Comparison of Transcriptome Responses between Sogatella furcifera Females That Acquired Southern Rice Black-Streaked Dwarf Virus and Not
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Plants
2.2. RNA Isolation, cDNA Library Preparation, and Transcriptome Sequencing
2.3. De Novo Assembly, Gene Annotation, and Functional Classification
2.4. Analysis for Differentially Expressed Genes
2.5. Expression of Caspase 1 and Female-Specific Genes
2.6. Caspase 1 Activity
2.7. Data Analysis
3. Results
3.1. Transcriptome Assembly and Annotation
3.2. GO and KEGG Analyses of DEGs
3.3. DEGs Associated with Apoptosis in S. furcifera Females
3.4. Expression of Female-Specific Genes in S. furcifera
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rubia-Sanchez, E.; Suzuki, Y.; Arimura, K.; Miyamoto, K.; Matsumura, M.; Watanabe, T. Comparing Nilaparvata lugens (Stal) and Sogatella furcifera (Horvath) (Homoptera: Delphacidae) feeding effects on rice plant growth processes at the vegetative stage. Crop Prot. 2003, 22, 967–974. [Google Scholar] [CrossRef]
- Fujita, D.; Kohli, A.; Horgan, F.G. Rice resistance to planthoppers and leafhoppers. Crit. Rev. Plant Sci. 2012, 32, 162–191. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, D.; Xu, D.; Zhang, M. Southern rice black-streaked dwarf virus: A white-backed planthopper-transmitted fijivirus threatening rice production in Asia. Front. Microbiol. 2013, 4, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.A.; Yang, J.A.; Zhou, G.H.; Zhang, H.M.; Chen, J.P.; Adams, M.J. The Complete genome sequence of two isolates of southern rice black-streaked dwarf virus, a new member of the genus Fijivirus. J. Phytopathol. 2010, 158, 733–737. [Google Scholar] [CrossRef]
- Hoang, A.T.; Zhang, H.M.; Yang, J.; Chen, J.P.; Hébrard, E.; Zhou, G.H.; Vinh, V.N.; Cheng, J.A. Identification, characterization, and distribution of southern rice black-streaked dwarf virus in Vietnam. Plant Dis. 2011, 95, 1063–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsukura, K.; Towata, T.; Sakai, J.; Onuki, M.; Okuda, M.; Matsumura, M. Dynamics of southern rice black-streaked dwarf virus in rice and implication for virus acquisition. Phytopathology 2013, 103, 509–512. [Google Scholar] [CrossRef] [Green Version]
- Lv, M.F.; Xie, L.; Wang, H.F.; Wang, H.D.; Chen, J.P.; Zhang, H.M. Biology of Southern rice black-streaked dwarf virus: A novel fijivirus emerging in East Asia. Plant Pathol. 2017, 66, 515–521. [Google Scholar] [CrossRef]
- Liu, D.; Li, P.; Gong, S.; An, X.; Liu, Y.; Hou, M. Effects of temperature, rice growth stages and nymph instar on the acquisition rate of Sourthern rice black-streaked dwarf virus by Sogatella furcifera. Plant Prot. 2016, 42, 129–133. (In Chinese) [Google Scholar]
