Functional Morphology of the Thorax of the Click Beetle Campsosternus auratus (Coleoptera, Elateridae), with an Emphasis on Its Jumping Mechanism
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Terminology
2.2. Dissection Methods
2.3. Micro-CT Scanning and 3D Reconstructions
2.4. High-Speed Filming
2.5. Experiment 1. Testing Functions of Essential Clicking-Related Muscles and Sclerites
2.6. Experiment 2. Observation of the Deformation of Structures in the Loading Phase
2.7. Recording of the Clicking Sounds
3. Results
3.1. The Functional Morphology of the Thorax of Campsosternus auratus
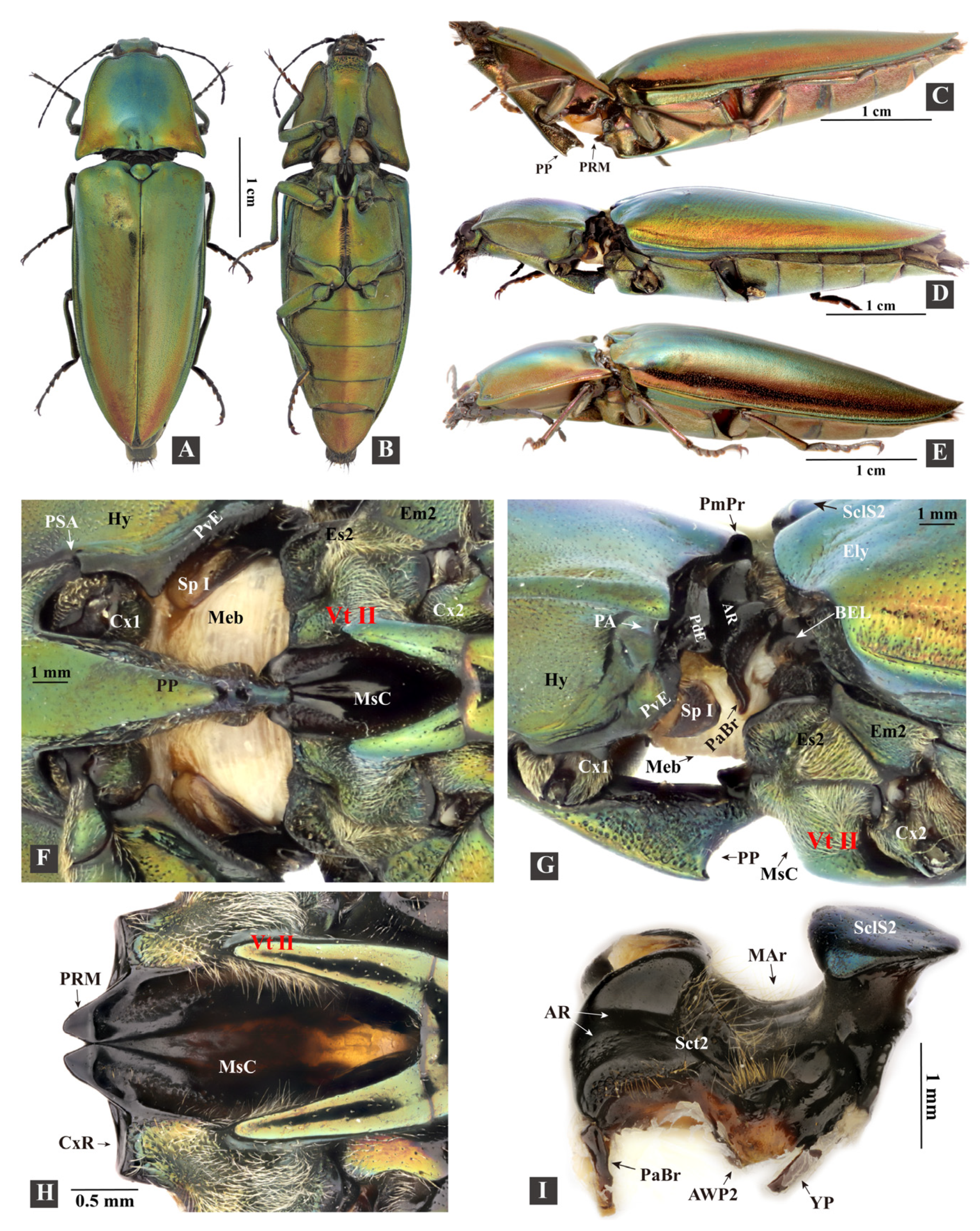
3.1.1. General Morphology
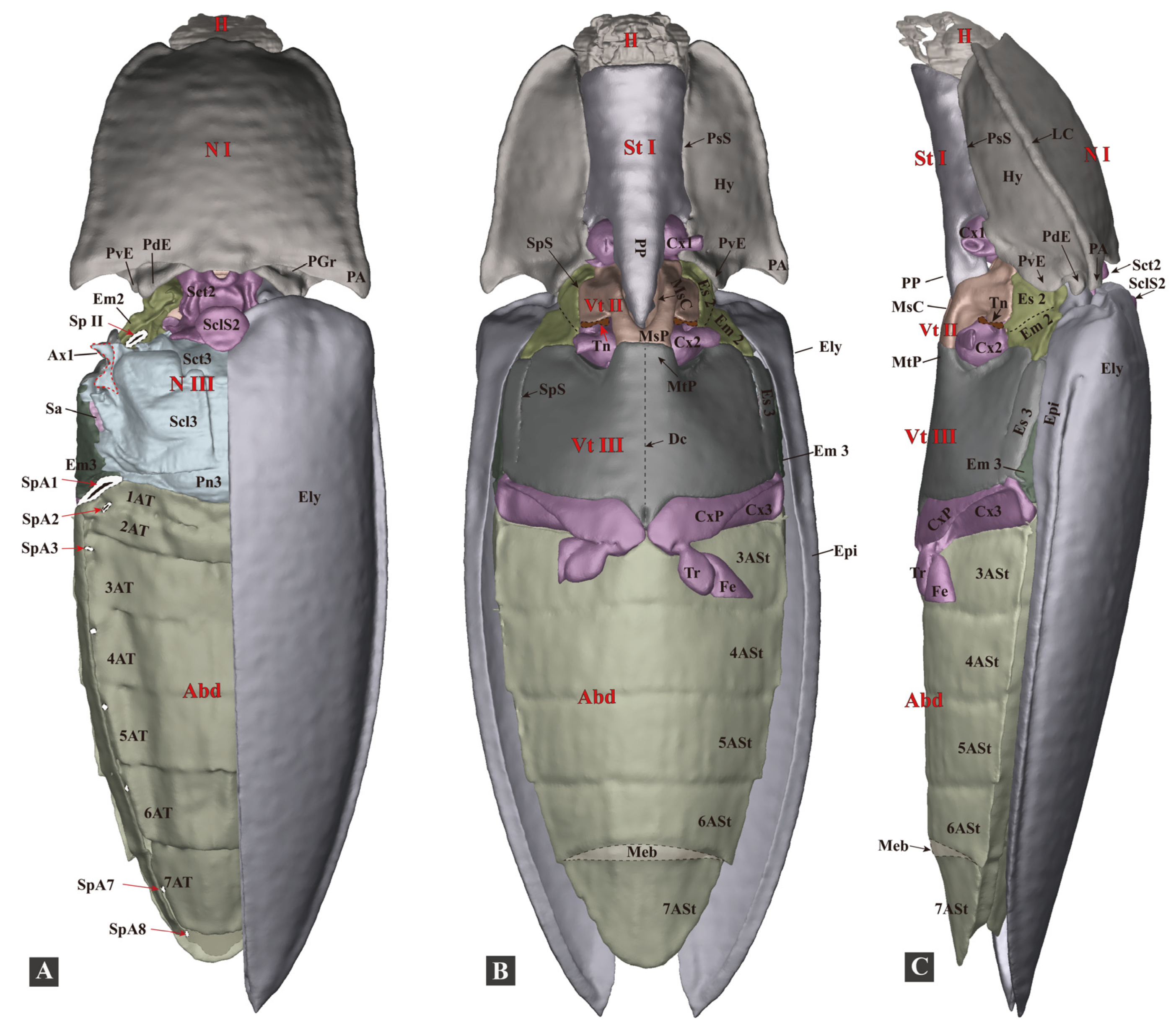
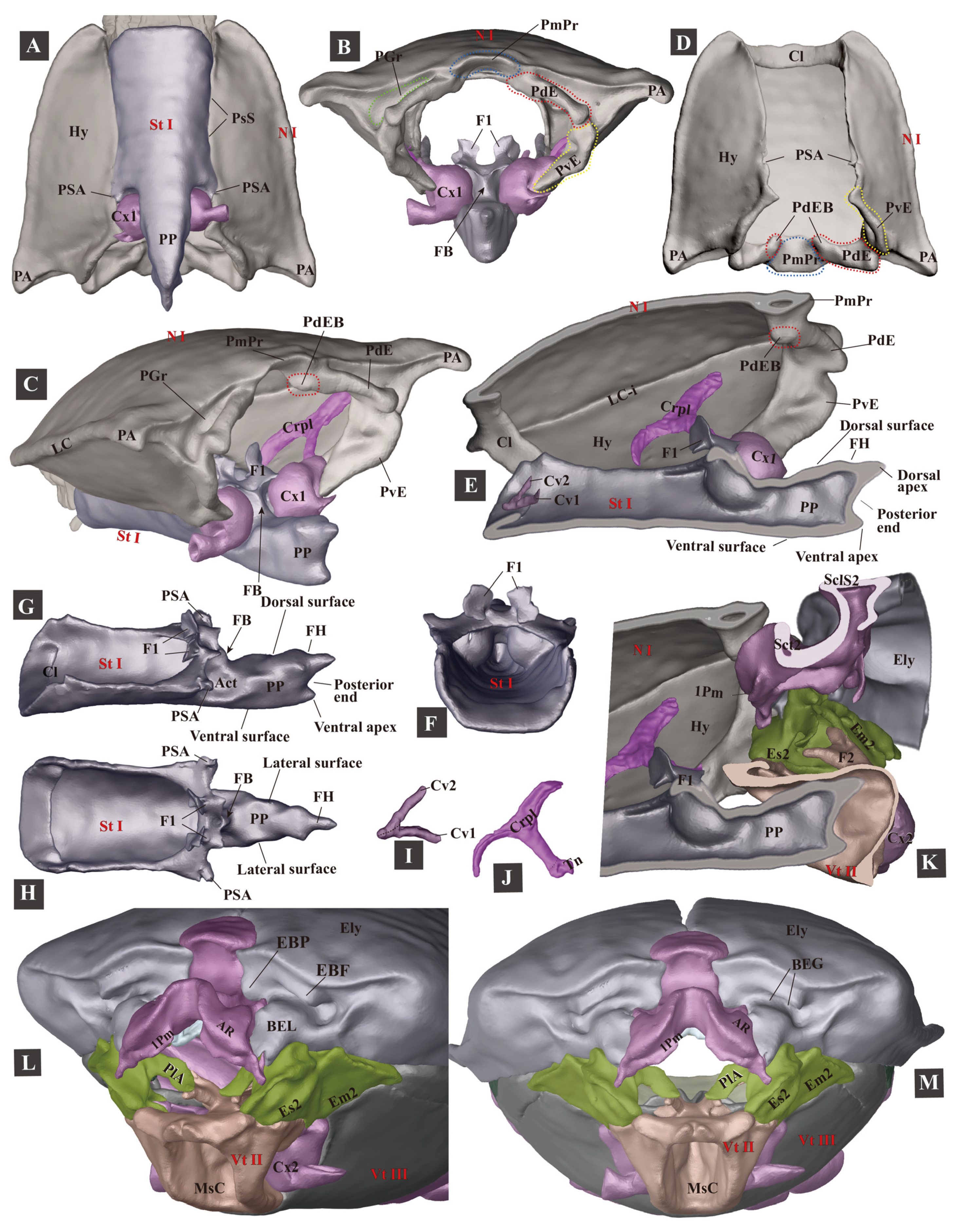
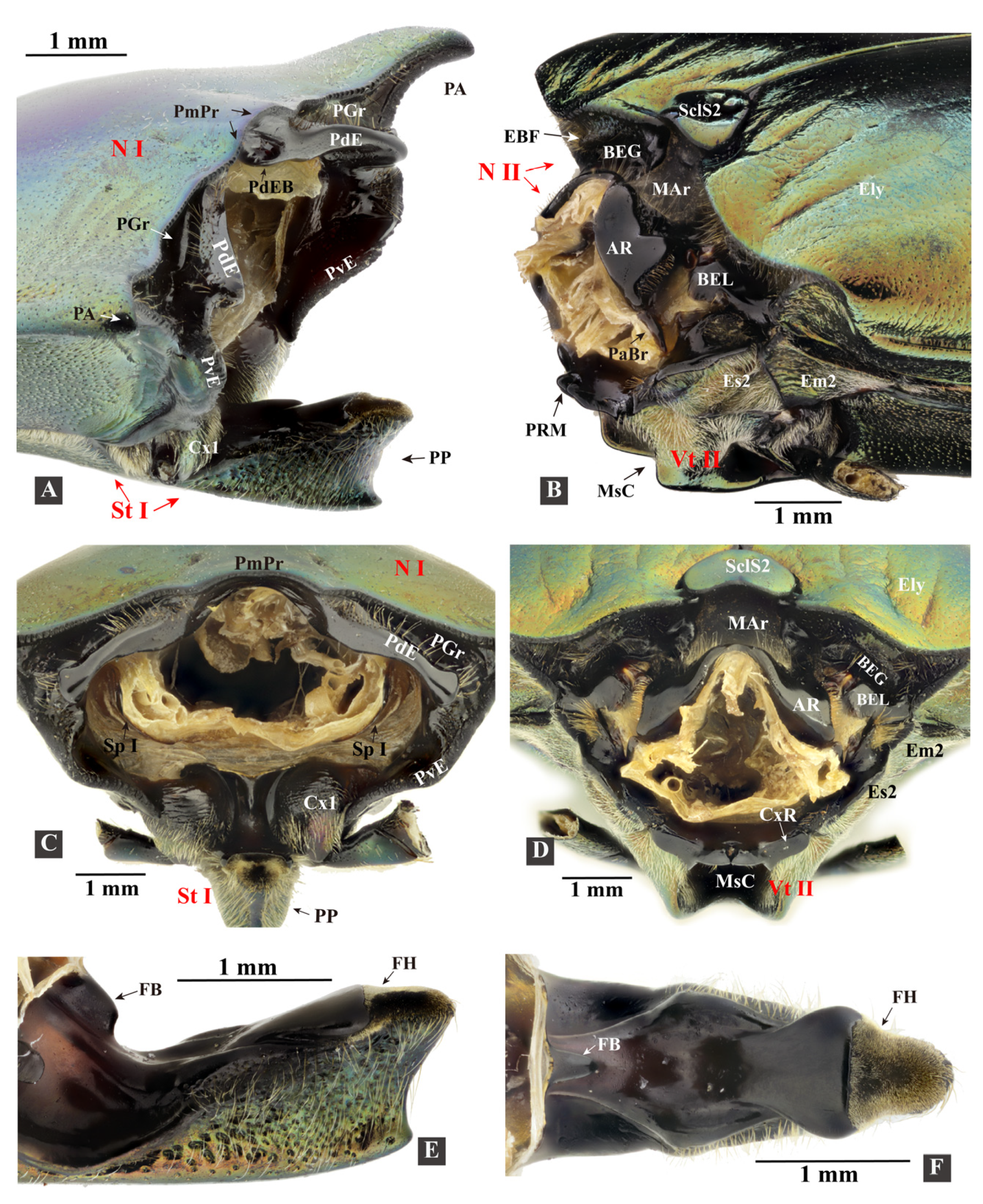
3.1.2. Head
3.1.3. Prothorax
- (1)
- Pronotum (Figure 4D)
- (2)
- Cryptopleuron (Figure 4J)
- (3)
- Prosternum (Figure 4F–H)
3.1.4. Mesothorax
- (1)
- (2)
- Elytron (Figure 5: Ely)
- (3)
- Mesopleuron (Figure 5: Es2, Em2)
- (4)
- Mesoventrite (Figure 5H–K: Vt II)


3.1.5. Metathorax
- (1)
- Metanotum (N III)
- (2)
- Metapleuron (Pl III)
- (3)
- Metaventrite (Vt III)
3.1.6. Thoracic Musculature
- (1)


- (2)
- (3)


3.1.7. Experiment 1. Testing Functions of Essential Clicking-Related Muscles and Sclerites
- (1)
- Clicking-Related Muscles
