Evolutionary Potential of Parthenogenesis—Bisexual Lineages within Triploid Apomictic Thelytoky in Cacopsylla ledi (Flor, 1861) (Hemiptera, Psylloidea) in Fennoscandia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Cytological Study
2.3. Molecular Analysis
3. Results
4. Discussion
4.1. Diploid Males and Females Show a Haplotype Different from Triploid Females
4.2. Diploids Show the Same Haplotype as Triploid Parthenogenetic Females
4.3. How Are the Bisexually Reproducing Populations Formed?
4.4. Evolutionary Potential of Triploid Apomictic Parthenogenesis in C. ledi
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Normark, B.B. The Evolution of Alternative Genetic Systems in Insects. Annu. Rev. Entomol. 2003, 48, 397–423. [Google Scholar] [CrossRef] [PubMed]
- Vershinina, A.O.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Entognatha and Non-Holometabolous Insects. J. Zool. Syst. Evol. Res. 2016, 54, 257–268. [Google Scholar] [CrossRef]
- Gokhman, V.E.; Kuznetsova, V.G. Parthenogenesis in Hexapoda: Holometabolous Insects. J. Zool. Syst. Evol. Res. 2018, 56, 23–34. [Google Scholar] [CrossRef]
- Bell, G. The Masterpiece of Nature The Evolution and Genetics of Sexuality; University of California Press: Berkeley, CA, USA, 1982. [Google Scholar]
- Nougué, O.; Rode, N.O.; Jabbour-zahab, R.; Ségard, A.; Chevin, L.-M.; Haag, C.R.; Lenormand, T. Automixis in Artemia: Solving a Century-Old Controversy. J. Evol. Biol. 2015, 28, 2337–2348. [Google Scholar] [CrossRef]
- Simon, J.-C.; Delmotte, F.; Rispe, C.; Crease, T. Phylogenetic Relationships between Parthenogens and Their Sexual Relatives: The Possible Routes to Parthenogenesis in Animals. Biol. J. Linn. Soc. 2003, 79, 151–163. [Google Scholar] [CrossRef]
- Butlin, R. The Costs and Benefits of Sex: New Insights from Old Asexual Lineages. Nat. Rev. Genet. 2002, 3, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Capdevielle Dulac, C.; Benoist, R.; Paquet, S.; Calatayud, P.-A.; Obonyo, J.; Kaiser, L.; Mougel, F. Spontaneous Parthenogenesis in the Parasitoid Wasp Cotesia Typhae: Low Frequency Anomaly or Evolving Process? Peer Community J. 2022, 2, e37. [Google Scholar] [CrossRef]
- Lynch, M. Destabilizing Hybridization, General-Purpose Genotypes and Geographic Parthenogenesis. Q. Rev. Biol. 1984, 59, 257–290. [Google Scholar] [CrossRef]
- Palmer, S.C.; Norton, R.A. Further Experimental Proof of Thelytokous Parthenogenesis in Oribatid Mites (Acari: Oribatida: Desmonomata). Exp. Appl. Acarol. 1990, 8, 149–159. [Google Scholar] [CrossRef]