- Xu, H.; He, X.; Zheng, X.; Yang, Y.; Tian, J.; Lu, Z. Southern rice black-streaked dwarf virus (SRBSDV) directly affects the feeding and reproduction behavior of its vector, Sogatella furcifera (Horváth) (Hemiptera: Delphacidae). Virol. J. 2014, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Li, P.; Han, Y.; Gong, S.; Yang, L.; Hou, M. EPG recordings reveal differential feeding behaviors in Sogatella furcifera in response to plant virus infection and transmission success. Sci. Rep. 2016, 6, 30240. [Google Scholar] [CrossRef]
- Tu, Z.; Ling, B.; Xu, D.; Zhang, M.; Zhou, G. Effects of southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol. J. 2013, 10, 145. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Liu, D.; Li, P.; Hou, M. Interactive Effects of southern rice black-streaked dwarf virus infection of host plant and vector on performance of the vector, Sogatella furcifera (Homoptera: Delphacidae). J. Econ. Entomol. 2014, 107, 1721–1727. [Google Scholar] [CrossRef]
- Higashi, C.H.V.; Bressan, A. Influence of a propagative plant virus on the fitness and wing dimorphism of infected and exposed insect vectors. Oecologia 2013, 172, 847–856. [Google Scholar] [CrossRef]
- Kaur, N.; Hasegawa, D.K.; Ling, K.S.; Wintermantel, W.M. Application of genomics for understanding plant virus-insect vector interactions and insect vector control. Phytopathology 2016, 106, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Luan, J.B.; Li, J.M.; Varela, N.; Wang, Y.L.; Li, F.F.; Bao, Y.Y.; Zhang, C.X.; Liu, S.S.; Wang, X.W. Global analysis of the transcriptional response of whitefly to tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J. Virol. 2011, 85, 3330–3340. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, P.; Li, W.; Zhang, J.; Huang, F.; Yang, J.; Bei, Y.; Lu, Y. De novo transcriptome sequencing in Frankliniella occidentalis to identify genes involved in plant virus transmission and insecticide resistance. Genomics 2013, 101, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhou, W.; Zhou, Y.; Wu, J.; Zhou, X. Transcriptome and comparative gene expression analysis of Sogatella furcifera (Horváth) in response to southern rice black-streaked dwarf virus. PLoS ONE 2012, 7, e36238. [Google Scholar] [CrossRef]
- Wang, L.; Tang, N.; Gao, X.; Guo, D.; Chang, Z.; Fu, Y.; Akinyemi, I.A.; Wu, Q. Understanding the immune system architecture and transcriptome responses to southern rice black-streaked dwarf virus in Sogatella furcifera. Sci. Rep. 2016, 6, 36254. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Zhou, G.; Zhang, S. Detection of Southern rice black-streaked dwarf virus using one-step dual RT-PCR. Acta Phytopathol. Sin. 2012, 42, 84–87. (In Chinese) [Google Scholar]
- Kechin, A.; Boyarskikh, U.; Kel, A.; Filipenko, M. cutPrimers: A new tool for accurate cutting of primers from reads of targeted next generation sequencing. J. Comput. Biol. 2017, 24, 1138–1143. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- An, X.K.; Hou, M.L.; Liu, Y.D. Reference gene selection and evaluation for gene expression studies using RT-qPCR in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Econ. Entomol. 2016, 109, 879–886. [Google Scholar] [CrossRef]
