and community composition of euglossine bee assemblages (Hymenoptera: Apidae) in western Amazonia. Biodivers. Conserv. 2011, 20, 2981–3001. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, W.; Zalewski, M.; Uvarov, A.V. Spatial distribution and species co-occurrence in soil invertebrate and plant communities on northern taiga islands. Ann. Zool. Fenn. 2012, 49, 161–173. [Google Scholar] [CrossRef]
- Andersen, A.N.; Del, T.I.; Parr, C.L. Savanna ant species richness is maintained along a bioclimatic gradient of increasing latitude and decreasing rainfall in northern Australia. J. Biogeogr. 2015, 42, 2313–2322. [Google Scholar] [CrossRef]
- Dunck, B.; Schneck, F.; Rodrigues, L. Patterns in species and functional dissimilarity: Insights from periphytic algae in subtropical floodplain lakes. Hydrobiologia 2016, 763, 237–247. [Google Scholar] [CrossRef]
- Kent, D.R.; Lynn, J.S.; Pennings, S.C.; Souza, L.A.; Smith, M.D.; Rudgers, J.A. Weak latitudinal gradients in insect herbivory for dominant rangeland grasses of North America. Ecol. Evol. 2020, 10, 6385–6394. [Google Scholar] [CrossRef]
- Janion-Scheepers, C.; Bengtsson, J.; Duffy, G.A.; Deharveng, L.; Leinaas, H.P.; Chown, S.L. High spatial turnover in springtails of the Cape Floristic Region. J. Biogeogr. 2019, 47, 1007–1018. [Google Scholar] [CrossRef]
- Jones, D.T.; Eggleton, P. Global Biogeography of Termites: A Compilation of Sources. Biol. Termit. A Mod. Synth. 2010, 477–498. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Thu, P.Q.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 1078. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.; Chu, H.; Jin, J.; Liu, X.; Wang, G. Soil carbon content drives the biogeographical distribution of fungal communities in the black soil zone of northeast China. Soil Biol. Biochem. 2015, 83, 29–39. [Google Scholar] [CrossRef]
- Carrillo, Y.; Ball, B.A.; Molina, M. Stoichiometric linkages between plant litter, trophic interactions and nitrogen mineralization across the litter–soil interface. Soil Biol. Biochem. 2016, 92, 102–110. [Google Scholar] [CrossRef]