- Maccari, M.; Gómez, A.; Hontoria, F.; Amat, F. Functional Rare Males in Diploid Parthenogenetic Artemia. J. Evol. Biol. 2013, 26, 1934–1948. [Google Scholar] [CrossRef]
- Nokkala, C.; Kuznetsova, V.; Nokkala, S. Meiosis in Rare Males in Parthenogenetic Cacopsylla myrtilli (Wagner, 1947) (Hemiptera, Psyllidae) Populations from Northern Europe. Comp. Cytogenet. 2013, 7, 241–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokkala, C.; Kuznetsova, V.G.; Nokkala, S. Rare Diploid Females Coexist with Rare Males: A Novel Finding in Triploid Parthenogenetic Populations in the Psyllid Cacopsylla myrtilli (W. Wagner, 1947) (Hemiptera, Psylloidea) in Northern Europe. Genetica 2015, 143, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Norton, R.A. Taxonomic, Geographic and Seasonal Distribution of Thelytokous Parthenogenesis in the Desmonomata (Acari: Oribatida). Exp. Appl. Acarol. 1991, 12, 67–81. [Google Scholar] [CrossRef]
- Norton, R.; Kethley, J.; Johnston, D.; O’Connor, B. Phylogenetic Perspectives on Genetic Systems and Reproductive Modes of Mites. In Evolution and Diversity of Sex Ratio in Haplodiploid Insects and Mites; Wrench, D., Ebbert, M., Eds.; Chapman & Hall: New York, NY, USA, 1993; pp. 8–99. [Google Scholar]
- Kearney, M. Hybridization, Glaciation and Geographical Parthenogenesis. Trends Ecol. Evol. 2005, 20, 495–502. [Google Scholar] [CrossRef]
- Peck, J.R.; Yearsley, J.M.; Waxman, D. Explaining the Geographic Distributions of Sexual and Asexual Populations. Nature 1998, 391, 889–892. [Google Scholar] [CrossRef]
- Normark, B.B. Modes of Reproduction. In The Evolution of Insect Mating Systems; Oxford University Press: Oxford, UK, 2014; pp. 1–19. [Google Scholar]
- Mark Welch, D.B.; Meselson, M. Evidence for the Evolution of Bdelloid Rotifers Without Sexual Reproduction or Genetic Exchange. Science (1979) 2000, 288, 1211–1215. [Google Scholar] [CrossRef] [Green Version]
- Heethoff, M.; Domes, K.; Laumann, M.; Maraun, M.; Norton, R.A.; Scheu, S. High Genetic Divergences Indicate Ancient Separation of Parthenogenetic Lineages of the Oribatid Mite Platynothrus Peltifer (Acari, Oribatida). J. Evol. Biol. 2007, 20, 392–402. [Google Scholar] [CrossRef]
- Domes, K.; Norton, R.A.; Maraun, M.; Scheu, S. Reevolution of Sexuality Breaks Dollo’s Law. Proc. Natl. Acad. Sci. USA 2007, 104, 7139–7144. [Google Scholar] [CrossRef] [Green Version]
- Maccari, M.; Amat, F.; Gómez, A. Origin and Genetic Diversity of Diploid Parthenogenetic Artemia in Eurasia. PLoS ONE 2013, 8, e83348. [Google Scholar] [CrossRef]
