Fine Structure of the Visual System of Arge similis (Hymenoptera, Argidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Compound Eye Preparation for Scanning Electron Microscopy (SEM)
2.3. Compound Eye Preparation for Transmission Electron Microscope (TEM)
2.4. Compound Eye Preparation for Light Microscopy (LM)
2.5. Data Obtaining and Analysis
3. Result
3.1. External Morphology of the Compound Eye and Ocelli in Arge similis
3.2. Ultrastructure of the Compound Eyes in Arge similis
3.3. Dioptric Apparatus
3.4. Photoreceptive Layer
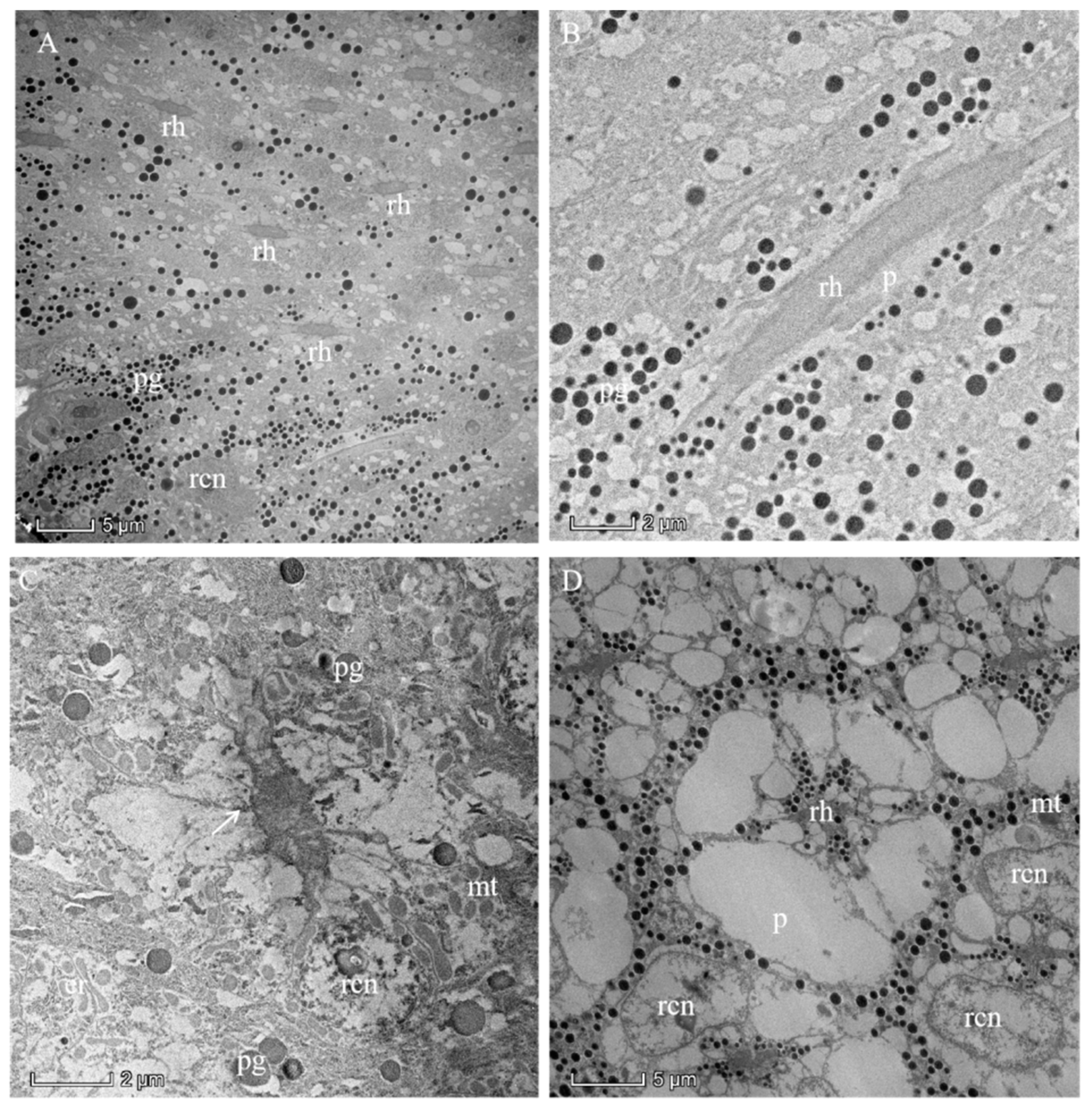
3.5. Pigments
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paterson, J.R.; Garcia-Bellido, D.C.; Lee, M.S.Y.; Brock, G.A.; Jaho, J.B.; Edgecombe, G.D. Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 2011, 480, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B. Compound eyes of insects and crustaceans: Some examples that show there is still a lot of work left to be done. Insect Sci. 2015, 22, 461–481. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F. The Insects: Structure and Function; Academic Press: New York, NY, USA, 1998; pp. 132–141. [Google Scholar] [CrossRef]
- Jia, L.; Liang, A. Fine structure of the compound eyes of Callitettix versicolor (Insecta: Hemiptera). Ann. Entomol. Soc. Am. 2015, 108, 316–324. [Google Scholar] [CrossRef]
- Prokopy, R.J.; Owens, E.D. Visual detection of plants by herbivorous insects. Annu. Rev. Entomol. 2003, 28, 337–364. [Google Scholar] [CrossRef]