- Burns, K.C. Is tree diversity different in the southern hemisphere? J. Veg. Sci. 2007, 18, 307–312. [Google Scholar] [CrossRef]
- Gaston, K.J.; Williams, P.H.; Eggleton, P.; Humphries, C.J. Large scale patterns of biodiversity: Spatial variation in family richness. Proc. R. Soc. Lond. 1995, 260, 149–154. [Google Scholar]
- Eggleton, P.; Williams, P.H.; Gaston, K.J. Explaining global termite diversity: Productivity or history? Biodivers. Conserv. 1994, 3, 318–330. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Gaston, K.J. Spatial patterns in the species richness of birds in the New World. Ecography 1996, 19, 369–376. [Google Scholar] [CrossRef]
- Platnick.; Norman, I. Patterns of biodiversity: Tropical vs temperate. J. Nat. His. 1991, 25, 1083–1088. [Google Scholar] [CrossRef]
- Rodriguero, M.S.; Gorla, D.E. Latitudinal gradient in species richness of the New World Triatominae (Reduviidae). Glob. Ecol. Biogeogr. 2004, 13, 75–84. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher predation risk for insect prey at low latitudes and elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yuan, Z.L.; Ren, S.Y.; Wei, B.L.; Jia, H.R.; Ye, Y.Z. Correlation analysis of soil and species of different life forms in Baotianman Nature Reserve. Chin. Sci. Bull. 2014, 59, 2367–2376. [Google Scholar]
- Turney, S.; Buddle, C.M. Pyramids of species richness: The determinants and distribution of species diversity across trophic levels. Oikos 2016, 125, 1224–1232. [Google Scholar] [CrossRef]
- Widenfalk, L.A.; Bengtsson, J.; Berggren, D.; Zwiggelaar, K.; Spijkman, E.; Huyer-Brugman, F.; Berg, M.P. Spatially structured environmental filtering of collembolan traits in late successional salt marsh vegetation. Oecologia 2015, 179, 537–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chase, J.M.; Leibold, M.A. Spatial scale dictates the productivity-biodiversity relationship. Nature 2002, 416, 427–430. [Google Scholar] [CrossRef]
- Levin, S.A. The problem of patterns and scale in ecology: The Robert H. MacArthur Award lecture. Ecology 1992, 73, 1943–1967. [Google Scholar] [CrossRef]