- Maccari, M.; Amat, F.; Hontoria, F.; Gómez, A. Laboratory Generation of New Parthenogenetic Lineages Supports Contagious Parthenogenesis in Artemia. PeerJ 2014, 2, e439. [Google Scholar] [CrossRef]
- Boyer, L.; Jabbour-Zahab, R.; Mosna, M.; Haag, C.R.; Lenormand, T. Not so Clonal Asexuals: Unraveling the Secret Sex Life of Artemia Parthenogenetica. Evol. Lett. 2021, 5, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Schön, I.; Gandolfi, A.; di Masso, E.; Rossi, V.; Griffiths, H.I.; Martens, K.; Butlin, R.K. Persistence of Asexuality through Mixed Reproduction in Eucypris Virens (Crustacea, Ostracoda). Heredity (Edinb.) 2000, 84, 161–169. [Google Scholar] [CrossRef]
- Delmotte, F.; Leterme, N.; Bonhomme, J.; Rispe, C.; Simon, J.-C. Multiple Routes to Asexuality in an Aphid Species. Proc. R. Soc. Lond. B Biol. Sci. 2001, 268, 2291–2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwander, T.; Henry, L.; Crespi, B.J. Molecular Evidence for Ancient Asexuality in Timema Stick Insects. Curr. Biol. 2011, 21, 1129–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nokkala, S.; Maryańska-Nadachowska, A.; Kuznetsova, V.G. First Evidence of Polyploidy in Psylloidea (Homoptera, Sternorrhyncha): A Parthenogenetic Population of Cacopsylla myrtilli (W. Wagner, 1947) from Northeast Finland Is Apomictic and Triploid. Genetica 2008, 133, 201–205. [Google Scholar] [CrossRef]
- Nokkala, S.; Kuznetsova, V.G.; Nokkala, C. Characteristics of Parthenogenesis in Cacopsylla ledi (Flor, 1861) (Hemiptera, Sternorryncha, Psylloidea): Cytological and Molecular Approaches. Comp. Cytogenet. 2017, 11, 807–817. [Google Scholar] [CrossRef] [Green Version]
- Ossiannilsson, F. The Psylloidea (Homoptera) of Fennoscandia and Denmark. Fauna Entomol. Scand. 1992, 26, 1–347. [Google Scholar]
- Nokkala, C.; Kuznetsova, V.G.; Rinne, V.; Nokkala, S. Description of Two New Species of the Genus Cacopsylla Ossiannilsson, 1970 (Hemiptera, Psylloidea) from Northern Fennoscandia Recognized by Morphology, Cytogenetic Characters and COI Barcode Sequence. Comp. Cytogenet. 2019, 13, 367–382. [Google Scholar] [CrossRef]
- Hall Tom, A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Schmitt, T.; Varga, Z. Extra-Mediterranean Refugia: The Rule and Not the Exception? Front. Zool. 2012, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Stewart, J.R.; Lister, A.M. Cryptic Northern Refugia and the Origins of the Modern Biota. Trends Ecol. Evol. 2001, 16, 608–613. [Google Scholar] [CrossRef]