- Moericke, V.R.; Prokopy, R.J.; Berlocher, S. Visual stimuli elicting attraction of Rhagoletis pomonella (Diptera: Tephritidae) flies to trees. Entomol. Exp. Appl. 2011, 18, 497–507. [Google Scholar] [CrossRef]
- Gokan, N.; Meyer-Rochow, V.B. Morphological comparisons of compound eyes in Scarabaeoidea (Coleoptera) related to the beetles’ daily activity maxima and phylogenetic positions. J. Agric. Sci. 2000, 45, 15–61. [Google Scholar]
- Exner, S. Die Physiologie der Facettierten Augen von Krebsen und Insekten; Franz Deuticke: Leipzig, Germany, 1891. [Google Scholar]
- Horridge, G.A. Optical mechanisms of clear-zone eyes. In The Compound Eye and Vision of Insects; Horridge, G.A., Ed.; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Warrant, E.J.; McIntyre, P.D. Arthropod eye design and the physical limits to spatial resolving power. Prog. Neurobiol. 1993, 40, 413–461. [Google Scholar] [CrossRef]
- Wen, C.; Ma, T.; Deng, Y.; Liu, C.; Liang, S.; Wen, J.; Wang, C.; Wen, X. Morphological and optical features of the apposition compound eye of Monochamus alternatus Hope (Coleoptera: Cerambycidae). Micron 2019, 128, 102769. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Waldvogel, H. Visual behaviour and the structure of dark and light-adapted larval and adult eyes of the New Zealand glowworm Arachnocampa luminosa (Mycetophilidae: Diptera). J. Insect Physiol. 1979, 25, 601–613. [Google Scholar] [CrossRef]
- Jia, L.; Liang, A. An apposition compound eye adapted for nocturnal vision in the moth midge Clogmia albipunctata (Williston) (Diptera: Psychodidae). J. Insect Physiol. 2017, 98, 188–198. [Google Scholar] [CrossRef]