- (2)
- Clicking-Related Sclerites
| The Muscle Removed | The Species Tested | The Ability to Click after the Operation |
|---|---|---|
| M2a + M2b (cut at posterior ends) | C. auratus (n = 1); Actenicerus sp. (n = 1); Cryptalaus larvatus (n = 1); Cardiophorus sp. (n = 1); Melanotus sp. (n = 4); and Sternocampsus coriaceus (n = 1). | Unable to click. Unable to bend the prothorax dorsad. If the prothorax was pushed dorsad manually, individuals could latch and click. |
| M4 (cut at posterior ends) | C. auratus (n = 1); Cryptalaus larvatus (n = 1); Melanotus sp. (n = 2); and Sternocampsus coriaceus (n = 1). | Unable to click or interlock the thorax. |
| M2b (cut at anterior ends) | C. auratus (n = 1, c = 3); Ludioschema obscuripes (n = 1, c = 3). | Able to click. |
| M1+ M2a + M8 (cut at anterior ends) | C. auratus (n = 1, c = 3); Actenicerus maculipennis (n = 1, c = 10); Ludioschema obscuripes (n = 2, h = 22.5 cm); Melanotus sp. (n = 1, h = 20.0 cm); and Priopus sp. (n = 1, h = 22.5 cm). | Able to click. |
| M5+ M6+ M7 (cut at anterior ends) | C. auratus (n = 1, c = 3); Ampedus sp. (n = 1, c = 3); and Melanotus sp. (n = 1, h = 4.0 cm). | Able to click. |
| M4x+ M11 (cut at posterior ends) | C. auratus (n = 1, h = 6.0 cm); Melanotus sp. (n = 3, h1 = 4.0 cm, h2 = 3.5 cm, and h3 = 15.5 cm); and Pectocera fortunei Candèze (n = 1, h = 2.0 cm). | Able to click. |