- Fu, S.L.; Zou, X.M.; Coleman, D. Highlights and perspectives of soil biology and ecology. Research in China. Soil Biol. Biochem. 2009, 41, 868–876. [Google Scholar] [CrossRef]
- Gao, M.X.; Lin, L.; Chang, L.; Sun, X.; Liu, D.; Wu, D.H. Spatial patterns and assembly rules in soil fauna communities. Biodivers. Sci. 2018, 26, 1034–1050. [Google Scholar] [CrossRef]
- Zhang, L.M.; Gao, M.X.; Liu, D.; Zhang, X.P.; Wu, D.H. Relative contributions of environmental filtering and dispersal limitation in species co-occurrence of above- and below-ground soil mite communities. Acta Ecol. Sin. 2016, 36, 3951–3959. [Google Scholar]
- Ponge, J.F.; Salmon, S. Spatial and taxonomic correlates of species and species trait assemblages in soil invertebrate communities. Pedobiologia 2013, 56, 129–136. [Google Scholar] [CrossRef]
- Terlizzi, A.; Anderson, M.J.; Bevilacqua, S.; Fraschetti, S.; Włodarska- Kowalczuk, M.; Ellingsen, K.E. Beta diversity and taxonomic sufficiency: Do higher-level taxa reflect heterogeneity in species composition? Divers. Distrib. 2009, 15, 450–458. [Google Scholar] [CrossRef]

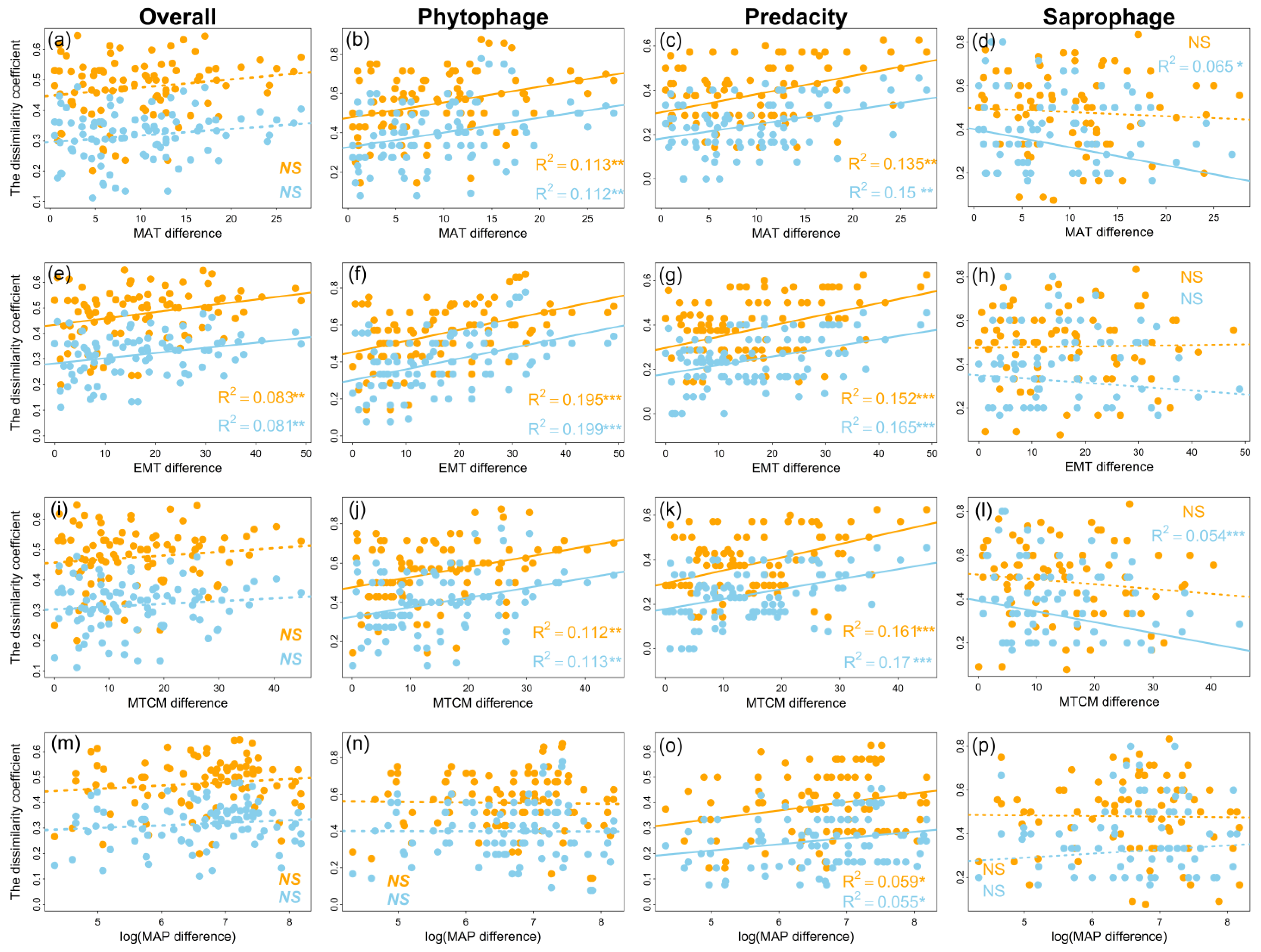
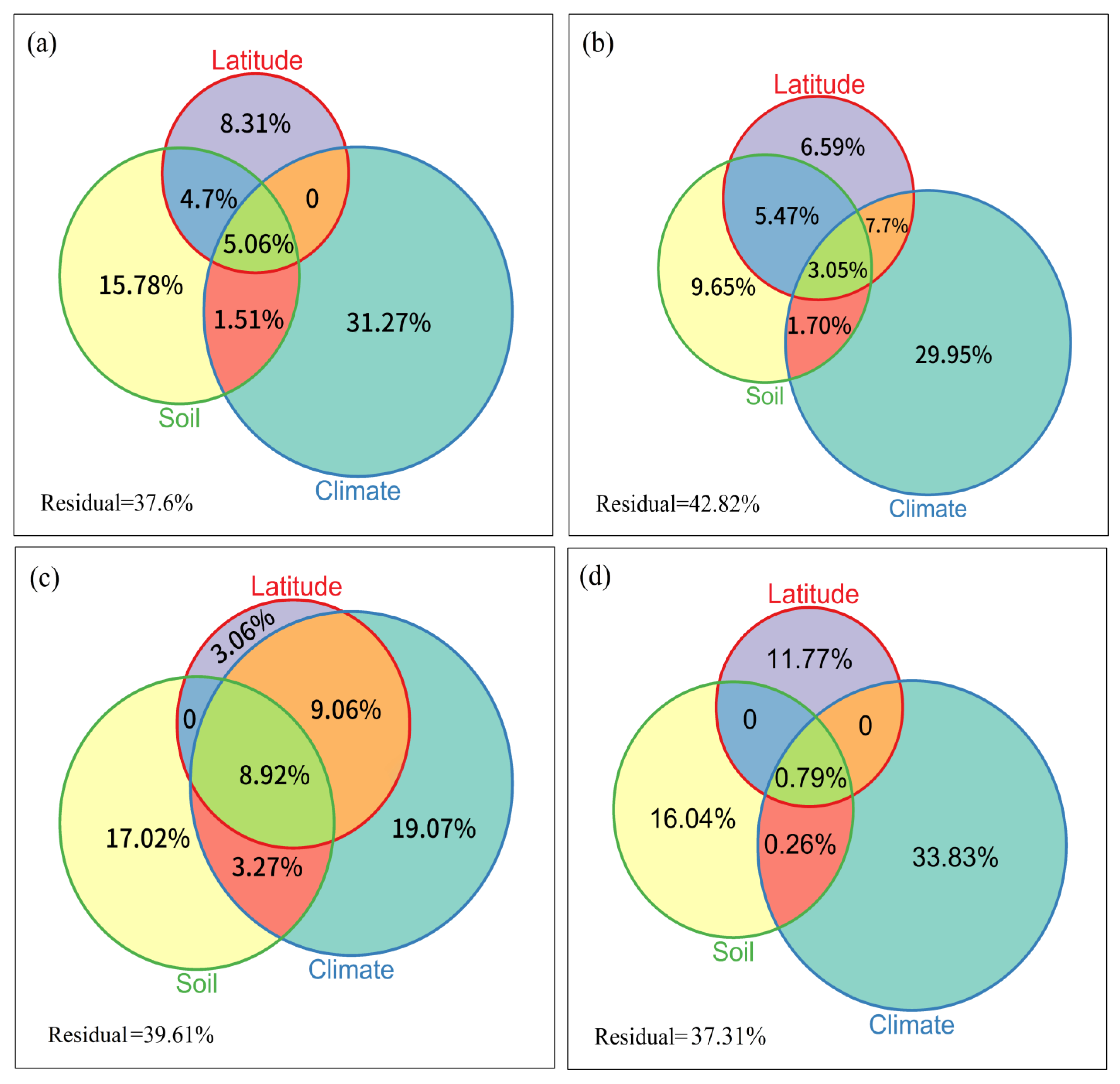
| Study | Location | Vegetation | Soil Type | Climatic Region |
|---|---|---|---|---|
| Huang et al. [44] | Tahe | Larix olgensis, Populus davidiana and Betula costata | Brown coniferous forest soil | Temperate zone |
| 52.33° N, 124.75° E | ||||
| Zhang et al. [45] | Aershan | Larix gmelini and Populus davidiana | Brown coniferous forest soil | Temperate zone |
| 47.18° N, 119.94° E | ||||
| Nakamura [46] | Sapporo | Abies fabri | Brown forest soil | Temperate zone |
| 42.87° N, 141.24° E | ||||
| Han [47] | Changbaishan 43.65° N, 127.62° E | Pinus koraiensis, Quercus mongolica, Acer mono, Populus davidiana and Betula platyphylla | Mountain dark brown soil | Temperate zone |
| Xu et al. [48] | Donglingshan | Quercus liaotungensis | Brown soil | Temperate zone |
| 40.03° N, 115.47° E | ||||
| Kwon [49] | Ganghwa | Pinus densiflora and Quercus mongolica | Mountain yellow soil | Temperate zone |
| 37.61° N, 126.45° E | ||||