- Land, M.F.; Gibson, G.; Horwood, J.; Zeil, J. Fundamental differences in the optical structure of the eyes of nocturnal and diurnal mosquitoes. J. Comp. Physiol. A 1999, 185, 91–103. [Google Scholar] [CrossRef]
- Greiner, B.; Ribi, W.; Warrant, E. Retinal and optical adaptations for nocturnal vision in the halictid bee Megalopta genalis. Cell Tissue Res. 2004, 316, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Kim, H.H.; Lee, D.W.; Lee, S.M.; Shin, H.C.; Choo, H.Y. Biological control of Arge captiva, Arge pagana papana, and Arge similis with entomopathogenic nematodes. J. Korean For. Soc. 2007, 96, 1–6. [Google Scholar]
- Shi, X.; Yang, S.; Zhang, Y.; Ma, T.; Jia, C.; Wen, X. Emergence and reproductive behaviors of Arge similis Vollenhoven (Hymenoptera: Argidae). For. Pest. Dis. 2018, 6, 20–23. (In Chinese) [Google Scholar] [CrossRef]
- Milne, R.; Davies, C.; Prickett, R.; Inns, L.; Chamberlain, D. Phylogeny of Rhododendron subgenus Hymenanthes based on chloroplast DNA markers: Between-lineage hybridisation during adaptive radiation? Plant Syst. Evol. 2010, 285, 233–244. [Google Scholar] [CrossRef]
- Shrestha, N.; Su, X.; Xu, X. The drivers of high rhododendron diversity in south-west China: Does seasonality matter? J. Biogeogr. 2017, 45, 438–447. [Google Scholar] [CrossRef]
- Jia, C.; Lin, B.; Zheng, Z.; Yuan, Y.; Wen, J.; Liang, J.; Chen, K. Spatial distribution pattern of Arge similis Vollenhoven eggs and sampling technique. J. Env. Entomol. 2003, 35, 744–748. (In Chinese) [Google Scholar] [CrossRef]
- Shi, X. The Preliminary Study of Biological Ecology of Arge similis. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar]