| M30 (cut at middle) | Cryptalaus larvatus (n = 2, c = 3); C. auratus (n = 1, c = 3). | Able to click. |
| The Structures Removed | The Species Tested | Influence on the Clicking Mechanism |
|---|---|---|
| Prosternal rest of the mesoventrite (PRM) | C. auratus (n = 1), Campsosternus gemma (n = 1), and Silesis sp. could not click; Melanotus sp. (n = 1, c = 3) and Ludioschema obscuripes (n = 1, c = 3) could click weakly. | Clicking mechanism was disrupted; the PP was stuck on the anterior cut edge of the mesoventrite. |
| Prosternal process (PP) (posterior part including the entire friction hold was removed) | C. auratus (n = 1), Ludioschema obscuripes (n = 1), and Silesis sp. (n = 1) could not click; Melanotus sp. (n = 1, c = 3) could click weakly. | Clicking mechanism was disrupted; the PP was stuck on the PRM. |
| Elytron (only one elytron was removed; auxiliary sclerites were not removed) | C. auratus (n = 1), Pectocera fortunei, Ludioschema obscuripes (n = 2), Melanotus sp., and Silesis sp. were all unable to click. | Clicking mechanism was disrupted; the PP was dislocated in the loading phase. |
| Posterodorsal evagination of the pronotum (PdE) (anterior bulged area was retained) | C. auratus (n = 2, c = 3), Ludioschema obscuripes (n = 1, c = 3), and Melanotus sp. (n = 1, c = 3) could click weakly; Priopus angulatus (n = 1, c = 3) could not click. | Clicking mechanism was disrupted; the loading motion was greatly weakened. |
| Posterior angles of the pronotum (PA) | C. auratus (n = 1, c = 3), Ludioschema obscuripes (n = 1, c = 5), and Ludioschema dorsale (n = 1, c = 3) could click normally. | Clicking mechanism was not affected. |
| Head was immobilized using epoxy resin (in the retracted position) | C. auratus (n = 2, c = 3) and Ludioschema obscuripes (n = 1, c = 3) could click normally. | Clicking mechanism was not affected. |
3.1.8. The Promesothoracic Interlocking Mechanism
3.2. The Jumping Mechanism of Campsosternus auratus
3.2.1. The Jumping Process
- (1)
- Latching Phase
- (2)
- Loading/Contraction Phase
- (3)
- Take-Off Phase


- (4)
- Airborne Phase
3.2.2. The Jumping Performance
- Righting Behavior
- Jumping Height
- The Somersaults during the Airborne Phase
- (1)
- Roll (rotate about the longitudinal axis). Rolling was present in six individuals and absent in one. In typical jumps, the body turns 360–980°. The rolling orientation is constant in the same individual. We hypothesize that the elytra shape could affect the rolling direction, as the counterforce from the ground acts on the base of the elytra.