- Lin, J.; He, J.; Liang, A.; Wang, F. Transcriptome profiling and dimorphic expression of sex-related genes in fifth-instar nymphs of Sogatella furcifera, an important rice pest. Genomics 2020, 112, 1105–1111. [Google Scholar] [CrossRef]
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant virus-insect vector interactions: Current and potential future research directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef]
- Li, D.; Zhang, C.; Tong, Z.; Su, D.; Zhang, G.; Zhang, S.; Zhao, H.; Hu, Z. Transcriptome response comparison between vector and non-vector aphids after feeding on virus-infected wheat plants. BMC Genom. 2020, 21, 638. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Studying apoptosis in Drosophila. Cold Spring Harb. Protoc. 2015, 2015, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Clem, R.J. The role of apoptosis in defense against baculovirus infection in insects. Curr. Top. Microbiol. 2005, 289, 113–129. [Google Scholar]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Degterev, A.; Boyce, M.; Yuan, J. A decade of caspases. Oncogene 2003, 22, 8543–8567. [Google Scholar] [CrossRef] [Green Version]
- Beug, S.T.; Beauregard, C.E.; Healy, C.; Sanda, T.; St-Jean, M.; Chabot, J.; Walker, D.E.; Mohan, A.; Earl, N.; Lun, X.; et al. Smac mimetics synergize with immune checkpoint inhibitors to promote tumour immunity against glioblastoma. Nat. Commun. 2017, 8, 14278. [Google Scholar] [CrossRef] [Green Version]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Richter, K.S.; Götz, M.; Winter, S.; Jeske, H. The contribution of translesion synthesis polymerases on geminiviral replication. Virology 2016, 488, 137–148. [Google Scholar] [CrossRef] [Green Version]
- He, Y.Z.; Wang, Y.M.; Yin, T.Y.; Fiallo-Olivé, E.; Liu, Y.Q.; Hanley-Bowdoin, L.; Wang, X.W. A plant DNA virus replicates in the salivary glands of its insect vector via recruitment of host DNA synthesis machinery. Proc. Natl. Acad. Sci. USA 2020, 117, 16928–16937. [Google Scholar] [CrossRef]





| Treatment | Raw Reads | Valid Reads | Valid Bases | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| CK1 | 56,680,154 | 54,835,900 | 7.69 G | 95.17 | 41.99 |
| CK2 | 55,922,502 | 53,222,474 | 7.45 G | 94.91 | 42.87 |
| CK3 | 54,429,046 | 51,774,068 | 7.25 G | 94.97 | 43.16 |
| Sf_NV1 | 57,203,084 | 54,911,608 | 7.70 G | 95.53 | 43.38 |
| Sf_NV2 | 48,169,010 | 43,964,184 | 6.15 G | 94.91 | 43.92 |
| Sf_NV3 | 56,369,404 | 49,752,106 | 6.95 G | 95.04 | 43.62 |
| Sf_V1 | 42,071,366 | 37,432,246 | 5.24 G | 94.7 | 44.16 |
| Sf_V2 | 58,402,142 | 54,280,658 | 7.61 G | 95.13 | 43.49 |
| Sf_V3 | 54,980,040 | 53,804,792 | 7.54 G | 95.18 | 41.26 |
| Index | No. Transcripts | No. Genes |
|---|---|---|