- Qi, S. Occurrence regularity and integrated control of Arge similis Vollenhoven. For. Pest Dis. 2000, 6, 24–25. (In Chinese) [Google Scholar]
- Meyer-Rochow, V.B. Structure and function of the larval eye of the sawfly Perga sp. (Pergidae, Hymenoptera). J. Insect Physiol. 1974, 20, 1565–1591. [Google Scholar] [CrossRef]
- Bouligand, Y. Sur une architecture torsadée repondue dans de nombreuses cuticles d’arthropodes. Comptes Rendus Acad. Sci. 1965, 261, 3665–3668. [Google Scholar]
- Jander, U.; Jander, R. Allometry and resolution of bee eyes (Apoidea). Arthropod Struct. Dev. 2002, 30, 179–193. [Google Scholar] [CrossRef]
- Fischer, S.; Müller, C.H.G.; Meyer-Rochow, V.B. How small can small be: The compound eye of the parasitoid wasp Trichogramma evanescens (Westwood, 1833) (Hymenoptera, Hexapoda), an insect of 0.3- to 0.4-mm total body size. Vis. Neurosci. 2011, 28, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Narendra, A.; Zeil, J. The properties of the visual system in the Australian desert ant Melophorus bagoti. Arthropod Struct. Dev. 2011, 40, 128–134. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.; Caveney, S. Superposition optics and the time of flight in onitine dung beetles. J. Comp. Physiol. A 1998, 183, 45–60. [Google Scholar] [CrossRef]
- Kirschfeld, K. The resolution of lens and compound eyes. In Neural Principles in Vision; Zettler, F., Weiler, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1976; pp. 354–370. ISBN 9783642664342. [Google Scholar]