- (2)
- Pitch (rotate about the transverse axis). All jumps of the seven individuals rotated head over tail. The body turns 360–540° in typical jumps.
- (3)
- Yaw (rotate about the dorsoventral axis). Yaw was absent in all seven individuals. According to Evans [13], yaw was also lacking in the jumps of Athous haemorrhoidalis.
- Escaping Behavior
3.2.3. What Triggers the Jump?
3.2.4. What Slows down the Oscillation of the Body?
3.2.5. Experiment 2. Observation of the Deformation of Thoracic Structures and Elastic Energy Storage in the Loading Phase

3.2.6. Recording of the Clicking Sounds

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennet-Clark, H.C.; Lucey, E.C.A. The jump of the flea: A study of the energetics and a model of the mechanism. J. Exp. Biol. 1967, 47, 59–76. [Google Scholar] [CrossRef] [PubMed]
- Sutton, G.P.; Burrows, M. Biomechanics of jumping in the flea. J. Exp. Biol. 2011, 214, 836–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heitler, W.J. The locust jump. J. Comp. Physiol. 1974, 89, 93–104. [Google Scholar] [CrossRef]
- Bennet-Clark, H.C. The energetics of the jump of the locust Schistocerca gregaria. J. Exp. Biol. 1975, 63, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Hao, Z.; Feng, X. Structures, properties, and energy-storage mechanisms of the semi-lunar process cuticles in locusts. Sci. Rep. 2016, 6, 35219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsignori, G.; Stefanini, C.; Scarfogliero, U.; Mintchev, S.; Benelli, G.; Dario, P. The green leafhopper, Cicadella viridis (Hemiptera, Auchenorrhyncha, Cicadellidae), jumps with near-constant acceleration. J. Exp. Biol. 2013, 216, 1270–1279. [Google Scholar] [CrossRef] [Green Version]
- Burrows, M.; Ghosh, A.; Sutton, G.P.; Yeshwanth, H.M.; Rogers, S.M.; Sane, S.P. Jumping in lantern bugs (Hemiptera, Fulgoridae). J. Exp. Biol. 2021, 224, jeb243361. [Google Scholar] [CrossRef]
- Nadein, K.; Betz, O. Jumping mechanisms and performance in beetles. I. Flea beetles (Coleoptera: Chrysomelidae: Alticini). J. Exp. Biol. 2016, 219, 2015–2027. [Google Scholar] [CrossRef] [Green Version]
- Nadein, K.; Betz, O. Jumping mechanisms and performance in beetles. II. Weevils (Coleoptera: Curculionidae: Rhamphini). Arthropod Struct. Dev. 2018, 47, 131–143. [Google Scholar] [CrossRef]
- Ruan, Y.; Konstantinov, A.S.; Shi, G.; Tao, Y.; Li, Y.; Johnson, A.J.; Luo, X.; Zhang, X.; Zhang, M.; Wu, J.; et al. The mechanical mechanism of the flea beetle jump, its application to bionics and preliminary design for a robotic jumping leg. ZooKeys 2020, 915, 87–105. [Google Scholar] [CrossRef]
- Muona, J.; Chang, H.; Ren, D. The clicking Elateroidea from Chinese Mesozoic deposits (Insecta, Coleoptera). Insects 2020, 11, 875. [Google Scholar] [CrossRef] [PubMed]
- Kundrata, R.; Packova, G.; Prosvirov, A.S.; Hoffmannova, J. The fossil record of Elateridae (Coleoptera: Elateroidea): Described species, current problems and future prospects. Insects 2021, 12, 286. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.G. The jump of the click beetle (Coleoptera, Elateridae)—A preliminary study. J. Zool. 1972, 167, 319–336. [Google Scholar] [CrossRef]
- Binaghi, G. Sulla meccanica del salto degli Elateridi. Boll. Soc. Ent. Ital. 1942, 74, 1–6. [Google Scholar]
- d’Aguilar, J. Recherches sur l’thologie des imagos d’Agriotes (Col. Elateridae). Annals Ểpiphyt. 1961, 11, 1–95. [Google Scholar]
- Larsén, O. On the morphology and function of locomotor organs of the Gyrinidae and other Coleoptera. Opusc. Entomol. 1966, 30, 1–241. [Google Scholar]
- Evans, M.E.G. The jump of the click beetle (Coleoptera: Elateridae)—Energetics and mechanics. J. Zool. 1973, 169, 181–194. [Google Scholar] [CrossRef]
- Ribak, G.; Weihs, D. Jumping without using legs: The jump of the click-beetles (Elateridae) is morphologically constrained. PLoS ONE 2011, 6, e20871. [Google Scholar] [CrossRef] [Green Version]
- Ribak, G.; Mordechay, O.; Weihs, D. Why are there no long distance jumpers among click-beetles (Elateridae)? Bioinspir. Biomim. 2013, 8, 036004. [Google Scholar] [CrossRef]