| Touyama [50] | Hiroshima | Pinus koraiensis, Picea asperata and Tilia amurensis | Brown forest soil | Subtropical zone |
| 34.52° N, 132.23° E | ||||
| Xu [51] | Baotianman 33.51° N, 111.94° E | Quercus variabilis, Quercus aliena var. acutidentata and Pinus armandii | Mountain yellow brown soil | Temperate zone |
| Yi [52] | Tiantongshan | Castanopsis fargesii, Schima superba and Pinus massoniana | Mountain yellow red soil | Subtropical zone |
| 29.80° N, 121.79° E | ||||
| He [53] | Badagongshan 29.74° N, 110.06° E | Fagus lucida, Liquidambar formosana and Castanopsis fargesii | Mountain yellow brown soil | Subtropical zone |
| Li et al. [54] | Shimentai 24.45° N, 113.3° E | Castanopsis eyrei, Schima superba, Pinus massoniana and Pinus elliottii | Mountainred soil | Subtropical zone |
| Chuan et al. [55] | Yushan | Tsuga chinensis and Yushania niitakayamensis | Mountain yellow red soil | Subtropical zone |
| 23.47° N, 120.89° E | ||||
| Yang et al. [56] | Xishuangbannan 21.68° N, 101.45° E | Hevea brasiliensis, Mallotus paniculatus, Ppometia tomentosa and Terminalia myricocarpa | Orthic acrisol | Tropical zone |
| Xiong [57] | Jianfengling 18.50° N, 109.00° E | Antidesma maclurei, Vatica mangachapoi, Lannea grandis and Aporosa chinensis | Laterite and Yellow soil | Tropical zone |
| Site | Total Number of Individuals | Sampling Volume/L | Individuals Per m3 | Sampling Quantity | Sampling Time/Month | Sampling Time/Year | Sampling Layer/cm | Number of Sampling Locations | Number of Repetitions |
|---|---|---|---|---|---|---|---|---|---|
| TH | 12841 | 126 | 101.9 | 336 | 6, 8, 10 | 2004 | Litter, 0–5–10–15 | 7 | 4 |
| ARS | 9684 | 48 | 201.8 | 128 | 8, 9 | 2004 | Litter, 0–5–10–15 | 4 | 4 |
| SPK | 19012 | 44 | 432.1 | 440 | 1, 5, 7, 9 | 1968 | 0–5 | 11 | 10 |
| CBS | 24325 | 67.5 | 360.4 | 180 | 5, 7, 9 | 2014 | Litter, 0–5–10–15 | 3 | 5 |
| DLS | 52673 | 270 | 195.1 | 480 | 4, 6, 8, 10 | 2012–2013 | Litter, 0–5–10–15 | 10 | 4 |
| GH | 47919 | 39.3 | 1220.8 | 400 | 9 | 2007 | 0–5 | 20 | 10 |
| HMA | 18244 | 70.7 | 258.1 | 120 | 5, 7, 9 | 1990 | Litter, 0–5 | 5 | 4 |