- Land, M.F. Visual acuity in insects. Annu. Rev. Entomol. 1977, 42, 147–177. [Google Scholar] [CrossRef] [Green Version]
- Laughlin, S.B.; Van Steveninck, R.R.D.; Anderson, J.C. The metabolic cost of neural information. Nat. Neurosci. 1998, 1, 36–41. [Google Scholar] [CrossRef]
- Laughlin, S.B. Energy as a constraint on the coding and processing of sensory information. Curr. Opin. Neurobiol. 2001, 1, 475–480. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Mishra, M. Structure and putative function of dark- and light-adapted as well as UV-exposed eyes of the food store pest Psyllipsocus ramburi Sélys-Longchamps (Insecta: Psocoptera: Psyllipsocidae). J. Insect Physiol. 2007, 53, 157–169. [Google Scholar] [CrossRef]
- Almach, L.A.; Lindgren, B.S.; Aukema, B.H. The role of vision in the host orientation behaviour of Hylobius warreni. Agric. For. Entomol. 2012, 14, 286–294. [Google Scholar] [CrossRef]
- Jakobsson, J.; Henze, M.J.; Svensson, G.P. Visual cues of oviposition sites and spectral sensitivity of Cydia strobilella L. J. Insect Physiol. 2017, 101, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Lau, T.F.S.; Gross, E.M.; Meyer-Rochow, V.B. Sexual dimorphism and light/dark adaptation in the compound eyes of male and female Acentria ephemerella (Lepidoptera: Pyraloidea: Crambidae). Eur. J. Entomol. 2007, 104, 459–470. [Google Scholar] [CrossRef] [Green Version]
- Raguso, R.A.; Willis, M.A. Synergy between visual and olfactory cues in nectar feeding by nave hawkmoths, Manduca sexta. Anim. Behav. 2002, 64, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Land, M.F. Optics and vision in invertebrates. In Hand-Book of Sensory Physiology; Autrum, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1981; pp. 471–592. [Google Scholar]