- Ribak, G.; Reingold, S.; Weihs, D. The effect of natural substrates on jump height in click-beetles. Funct. Ecol. 2012, 26, 493–499. [Google Scholar] [CrossRef]
- Bolmin, O.; Duan, C.; Urrutia, L.; Abdulla, A.M.; Hazel, A.M.; Alleyne, M.; Dunn, A.C.; Wissa, A. Pop! Observing and modeling the legless self-righting jumping mechanism of click beetles. In Living Machines; Mangan, M., Cutkosky, M., Mura, A., Verschure, P.F.M.J., Prescott, T., Lepora, N., Eds.; Springer: Cham, Switzerland, 2017; pp. 35–47. [Google Scholar]
- Bolmin, O.; Wei, L.; Hazel, A.M.; Dunn, A.C.; Wissa, A.; Alleyne, M. Latching of the click beetle (Coleoptera: Elateridae) thoracic hinge enabled by the morphology and mechanics of conformal structures. J. Exp. Biol. 2019, 222, jeb196683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolmin, O.; Socha, J.J.; Alleyne, M.; Dunn, A.C.; Fezzaa, K.; Wissa, A. Nonlinear elasticity and damping govern ultrafast dynamics in click beetles. Proc. Natl. Acad. Sci. USA 2021, 118, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, A.; Kawaguchi, Y. Click beetle-like jumping device for entertainment. In Proceedings of the SIGGRAPH Asia 2014 Emerging Technologies, Shenzhen, China, 3–6 December 2014; pp. 1–3. [Google Scholar] [CrossRef]
- Chen, G.; Tu, J.; Ti, X.; Hu, H. A single-legged robot inspired by the jumping mechanism of click beetles and its hopping dynamics analysis. J. Bionic. Eng. 2020, 17, 1–17. [Google Scholar] [CrossRef]
- Cate, P.C.; Sánchez-Ruiz, A.; Löbl, I.; Smetana, A. Elateridae. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2007; Volume 4, pp. 89–209. [Google Scholar]
- Kundrata, R.; Gunter, N.L.; Janosikova, D.; Bocak, L. Molecular evidence for the subfamilial status of Tetralobinae (Coleoptera: Elateridae), with comments on parallel evolution of some phenotypic characters. Arthropod Syst. Phylogeny 2018, 76, 137–145. [Google Scholar]
- Douglas, H.B.; Kundrata, R.; Brunke, A.J.; Escalona, H.E.; Chapados, J.T.; Eyres, J.; Richter, R.; Savard, K.; Ślipiński, A.; McKenna, D.; et al. Anchored phylogenomics, evolution and systematics of Elateridae: Are all bioluminescent Elateroidea derived click beetles? Biology 2021, 10, 451. [Google Scholar] [CrossRef]
- Matsuda, R. Morphology and evolution of the insect thorax. Mem. Entomol. Soc. Can. 1970, 102, 5–431. [Google Scholar] [CrossRef]
- Verhoeff, K.W. Zur vergleichenden Morphologie des Abdomens der Coleopteren und über die phylogenetische Bedeutung desselben, zugleich ein zusammenfassender kritischer Rückblick und neuer Beitrag. Z. Wiss. Zool. 1918, 117, 130–204. [Google Scholar]
- Horst, A. Zur Kenntnis der Biologie und Morphologie einiger Elateriden und ihrer Larven. Arch. Nat. 1922, 88, 1–90. [Google Scholar]
- Douglas, H.B. Revision of Cardiophorus (Coleoptera: Elateridae) species of eastern Canada and United States of America. Can. Entomol. 2003, 135, 493–548. [Google Scholar] [CrossRef]
- Šípek, P.; Fabrizi, S.; Eberle, J.; Ahrens, D. A molecular phylogeny of rose chafers (Coleoptera: Scarabaeidae: Cetoniinae) reveals a complex and concerted morphological evolution related to their flight mode. Mol. Phylogenet. Evol. 2016, 101, 163–175. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ogawa, N.; Yoshizawa, K. Morphology of the elytral base sclerites. Arthropod Struct. Dev. 2018, 47, 423–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kukalová-Peck, J.; Lawrence, J.F. Relationship among coleopteran suborders and major endoneopteran lineages: Evidence from hind wing characters. Eur. J. Entomol. 2004, 101, 95–144. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.F.; Beutel, R.G.; Leschen, R.A.B.; Ślipiński, A. Glossary of morphological terms. In Handbook of Zoology—Arthropoda: Insecta—Coleoptera, Beetles; Beutel, R.G., Lawrence, J.F., Leschen, R.A.B., Eds.; De Gruyter: Berlin, Germany, 2010; Volume 2, pp. 9–20. [Google Scholar]
- Gurjeva, E.L. Stroenie grudnogo otdela zhukov-shchelkunov (Coleoptera, Elateridae) I znachenie ego priznakov dlya sistemy semeistva [Thoracic structure of click beetles (Coleoptera, Elateridae) and the significance of the structural characters for the system of the family]. Ent. Obozr. 1974, 53, 96–113. (In Russian) [Google Scholar]