- Provan, J.; Bennett, K.D. Phylogeographic Insights into Cryptic Glacial Refugia. Trends Ecol. Evol. 2008, 23, 564–571. [Google Scholar] [CrossRef]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia Revisited: Individualistic Responses of Species in Space and Time. Proc. R. Soc. B Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, H. The Genetic Legacy of the Quaternary Ice Ages. Nature 2000, 405, 907–913. [Google Scholar]
- Ibrahim, K.M.; Nichols, R.A.; Hewitt, G.M. Spatial Patterns of Genetic Variation Generated by Different Forms of Dispersal during Range Expansion. Heredity (Edinb.) 1996, 77, 282–291. [Google Scholar] [CrossRef]
- Nokkala, C.; Kuznetsova, V.G.; Shapoval, N.A.; Nokkala, S. Phylogeography and Wolbachia Infections Reveal Postglacial Recolonization Routes of the Parthenogenetic Plant Louse Cacopsylla myrtilli (W. Wagner 1947), (Hemiptera, Psylloidea). J. Zool. Syst. Evol. Res. 2022, 2022, 5458633. [Google Scholar] [CrossRef]
- Taberlet, P.; Fumagalli, L.; Wust-Saucy, A.; Cosson, J. Comparative Phylogeography and Postglacial Colonization Routes in Europe. Mol. Ecol. 1998, 7, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, G.M. Postglacial Distribution and Species Structure: Lessons from Pollen, Insects and Hybrid Zones. In Evolutionary Patterns and Processes. Linnean Society Symposium Series; Lees, D.R., Edwards, D., Eds.; Academic Press: London, UK, 1993; Volume 14, pp. 97–123. [Google Scholar]
- Poutanen, M.; Steffen, H. Land Uplift at Kvarken Archipelago/High Coast UNESCO World Heritage Area. Geophysica 2014, 50, 49–64. [Google Scholar]
- Nordman, M.; Peltola, A.; Bilker-Koivula, M.; Lahtinen, S. Past and Future Sea Level Changes by Nodman et al. In International Association of Geodesy Symposia; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–7. [Google Scholar]
- Hewitt, G.M. The Structure of Biodiversity—Insights from Molecular Phylogeography. Front. Zool. 2004, 1, 4. [Google Scholar] [CrossRef] [Green Version]
- van der Kooi, C.J.; Schwander, T. On the Fate of Sexual Traits under Asexuality. Biol. Rev. 2014, 89, 805–819. [Google Scholar] [CrossRef]
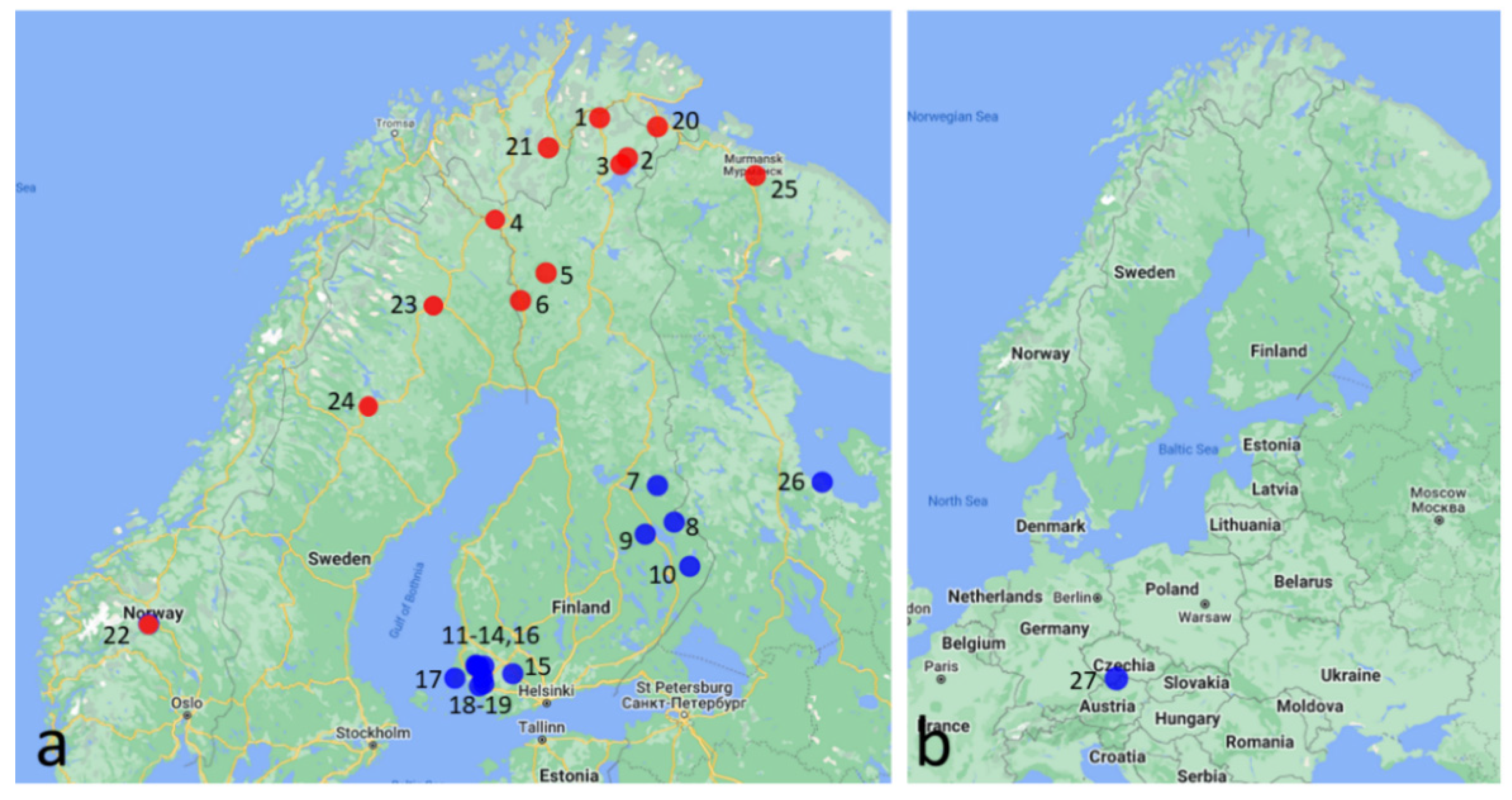


| Location | Coordinates | Females | Males | Male% | Date | |
|---|---|---|---|---|---|---|
| Finland | ||||||
| 1 | Utsjoki, Hietala | 69.851667 | 28 | 2 | 6.7 | 15 August 2017 |
| 27.009444 | ||||||
| 2 | Inari, Nitsijärvi | 69.302 | 41 | 3 | 7.3 | 14 August 2021 |
| 28.107 | ||||||
| 3 | Inari, Sevettijärvi | 69.2161111 | 160 | 10 | 5.9 | 17 August2017 |