- Varela, F.G.; Porter, K.R. Fine structure of the visual systems of the honey bee (Apis mellifera). J. Ultrastruct. Res. 1969, 29, 236–259. [Google Scholar] [CrossRef]
- Brunnert, A.; Wehner, R. Fine structure of light- and dark-adapted eyes of desert ants, Cataglyphis bicolor (Formicidae, Hymenoptera). J. Morphol. 1973, 140, 15–29. [Google Scholar] [CrossRef]
- Ribi, W.A. Colour receptors in the eye of the digger wasp, Sphex cognatus Smith: Evaluation by selective adaptation. Cell Tissue Res. 1978, 195, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.A. Ommatidia numbers and eyes in scolytid beetles. Ann. Entomol. Soc. Am. 1972, 65, 550–553. [Google Scholar] [CrossRef]
- Makarova, A.A.; Meyer-Rochow, V.B.; Polilov, A.A. Morphology and scaling of compound eyes in the smallest beetles (Coleoptera: Ptiliidae). Arthropod Struct. Dev. 2019, 48, 83–97. [Google Scholar] [CrossRef]
- Barlow, H.B. Size of ommatidia in apposition eyes. J. Exp. Biol. 1952, 29, 667–674. [Google Scholar] [CrossRef]
- Mishra, M. Eye ultrastructure investigation of Scaphidium japonum reitter (Coleoptera: Staphylinidae: Scaphidiidae). J. Entomol. Zool. Stud. 2013, 1, 8–16. [Google Scholar]
- Meyer-Rochow, V.B.; Kashiwagi, T.; Eguchi, E. Selective photoreceptor damage in four species of insects induced by experimental exposures to UV-irradiation. Micron 2002, 33, 23–31. [Google Scholar] [CrossRef]