- Friedrich, F.; Beutel, R.G. The pterothoracic skeletomuscular system of Scirtoidea (Coleoptera: Polyphaga) and its implications for the relationships of the beetle suborders. J. Zool. Syst. Evol. Res. 2006, 44, 290–315. [Google Scholar] [CrossRef]
- Crowson, R.A. The Natural Classification of the Families of Coleoptera; EW. Classey Ltd.: Middlesex, UK, 1967; p. 214. [Google Scholar]
- Calder, A.A. Click Beetles: Genera of the Australian Elateridae (Coleoptera); Monographs on Invertebrate Taxonomy; CSIRO Publishing: Clayton, Australia, 1996; p. 401. [Google Scholar]
- Douglas, H.B. Phylogenetic relationships of Elateridae inferred from adult morphology, with special reference to the position of Cardiophorinae. Zootaxa 2011, 2900, 1–45. [Google Scholar] [CrossRef]
- Douglas, H.B. World reclassification of the Cardiophorinae (Coleoptera, Elateridae), based on phylogenetic analyses of morphological characters. ZooKeys 2017, 655, 1–130. [Google Scholar] [CrossRef] [Green Version]
- Baehr, M. Zur Funktionsmorphologie und evolutiven Bedeutung der elytralen Sperrmechanismen der Scaritini (Coleoptera: Carabidae). Entomol. Gen. 1980, 6, 311–333. [Google Scholar]
- Friedrich, F.; Beutel, R.G. The thorax of Zorotypus (Hexapoda, Zoraptera) and a new nomenclature for the musculature of Neoptera. Arthropod Struct. Dev. 2008, 37, 29–54. [Google Scholar] [CrossRef]
- Belkaceme, T. Skelet und Muskulatur des Kopfes und Thorax von Noterus laevis Sturm. Ein Beitrag zur Morphologie und Phylogenie der Noteridae (Coleoptera: Adephaga). Stuttg. Beitr. Naturk. 1991, 462, 1–94. [Google Scholar]
- Lawrence, J.F.; Ślipiński, A.; Seago, A.E.; Thayer, M.K.; Newton, A.F.; Marvaldi, A.E. Phylogeny of the Coleoptera based on morphological characters of adults and larvae. Ann. Zool. 2011, 61, 1–217. [Google Scholar] [CrossRef]
- Jordan, A.; Broad, G.R.; Stigenberg, J.; Hughes, J.; Stone, J.; Bedford, I.; Penfield, S.; Wells, R. The potential of the solitary parasitoid Microctonus brassicae for the biological control of the adult cabbage stem flea beetle Psylliodes chrysocephala. Entomol. Exp. Appl. 2020, 168, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Patek, S.N.; Korff, W.L.; Caldwell, R.L. Biomechanics: Deadly strike mechanism of a mantis shrimp. Nature 2004, 428, 819–820. [Google Scholar] [CrossRef]
- Patek, S.N.; Rosario, M.V.; Taylor, J.R. Comparative spring mechanics in mantis shrimp. J. Exp. Biol. 2013, 216, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadayon, M.; Amini, S.; Wang, Z.A.; Miserez, A. Biomechanical design of the mantis shrimp saddle: A biomineralized spring used for rapid raptorial strikes. iScience 2018, 8, 271–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, J. Woodpeckers and head injury. Lancet 1976, 307, 640. [Google Scholar] [CrossRef]
- May, P.R.; Fuster, J.M.; Haber, J.; Hirschman, A. Woodpecker drilling behavior: An endorsement of the rotational theory of impact brain injury. Arch. Neurol. 1979, 36, 370–373. [Google Scholar] [CrossRef]
- Wang, L.; Cheung, J.T.M.; Pu, F.; Li, D.; Zhang, M.; Fan, Y. Why do woodpeckers resist head impact injury: A biomechanical investigation. PLoS ONE 2011, 6, e26490. [Google Scholar] [CrossRef] [Green Version]
- Kundrata, R.; Bocak, L. The phylogeny and limits of Elateridae (Insecta, Coleoptera): Is there a common tendency of click beetles to soft-bodiedness and neoteny? Zool. Scr. 2011, 40, 364–378. [Google Scholar] [CrossRef]
| Species Name and Numbers of Individuals Used | Subfamily/Family | |
|---|---|---|
| Dissected Elateridae specimens | Campsosternus auratus (ethanol-preserved specimens, n = 11; dry specimens, n = 14) | Dendrometrinae |
| Sinelater perroti (dry specimens, n = 2) | Tetralobinae | |
| Phorocardius unguicularis (Fleutiaux) (dry specimen, n = 1) | Cardiophorinae | |
| Specimens used for micro-CT scans | Campsosternus auratus (n = 2) | Dendrometrinae |
| Specimens used for high-speed filming and observation of jumping performance | Campsosternus auratus (n = 24) | Dendrometrinae |
| Sinelater perroti (n = 11) | Tetralobinae | |