| BTM | 13063 | 47.1 | 277.4 | 640 | 5, 9 | 2018–2019 | Litter, 0–5–10–15 | 16 | 5 |
| TTS | 13937 | 21.6 | 645.2 | 216 | 4, 6, 8, 10 | 2003–2004 | 0–5–10–15 | 6 | 3 |
| BDGS | 12933 | 52.3 | 247.3 | 360 | 4, 7, 11 | 2016 | Litter, 0–15 | 15 | 4 |
| SMT | 20045 | 100 | 200.5 | 400 | 9, 10 | 2001 | Litter, 0–5 | 20 | 5 |
| YS | 12860 | 32.4 | 396.9 | 324 | 4, 6, 8,10 | 1998–1999 | 0–5–10–15 | 3 | 3 |
| XSBN | 14434 | 23.6 | 612.9 | 180 | 7, 8, 9 | 1997 | 0–5–10–15 | 4 | 5 |
| JFL | 37083 | 54 | 686.7 | 540 | 1, 4, 7, 10 | 1993–1994 | 0–5–10–15 | 15 | 3 |
| Class | Order Name | Code | Traits | Class | Order Name | Code | Traits |
|---|---|---|---|---|---|---|---|
| Chilopoda | Geophilomorpha | Geo | Pre | Insecta | Blattaria | Bla | Omn |
| Chilopoda | Lithobiomorpha | Lit | Pre | Insecta | Coleoptera | Cole | Pre |
| Chilopoda | Scolopendromorpha | Sco | Pre | Insecta | Dermaptera | Der | Omn |
| Chilopoda | Scutigeromorpha | Scu | Pre | Insecta | Diplura | Dipl | Phy |
| Copepoda | Harpacticoida | Har | Omn | Insecta | Diptera | Dipt | Omn |
| Diplopoda | Chordeumatida | Cho | Sap | Insecta | Hemiptera | Hem | Omn |
| Diplopoda | Glomerida | Glo | Sap | Insecta | Homoptera | Hom | Phy |
| Diplopoda | Julida | Julid | Sap | Insecta | Hymenoptera | Hym | Omn |
| Diplopoda | Juliformia | Julif | Sap | Insecta | Isoptera | Isopt | Pre |
| Diplopoda | Polydesmida | Polyd | Sap | Insecta | Lepidoptera | Lep | Phy |
| Diplopoda | Polyxenida | Polyx | Sap | Insecta | Microcoryphia | Mic | Phy |
| Diplopoda | Sphaerotheriida | Sph | Sap | Insecta | Neuroptera | Neu | Pre |
| Diplopoda | Spirobolida | Spi | Sap | Insecta | Orthoptera | Ort | Phy |
| Pauropoda | Tetramerocerata | Tet | Sap | Insecta | Plecoptera | Ple | Phy |
| Gastropoda | Archaeogastropoda | Arc | Omn | Insecta | Psocoptera | Pso | Phy |
| Gastropoda | Mesogastropoda | Mesog | Phy | Insecta | Thysanoptera | Thy | Phy |
| Gastropoda | Stylommatophora | Sty | Omn | Insecta | Thysanura | Thysanura | Phy |
| Crustacea | Amphipoda | Amp | Sap | Oligochaeta | Lumbricida | Lum | Sap |