- Gokan, N.; Meyer-Rochow, V.B. Fine structure of the compound eye of the buprestid beetle Curis caloptera (Coleoptera; Buprestidae). Z Mikrosk Anat 1984, 98, 17–35. [Google Scholar] [CrossRef]
- Gribakin, F.G. Functional morphology of the compound eye of the bee. In The Compound Eye and Vision of Insects; Horridge, G.A., Ed.; Clarendon Press: Oxford, UK, 1975; pp. 154–176. [Google Scholar]
- Ribi, W.A.; Zeil, J. Diversity and common themes in the organization of ocelli in Hymenoptera, Odonata and Diptera. J. Comp. Physiol. A 2018, 204, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Rochow, V.B. Electrophysiologically determined spectral efficiencies of the compound eye and median ocellus in the bumblebee Bombus hortorum tarhakimalainen (Hymenoptera, Insecta). J. Comp. Physiol. A 1980, 139, 261–266. [Google Scholar] [CrossRef]
- Van Kleef, J.; James, A.C.; Stange, G. A spatiotemporal white noise analysis of photoreceptor responses to UV and green light in the dragonfly median ocellus. J. Gen. Physiol. 2005, 126, 481–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
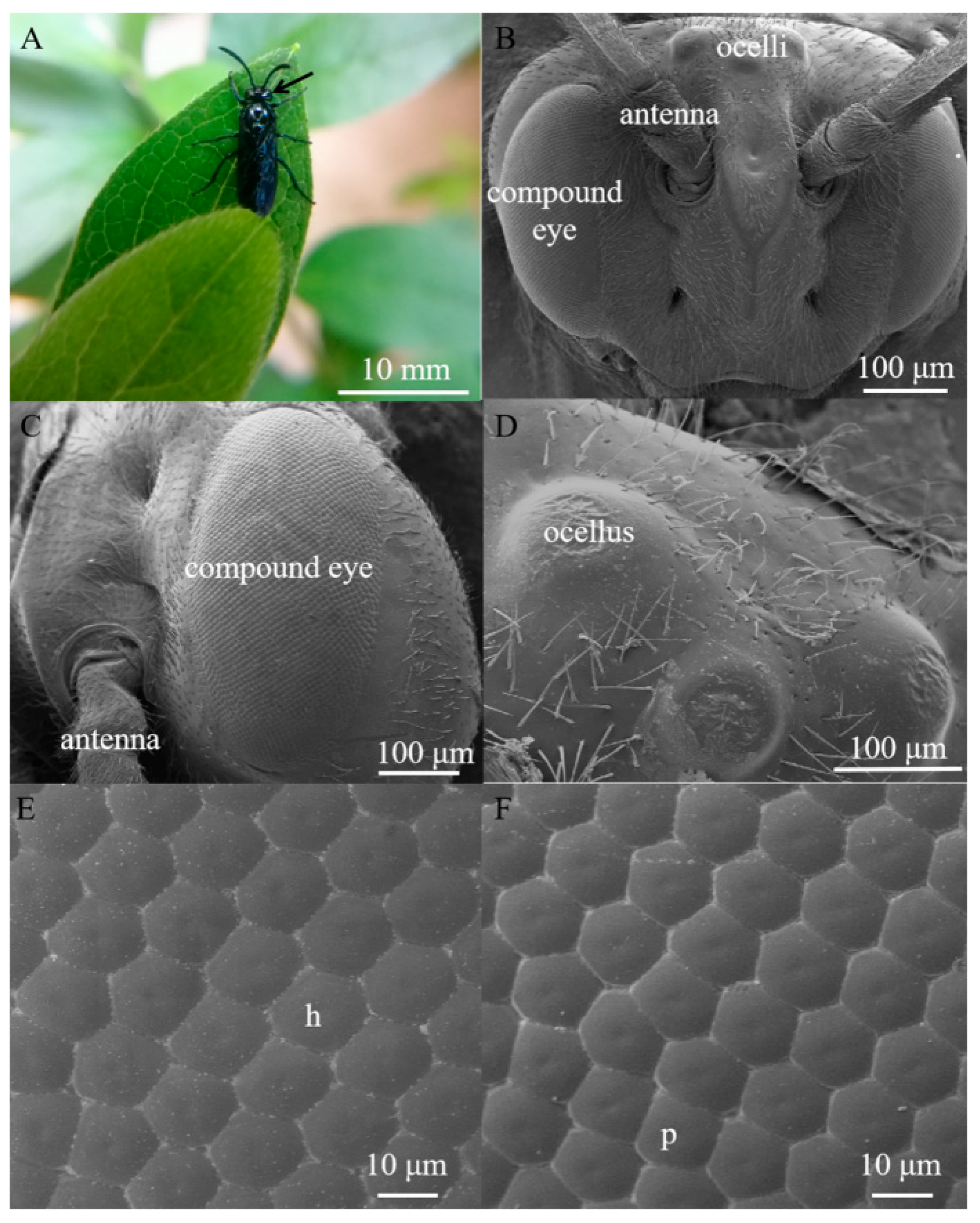
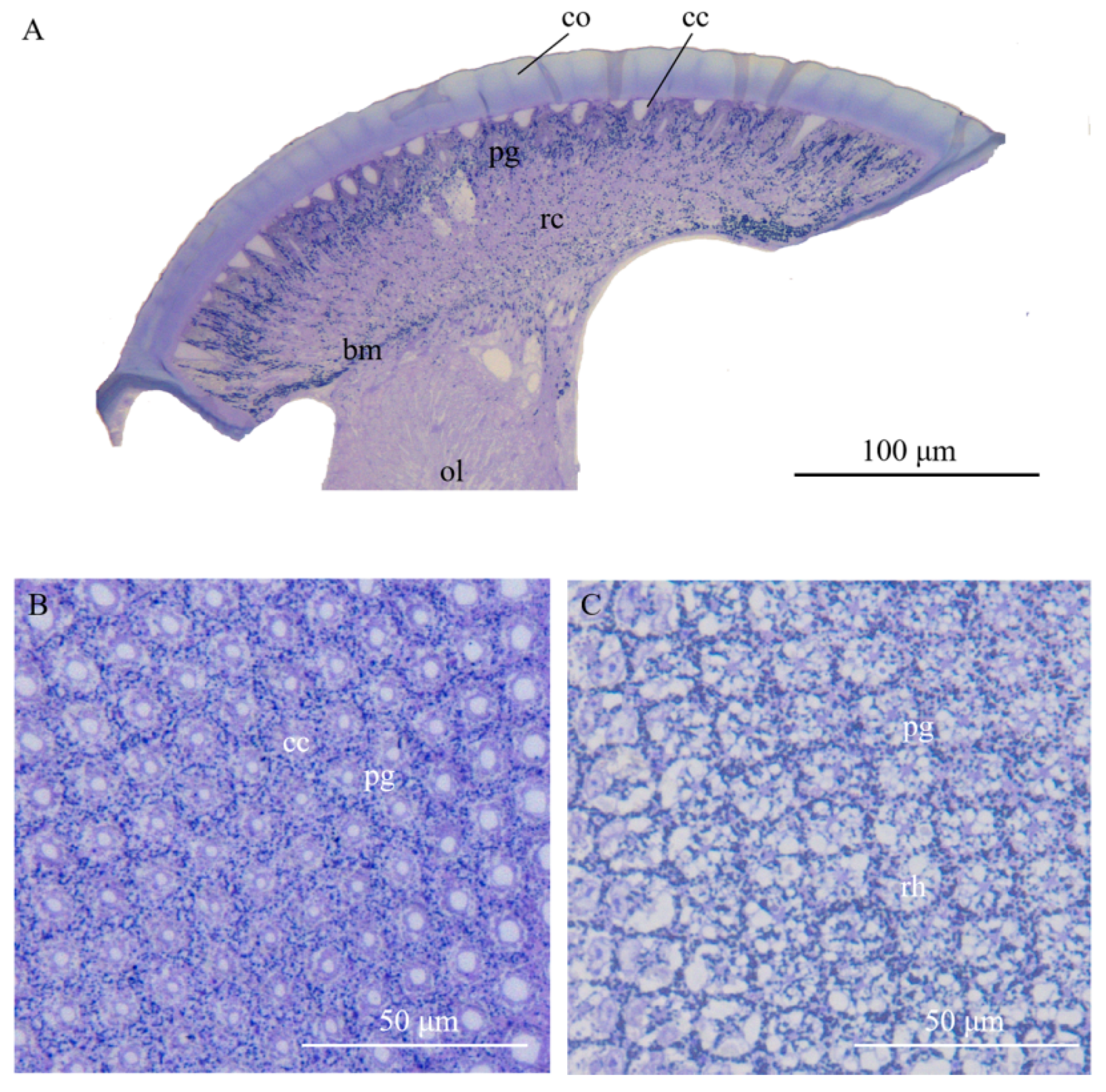
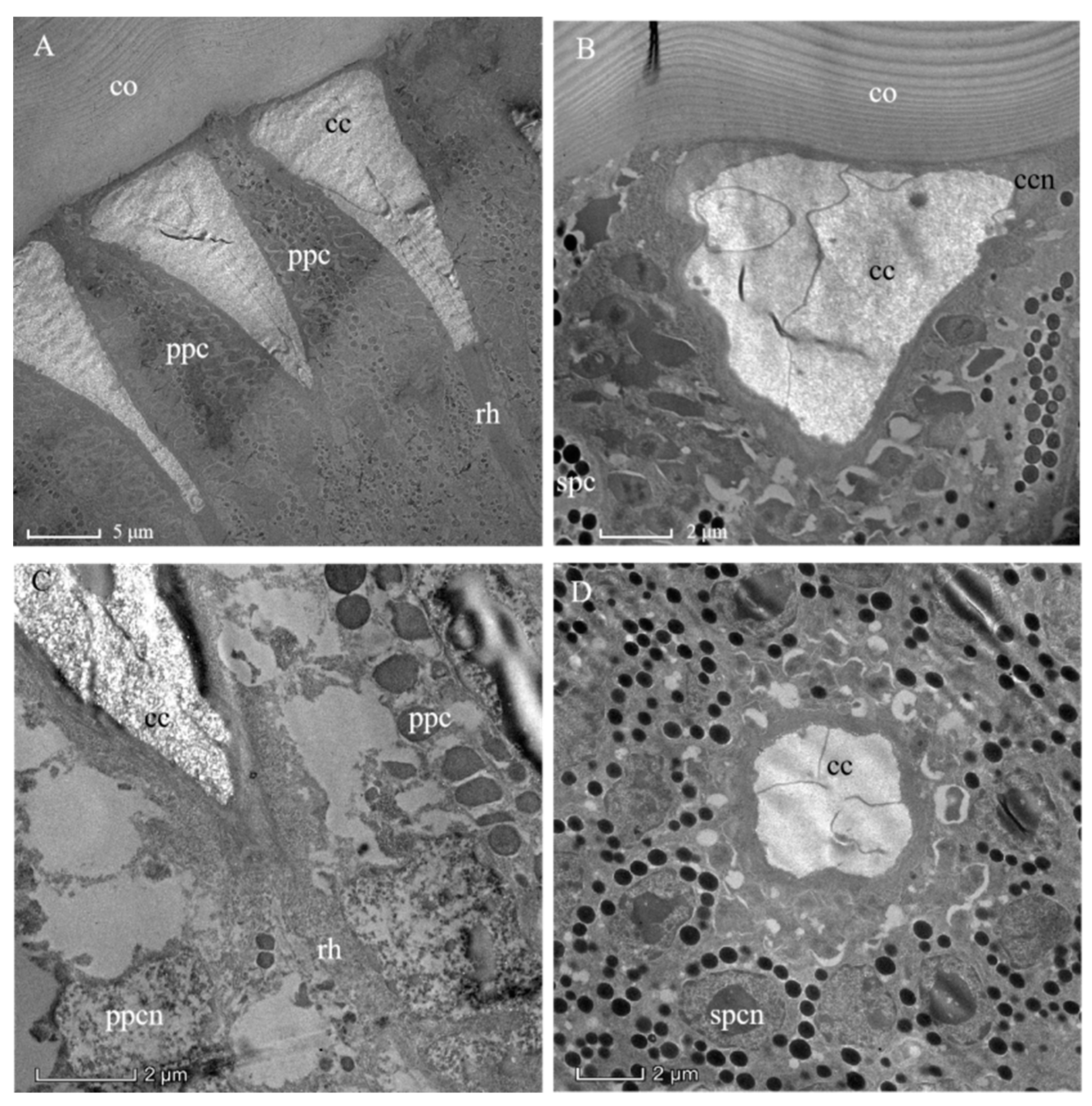
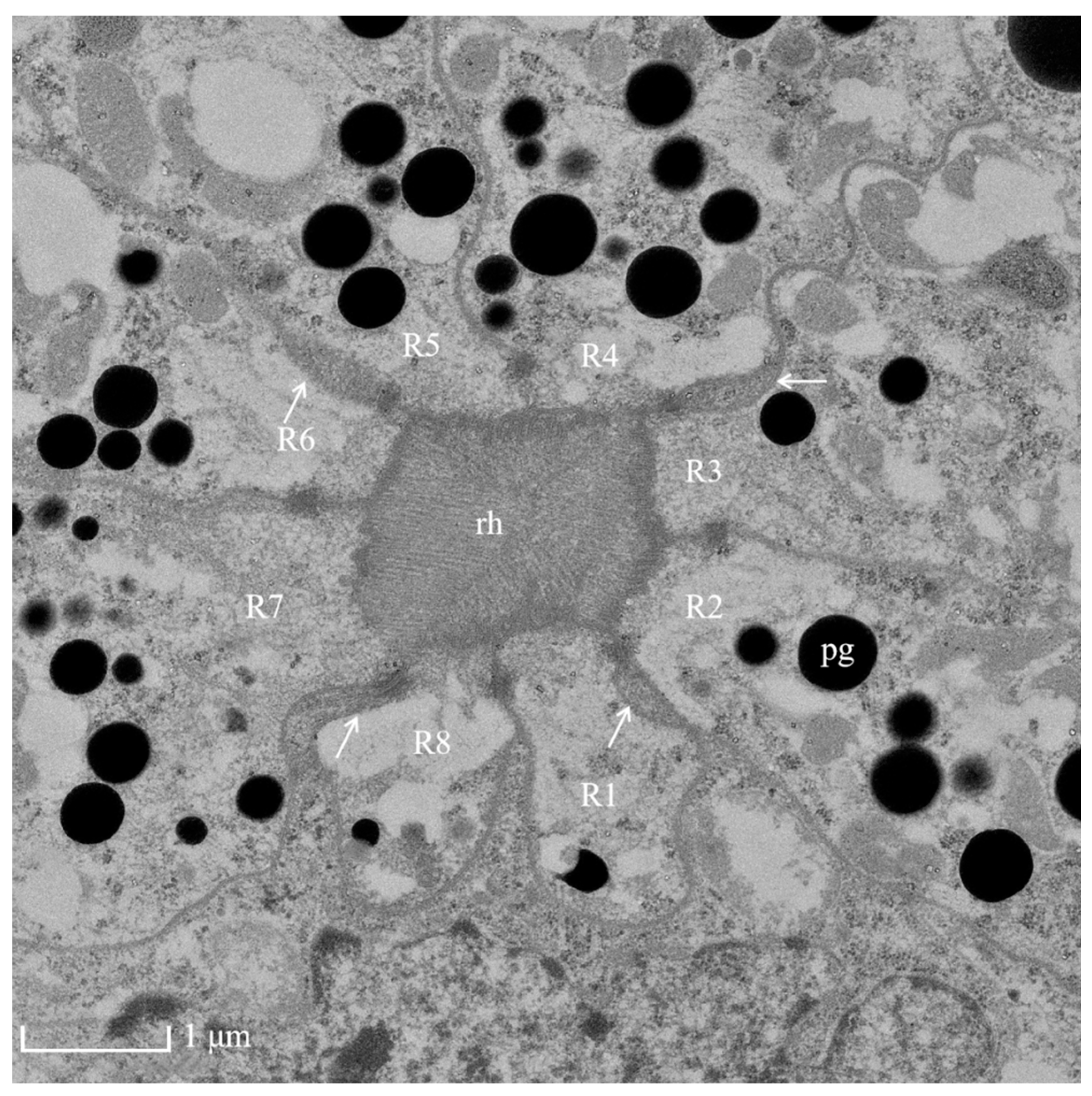

| Parameter | N | Unit | Range | Average |
|---|---|---|---|---|
| Facet number in males per eye | 3 | - | 1819–2195 | 2022 ± 89 |
| Facet number in females per eye | 3 | - | 2095–2288 | 2223 ± 52 |
| Compound eye major axis diameter in males | 3 | μm | 1129.10–1225.70 | 1165.67 ± 24.70 |
| Compound eye major axis diameter in females | 3 | μm | 1173.58–1210.60 | 1192.20 ± 8.73 |