| 27.8705556 | ||||||
| 4 | Enontekiö, Käsivarrentie | 68.32450 | 11 | 0 | 0 | 14 August 2022 |
| 22.993397 | ||||||
| 5 | Kittilä | 67. 628 | 184 | 0 | 0 | 12 August 2021 |
| 24.933 | ||||||
| 6 | Kolari | 67.209167 | 51 | 1 | 1.9 | 9 August 2018 |
| 23.906389 | ||||||
| 7 | Kuhmo, Syväjärvi | 64.1946 | 161 | 64 | 28.4 | 4 August 2019 |
| 29.2932 | ||||||
| 8 | Lieksa, Nurmijärvi | 63.5430 | 77 | 1 | 1.3 | 5 August 2019 |
| 29.9635 | ||||||
| 9 | Nurmes, Panjavaara | 63.335394 | 94 | 6 | 6.0 | 1 August 2020 |
| 28.828826 | ||||||
| 10 | Ilomantsi, Tokrajärvi | 62.7544 | 68 | 0 | 0 | 5 August 2019 |
| 30.5773 | ||||||
| 11 | Eura, Isosuo | 60.901389 | 107 | 18 | 14.4 | 24 August 2018 |
| 22.161944 | ||||||
| 12 | Eura, Laustinrahka | 60.889722 | 85 | 13 | 13.3 | 24 August 2018 |
| 22.146667 | ||||||
| 13 | Pöytyä, Levonsuo | 60.886111 | 102 | 5 | 4.7 | 15 August 2019 |
| 22.444722 | ||||||
| 14 | Pöytyä, Valastensuo | 60.856000 | 34 | 29 | 46.0 | 21 August 2020 |
| 22.273000 | ||||||
| 15 | Tammela, Torronsuo | 60.738889 | 56 | 6 | 9.7 | 18 July 2019 |
| 23.585778 | ||||||
| 16 | Pöytyä, Lammenrahka | 60.676944 | 182 | 107 | 37.0 | 22 August 2018 |
| 22.425556 | ||||||
| 17 | Kustavi, Kaurissalo | 60.655556 | 313 | 7 | 2.2 | 25 August 2019 |
| 21.303333 | ||||||
| 18 | Marttila, Onnenperänrahka | 60.544444 | 202 | 27 | 11.8 | 19 August 2019 |
| 22.444722 | ||||||
| 19 | Turku, Runosmäki | 60.498889 | 1141 | 100 | 8.8 | [29] |
| 22.265278 | ||||||
| Norway | ||||||
| 20 | Neiden, Jäälä | 69.732586 | 42 | 0 | 0 | 28 July 2020 |
| 29.296901 | ||||||
| 21 | Mohkejogas, Finmark | 69.443289 | 218 | 28 | 12.8 | 26 July 2020 |
| 25.193346 | ||||||
| 22 | Sjoa, Stålane | 61.6875000 | 3 | 0 | 0 | 1 August 2009 |
| 9.2408333 | ||||||
| Sweden | ||||||
| 23 | Jokkmokk, Kåbdalis | 66.027386 | 9 | 0 | 0 | 14 August 2022 |
| 19.908879 | ||||||
| 24 | Sorsele, Europaväg 45 | 65.571807 | 12 | 0 | 0 | 14 August 2022 |
| 18.045919 | ||||||
| Russsia | ||||||
| 25 | Murmansk, Mokhnatkina Pakhta | 69.050851 | 115 | 66 | 36.5 | 31 July2020 |
| 33.161461 | ||||||
| 26 | Kolezma | 64.246033 | 44 | 0 | 29 September 2020 | |
| 35.813505 | ||||||
| Czech Republic | ||||||
| 27 | Cervene Blato Natl. Nat. Res. | 48.858611 | 10 | 8 | 44.4 | 20 July 2021 |
| 14.803889 | ||||||
| Population (Freq. of Males) | 3n Females | 2n Females | 2n Males | Principal Type of Reproduction | |
|---|---|---|---|---|---|
| 1 | Inari, Sevettijärvi (5.9%) | H1 N = 36 | H1 N = 5 | H1 N = 7 | Parthenogenetic |