| High-speed filming of Elaterid species used for comparison purposes | Agrypnus bipapulatus (Candèze) (n = 2), Agrypnus costicollis (Candèze) (n = 1), Cryptalaus berus (Candèze) (n = 1), and Cryptalaus larvatus (Candèze) (n = 2) | Agrypninae |
| Cardiophorus sp. (n = 1) | Cardiophorinae | |
| Ludioschema obscuripes (Gyllenhal) (n = 1), Melanotus sp. (n = 2) | Elaterinae | |
| Specimens used for ‘Experiment 1’ and ‘Experiment 2’ | Campsosternus auratus (n = 24), Campsosternus gemma Candèze (n = 1), Actenicerus maculipennis (Schwarz) (n = 2), Pectocera fortunei Candèze (n = 2), and Sternocampsus coriaceus Liu et Jiang (n = 2) | Dendrometrinae |
| Ampedus sp. (n = 1), Ludioschema dorsale (Candèze) (n = 1), Ludioschema obscuripes (n = 5), Melanotus sp. (n = 18), Priopus angulatus (Candèze) (n = 1), Priopus sp. (n = 1), and Silesis sp. (n = 3) | Elaterinae | |
| Cardiophorus sp. (n = 1) | Cardiophorinae | |
| Cryptalaus larvatus (n = 5) | Agrypninae | |
| Sinelater perroti (n = 2) | Tetralobinae | |
| Specimens used forthe recording of the clicking sounds | Campsosternus auratus (n = 3) | Dendrometrinae |
| Specimens used foran additional test to observe the displacement of the mesonotum in the loading phase | Campsosternus auratus (n = 1) | Dendrometrinae |
| Sinelater perroti (n = 1) | Tetralobinae | |
| Specimens used for observation of the interlocking mechanism of the thorax | Campsosternus auratus (n = 4) | Agrypninae |
| Sinelater perroti (n = 1) | Tetralobinae | |
| Callirhipis sp. (n = 2) | Callirhipidae | |
| Eulichas cf. funebris (Westwood) (n = 2) | Eulichadidae | |
| Chalcophora yunnana Fairmaire (n = 5) | Buprestidae |
| Complementary Structures | Interaction of the Structures | Contributions to the Following Mechanisms |
|---|---|---|
| Flange of the elytral base (EBF) + posterodorsal groove of the pronotum (PGr) | Conformal contact | Thoracic interlocking. |
| Posteroventral evagination of the pronotum (PdE) + basal elytral groove (BEG) | Conformal contact | Thoracic interlocking. |
| Posteroventral evagination of the pronotum (PdE) + anterolateral region of the mesonotum (AR) | Conformal contact | Clicking and thoracic interlocking. The PdE and AR form the thoracic hinge for the clicking mechanism. |
| Posteroventral evagination of the pronotum (PvE) + mesanepisternum (Es2) | Conformal contact | Thoracic interlocking. Its involvement in the clicking mechanism is unknown. |
| Mesal basal part of the elytra + posterior part of the mesonotum | Conformal contact; interlock with each other | Clicking, thoracic interlocking, and elytral-mesoscutellar interlocking. |
| Friction hold (FH) of the prosternal process + prosternal rest of the mesoventrite (PRM) | Conformal contact | Clicking. |
| Prosternal process (PP) + mesoventral cavity (MsC) | Conformal contact | Clicking. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Y.; Zhang, M.; Kundrata, R.; Qiu, L.; Ge, S.; Yang, X.; Chen, X.; Jiang, S. Functional Morphology of the Thorax of the Click Beetle Campsosternus auratus (Coleoptera, Elateridae), with an Emphasis on Its Jumping Mechanism. Insects 2022, 13, 248. https://doi.org/10.3390/insects13030248
Ruan Y, Zhang M, Kundrata R, Qiu L, Ge S, Yang X, Chen X, Jiang S. Functional Morphology of the Thorax of the Click Beetle Campsosternus auratus (Coleoptera, Elateridae), with an Emphasis on Its Jumping Mechanism. Insects. 2022; 13(3):248. https://doi.org/10.3390/insects13030248
Chicago/Turabian StyleRuan, Yongying, Mengna Zhang, Robin Kundrata, Lu Qiu, Siqin Ge, Xingke Yang, Xiaoqin Chen, and Shihong Jiang. 2022. "Functional Morphology of the Thorax of the Click Beetle Campsosternus auratus (Coleoptera, Elateridae), with an Emphasis on Its Jumping Mechanism" Insects 13, no. 3: 248. https://doi.org/10.3390/insects13030248
APA StyleRuan, Y., Zhang, M., Kundrata, R., Qiu, L., Ge, S., Yang, X., Chen, X., & Jiang, S. (2022). Functional Morphology of the Thorax of the Click Beetle Campsosternus auratus (Coleoptera, Elateridae), with an Emphasis on Its Jumping Mechanism. Insects, 13(3), 248. https://doi.org/10.3390/insects13030248