| Compound eye minor axis diameter in males | 3 | μm | 549.89–666.20 | 607.11 ± 27.43 |
| Compound eye minor axis diameter in females | 3 | μm | 595.29–611.37 | 603.66 ± 3.80 |
| Compound eye area in males | 3 | mm2 | 0.58–0.60 | 0.59 ± 0.01 |
| Compound eye area in females | 3 | mm2 | 0.55–0.65 | 0.60 ± 0.02 |
| Facet diameter in males | 120 | μm | 10.06–25.75 | 17.40 ± 0.19 |
| Facet diameter in females | 120 | μm | 13.32–22.86 | 18.26 ± 0.17 |
| Crystalline cone length | 3 | μm | 20.30–23.38 | 22.10 ± 0.76 |
| Diameter of PPC pigment granules | 50 | μm | 0.23–1.01 | 0.60 ± 0.02 |
| Diameter of SPC pigment granules | 50 | μm | 0.22–0.63 | 0.41 ± 0.01 |
| Diameter of Ret C pigment granules | 50 | μm | 0.15–0.68 | 0.34 ± 0.02 |
| Diameters of median ocelli | 3 | μm | 86.45–122.68 | 106.92 ± 8.75 |
| Diameters of ocelli on both sides | 3 | μm | 78.89–97.22 | 87.14 ± 4.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Pan, Z.; Liang, S.; Shen, L.; Wen, X.; Wang, C. Fine Structure of the Visual System of Arge similis (Hymenoptera, Argidae). Insects 2022, 13, 152. https://doi.org/10.3390/insects13020152
Wen C, Pan Z, Liang S, Shen L, Wen X, Wang C. Fine Structure of the Visual System of Arge similis (Hymenoptera, Argidae). Insects. 2022; 13(2):152. https://doi.org/10.3390/insects13020152
Chicago/Turabian StyleWen, Chao, Zijian Pan, Shiping Liang, Liming Shen, Xiujun Wen, and Cai Wang. 2022. "Fine Structure of the Visual System of Arge similis (Hymenoptera, Argidae)" Insects 13, no. 2: 152. https://doi.org/10.3390/insects13020152