| All | 96,752 | 53,084 |
| GC% | 38.41 | 38.16 |
| Min length (bp) | 201 | 201 |
| Median length (bp) | 416 | 395 |
| Max length (bp) | 11,242 | 11,242 |
| Total assembled Bases (bp) | 69,880,720 | 37,703,068 |
| N50 length (bp) | 1164 | 1171 |
| Data Base | Number | Ratio (%) |
|---|---|---|
| All | 53,084 | 100 |
| GO | 10,802 | 20.35 |
| KEGG | 10,059 | 18.95 |
| Pfam | 10,543 | 19.86 |
| SwissProt | 9650 | 18.18 |
| eggNOG | 13,198 | 24.86 |
| NR | 17,102 | 32.22 |
| Gene ID | Annotation | log2 FC | ||
|---|---|---|---|---|
| Sf–NV vs. CK | Sf–V vs. CK | Sf–V vs. Sf–NV | ||
| DN17359 | actin 1 | −1.33 | 4.33 | 5.69 |
| DN19763 | tubulin alpha-1A chain-like | 1.71 | / | / |
| DN22978 | Ras GTPase-activating protein | 1.18 | 1.67 | / |
| DN24440 | actin | −1.13 | / | / |
| DN17338 | dynamin-1-like protein | 1.02 | / | / |
| DN17887 | Stress-activated protein kinase JNK | 1.36 | / | / |
| DN18498 | cellular tumor antigen p53-like | 1.64 | / | / |
| DN19626 | caspase 1 | 1.15 | 1.43 | / |
| DN19706 | serine-protein kinase | 1.11 | 1.83 | / |
| DN20330 | cellular tumor antigen p53-like | 1.16 | / | / |
| DN21372 | baculoviral IAP repeat-containing protein 6-like | 1.11 | / | / |
| DN22189 | Poly [ADP-ribose] polymerase | 1.09 | 1.65 | / |
| DN22345 | eukaryotic translation initiation factor 2-alpha kinase-like | 1.29 | / | / |
| DN17707 | Apoptosis inhibitor IAP OS | 1.14 | / | / |
| DN25173 | protein S100-A9 | 7.52 | / | −7.44 |
| DN17988 | Cell division cycle and apoptosis regulator protein 1 | 1.39 | / | / |
| DN20596 | Dynamin-1-like protein | / | 1.55 | / |
| Gene ID | Annotation | log2 FC | ||
|---|---|---|---|---|
| Sf–NV vs. CK | Sf–V vs. CK | Sf–V vs. Sf–NV | ||
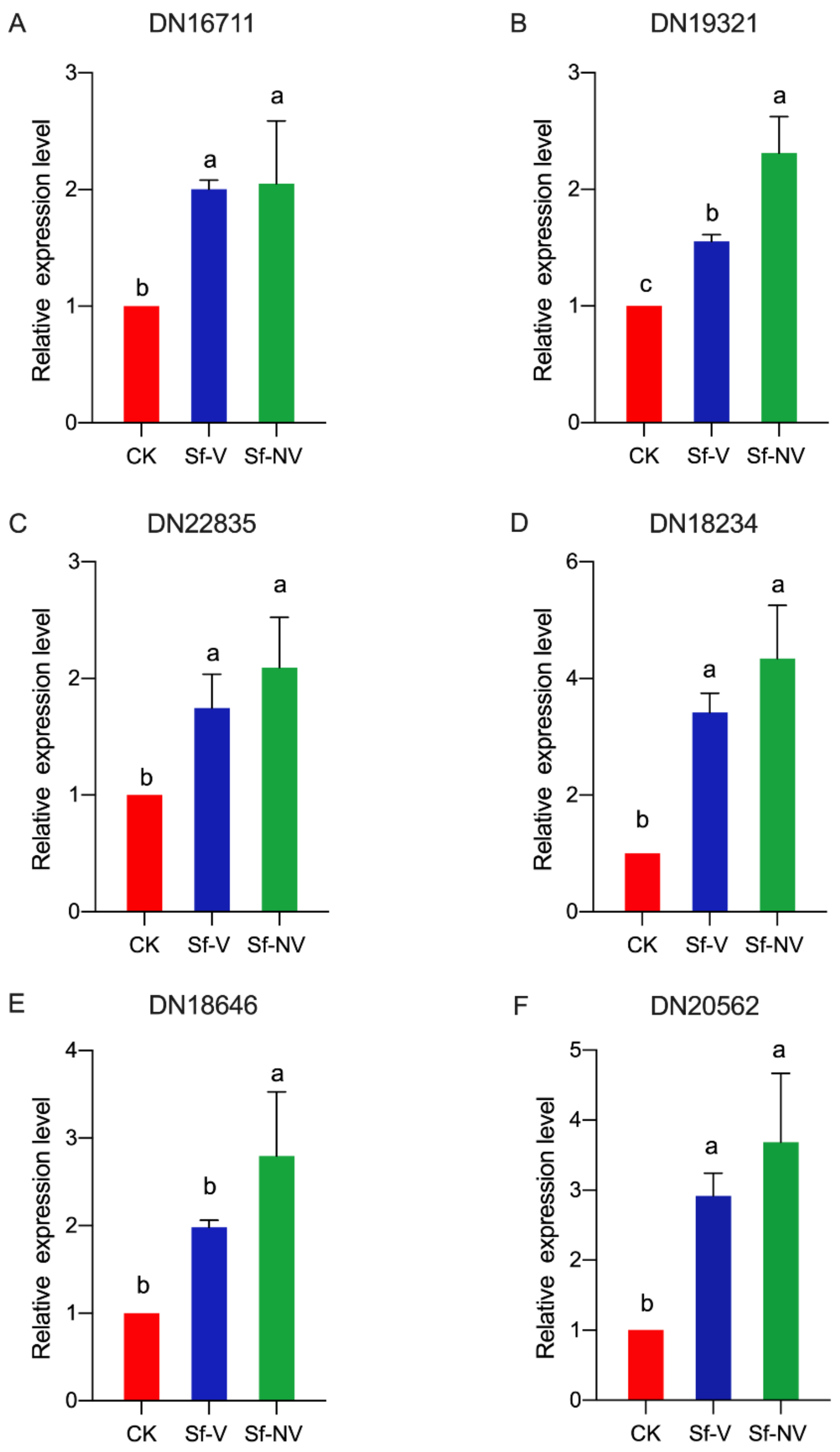
| DN18646 | Coiled-coil domain-containing protein 50 | 1.44 | / | / |
| DN22835 | Chromodomain-helicase-DNA-binding protein 7 | 1.16 | 1.55 | / |
| DN16711 | PiggyBac transposable element-derived protein 4-like | 2.85 | 1.86 | / |
| DN18234 | DNA excision repair protein haywire | 1.11 | / | / |
| DN19321 | DNA helicase | 1.56 | / | / |
| DN20562 | Collagen alpha-1(II) | 2.77 | 1.56 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, Z.; Hou, M. Comparison of Transcriptome Responses between Sogatella furcifera Females That Acquired Southern Rice Black-Streaked Dwarf Virus and Not. Insects 2022, 13, 182. https://doi.org/10.3390/insects13020182
Liu D, Li Z, Hou M. Comparison of Transcriptome Responses between Sogatella furcifera Females That Acquired Southern Rice Black-Streaked Dwarf Virus and Not. Insects. 2022; 13(2):182. https://doi.org/10.3390/insects13020182
Chicago/Turabian StyleLiu, Dandan, Zhengxi Li, and Maolin Hou. 2022. "Comparison of Transcriptome Responses between Sogatella furcifera Females That Acquired Southern Rice Black-Streaked Dwarf Virus and Not" Insects 13, no. 2: 182. https://doi.org/10.3390/insects13020182