| Entognatha | Collembola | Coll | Omn | Oligochaeta | Tubificida | Tub | Sap |
| Entognatha | Protura | Pro | Sap | Arachnida | Acarina | Aca | Sap |
| Malacostraca | Isopoda | Isopo | Omn | Arachnida | Araneae | Ara | Pre |
| Protura | Eosentomata | Eos | Sap | Arachnida | Mesostigmata | Mes | Pre |
| Protura | Sinentomata | Sin | Sap | Arachnida | Opiliones | Opi | Pre |
| Adenophorea | Rhabditida | Rha | Phy | Arachnida | Pseudoscorpiones | Pse | Pre |
| Symphyla | Symphyla | Sym | Sap | Arachnida | Schizomida | Sch | Sap |
| Site Name | Code | MAT | MTCM | EMT | MAP | SOC | SBD | pH |
|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (°C) | (mm) | (g/kg) | (kg/m3) | |||
| Tahe | TH | −2.4 | −25.5 | −32.6 | 428.0 | 2.8 | 11.1 | 5.5 |
| Aershan | ARS | −3.2 | −21 | −31.5 | 441.4 | 2.2 | 11.8 | 6.5 |
| Changbaishan | CBS | 3.4 | −15.6 | −24.8 | 758.0 | 2.6 | 11.2 | 5.7 |
| DongLingshan | DLS | 8 | −7 | −6.0 | 575.0 | 1.8 | 10.5 | 6.9 |
| Baotianman | BTM | 15.1 | 1.5 | −17.0 | 885.6 | 2 | 11.2 | 5.9 |
| Badagongshan | BDGS | 11.5 | 0.1 | −0.2 | 2105.4 | 1.2 | 11.9 | 5.9 |
| Tiantongshan | TTS | 16.2 | 4.2 | 1.1 | 1374.7 | 1.4 | 12.7 | 5.8 |
| Shimentai | SMT | 20.8 | 10.9 | 4.5 | 2000.0 | 1.6 | 11.4 | 5.1 |
| Jianfengling | JFL | 24.5 | 19.4 | 16.4 | 2265.8 | 0.4 | 13.9 | 6.2 |
| Xishuangbanna | XSBN | 21.8 | 10 | 5.00 | 1556.0 | 1 | 12 | 6 |
| Guanghwa | GH | 10.3 | −8.6 | −8.6 | 1450.5 | 1.2 | 12.9 | 6 |
| Sapporo | SPK | 8.5 | −4 | −14.1 | 738.0 | 5 | 9.2 | 5.1 |
| Hiroshima | HMA | 13.9 | 5 | −2.0 | 1700.0 | 1.8 | 11.1 | 5.1 |
| Yushan | YS | 9 | 4.3 | −6.00 | 4000.0 | 1.6 | 11 | 4.4 |
| Overall | Phytophagous | Predatory | Saprophagous | |||||
|---|---|---|---|---|---|---|---|---|
| R2 | Pr (>r) | R2 | Pr (>r) | R2 | Pr (>r) | R2 | Pr (>r) | |
| Latitude | 0.5227 | 0.02 * | 0.6539 | 0.004 ** | 0.6086 | 0.01 ** | 0.406 | 0.046 * |
| MAT | 0.4886 | 0.028 * | 0.5362 | 0.016 * | 0.5298 | 0.019 * | 0.1762 | 0.34 |
| MTCM | 0.4714 | 0.035 * | 0.566 | 0.012 * | 0.6468 | 0.004 ** | 0.4775 | 0.019 * |
| EMT | 0.3641 | 0.085 | 0.3293 | 0.098 | 0.5203 | 0.01 ** | 0.1626 | 0.35 |