| 2 | Pöytyä, Lammenrahka (37.0%) | H1 N = 1 | H2 N = 43 | H2 N =30 | Bisexual |
| 3 | Eura, Laustinrahka (13.3%) | H1 N = 16 | H2 N = 23 | H1 N = 2 H2 N = 11 | Mixed |
| 4 | Pöytyä, Levonsuo (4.7%) | H1 N = 44 | H2 N = 1 | H1 N = 1 H2 N = 4 | Mixed |
| Sampling Location | Females | Males | Type of Reprouction | |||
|---|---|---|---|---|---|---|
| 3n | 2n | |||||
| H1 | H1 | H2 | H1 | H2 | ||
| Inari, Nitsijärvi | 2 | parthenogenetic | ||||
| Inari, Sevettijärvi | 36 | 5 | 7 | parthenogenetic | ||
| Enontekiö, Käsivarrentie | 11 | parthenogenetic | ||||
| Kittilä | 10 | parthenogenetic | ||||
| Kolari | 4 | 1 | parthenogenetic | |||
| Kuhmo, Syväjärvi | 24 | 17 | 35 | mixed | ||
| Nurmes, Panjavaara | 6 | mixed | ||||
| Eura, Isosuo | 7 | mixed | ||||
| Eura, Laustinrahka | 16 | 23 | 2 | 11 | mixed | |
| Pöytyä, Levonsuo | 44 | 1 | 1 | 4 | mixed | |
| Pöytyä, Valastensuo | 11 | 11 | bisexual | |||
| Tammela, Torronsuo | 6 | mixed | ||||
| Pöytyä, Lammenrahka | 1 | 43 | 30 | bisexual | ||
| Kustavi, Kaurissalo | 6 | 6 | mixed | |||
| Marttila, Onnenperänrahka | 16 | mixed | ||||
| Turku, Runosmäki | 61 | 6 | 5 | mixed | ||
| Jokkmokk, Kåbdalis | 9 | parthenogenetic | ||||
| Sorsele, Europavägen 45 | 12 | parthenogenetic | ||||
| Neiden, Jäälä | 11 | parthenogenetic | ||||
| Mohkejogas, Finmark | 20 | 12 | mixed | |||
| Sjoa, Stålane | 2 | parthenogenetic | ||||
| Murmansk, Mokhnatkina Pakhta | 30 | 30 | bisexual | |||
| Kolezma | 17 | 1 | mixed | |||
| Cervene blato Natl.Nat.Res. | 6 | 2 | bisexual | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nokkala, S.; Kuznetsova, V.G.; Pietarinen, P.; Nokkala, C. Evolutionary Potential of Parthenogenesis—Bisexual Lineages within Triploid Apomictic Thelytoky in Cacopsylla ledi (Flor, 1861) (Hemiptera, Psylloidea) in Fennoscandia. Insects 2022, 13, 1140. https://doi.org/10.3390/insects13121140
Nokkala S, Kuznetsova VG, Pietarinen P, Nokkala C. Evolutionary Potential of Parthenogenesis—Bisexual Lineages within Triploid Apomictic Thelytoky in Cacopsylla ledi (Flor, 1861) (Hemiptera, Psylloidea) in Fennoscandia. Insects. 2022; 13(12):1140. https://doi.org/10.3390/insects13121140
Chicago/Turabian StyleNokkala, Seppo, Valentina G. Kuznetsova, Peppi Pietarinen, and Christina Nokkala. 2022. "Evolutionary Potential of Parthenogenesis—Bisexual Lineages within Triploid Apomictic Thelytoky in Cacopsylla ledi (Flor, 1861) (Hemiptera, Psylloidea) in Fennoscandia" Insects 13, no. 12: 1140. https://doi.org/10.3390/insects13121140