| MAP | 0.7765 | 0.001 *** | 0.7527 | 0.001 *** | 0.7322 | 0.001 *** | 0.3843 | 0.062 |
| SOC | 0.6292 | 0.002 ** | 0.5848 | 0.001 *** | 0.3942 | 0.04 * | 0.6665 | 0.002 ** |
| SBD | 0.7279 | 0.001 *** | 0.4508 | 0.028 * | 0.2802 | 0.162 | 0.6523 | 0.003 ** |
| pH | 0.0631 | 0.692 | 0.1238 | 0.5 | 0.2282 | 0.25 | 0.1134 | 0.35 |
| Overall | Phytophagous | Predatory | Saprophagous | |||||||||||||
| βj | βs | βj | βs | βj | βs | βj | βs | |||||||||
| Slope | p | Slope | p | Slope | p | Slope | p | Slope | p | Slope | p | Slope | p | Slope | p | |
| Lat | 0.025 | * | 0.019 | * | 0.035 | * | 0.036 | * | 0.054 | *** | 0.039 | *** | 0.014 | NS | 0.006 | NS |
| MAT | 0.003 | NS | 0.002 | NS | 0.008 | ** | 0.008 | ** | 0.008 | ** | 0.006 | ** | 0.002 | NS | 0.010 | * |
| EMT | 0.002 | ** | 0.002 | ** | 0.006 | *** | 0.006 | *** | 0.005 | *** | 0.004 | *** | 0.000 | NS | 0.001 | NS |
| MTCM | 0.001 | NS | 0.001 | NS | 0.005 | ** | 0.005 | ** | 0.006 | *** | 0.004 | *** | 0.002 | NS | 0.005 | * |
| MAP | 0.013 | NS | 0.010 | NS | 0.04 | NS | 0.001 | NS | 0.034 | * | 0.025 | * | 0.000 | NS | 0.022 | NS |
| SOC | 0.039 | *** | 0.033 | *** | 0.039 | * | 0.035 | * | 0.062 | *** | 0.049 | *** | 0.035 | * | 0.059 | ** |
| SBD | 0.018 | NS | 0.016 | NS | 0.015 | NS | 0.016 | NS | 0.043 | ** | 0.033 | ** | 0.019 | NS | 0.055 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Zhang, J.; Ding, S.; Yan, P.; Zhang, P.; Ding, S. Environmental Effects on Taxonomic Turnover in Soil Fauna across Multiple Forest Ecosystems in East Asia. Insects 2022, 13, 1103. https://doi.org/10.3390/insects13121103
Li P, Zhang J, Ding S, Yan P, Zhang P, Ding S. Environmental Effects on Taxonomic Turnover in Soil Fauna across Multiple Forest Ecosystems in East Asia. Insects. 2022; 13(12):1103. https://doi.org/10.3390/insects13121103
Chicago/Turabian StyleLi, Peikun, Jian Zhang, Shunping Ding, Peisen Yan, Panpan Zhang, and Shengyan Ding. 2022. "Environmental Effects on Taxonomic Turnover in Soil Fauna across Multiple Forest Ecosystems in East Asia" Insects 13, no. 12: 1103. https://doi.org/10.3390/insects13121103





