ddRAD Sequencing Identifies Pesticide Resistance-Related Loci and Reveals New Insights into Genetic Structure of Bactericera cockerelli as a Plant Pathogen Vector
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Psyllid Collections and Colony Maintenance
2.2. Sample Collection and DNA Extraction
2.3. Data Analysis
2.3.1. SNP Calling
2.3.2. Genetic Basis of Insecticide Resistance Using Association Test and Annotation of De Novo Assembled Contigs
2.3.3. Genetic Structure and Variation
3. Results and Discussion
3.1. Genetic Basis of Insecticide Resistance
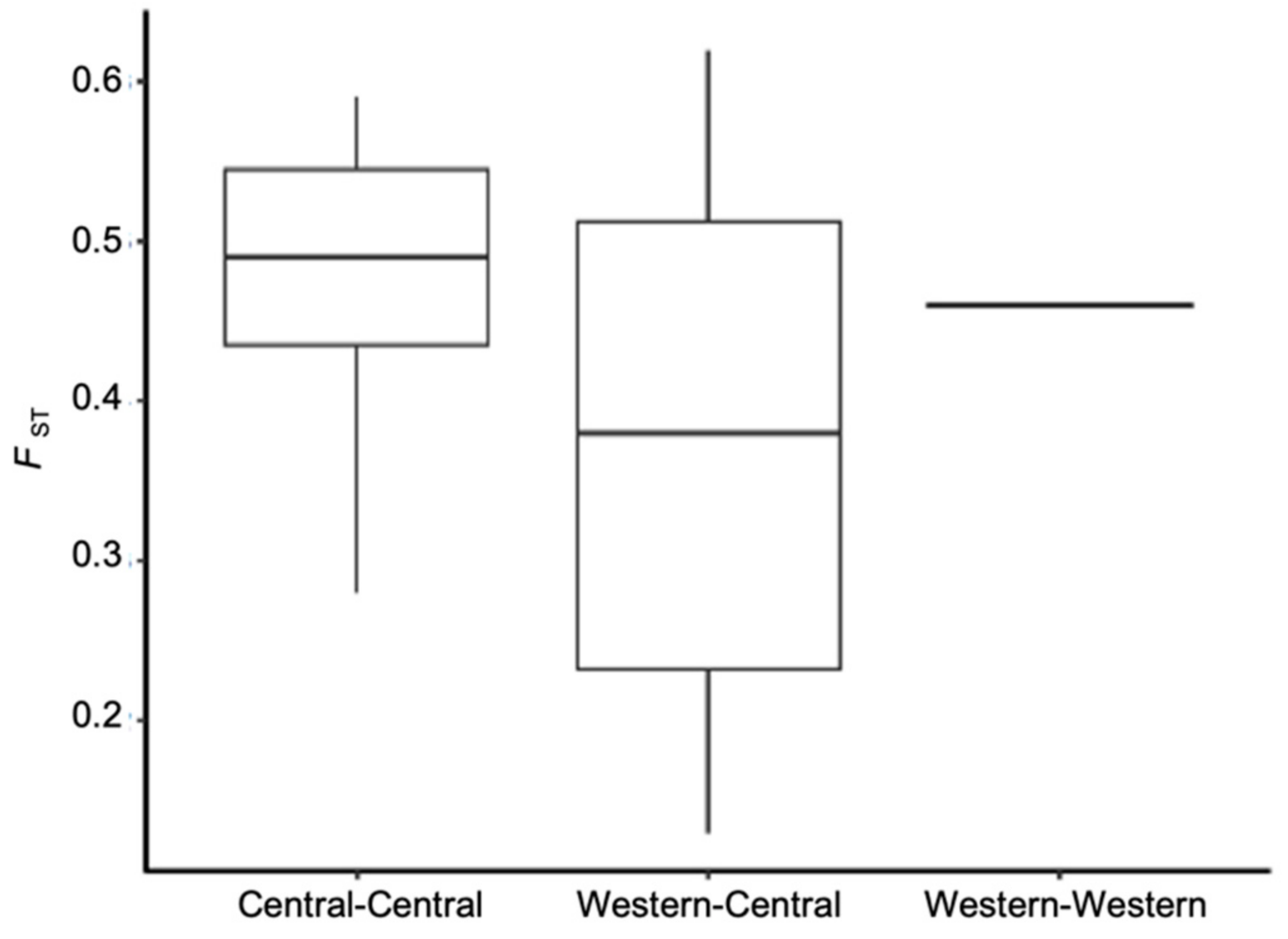
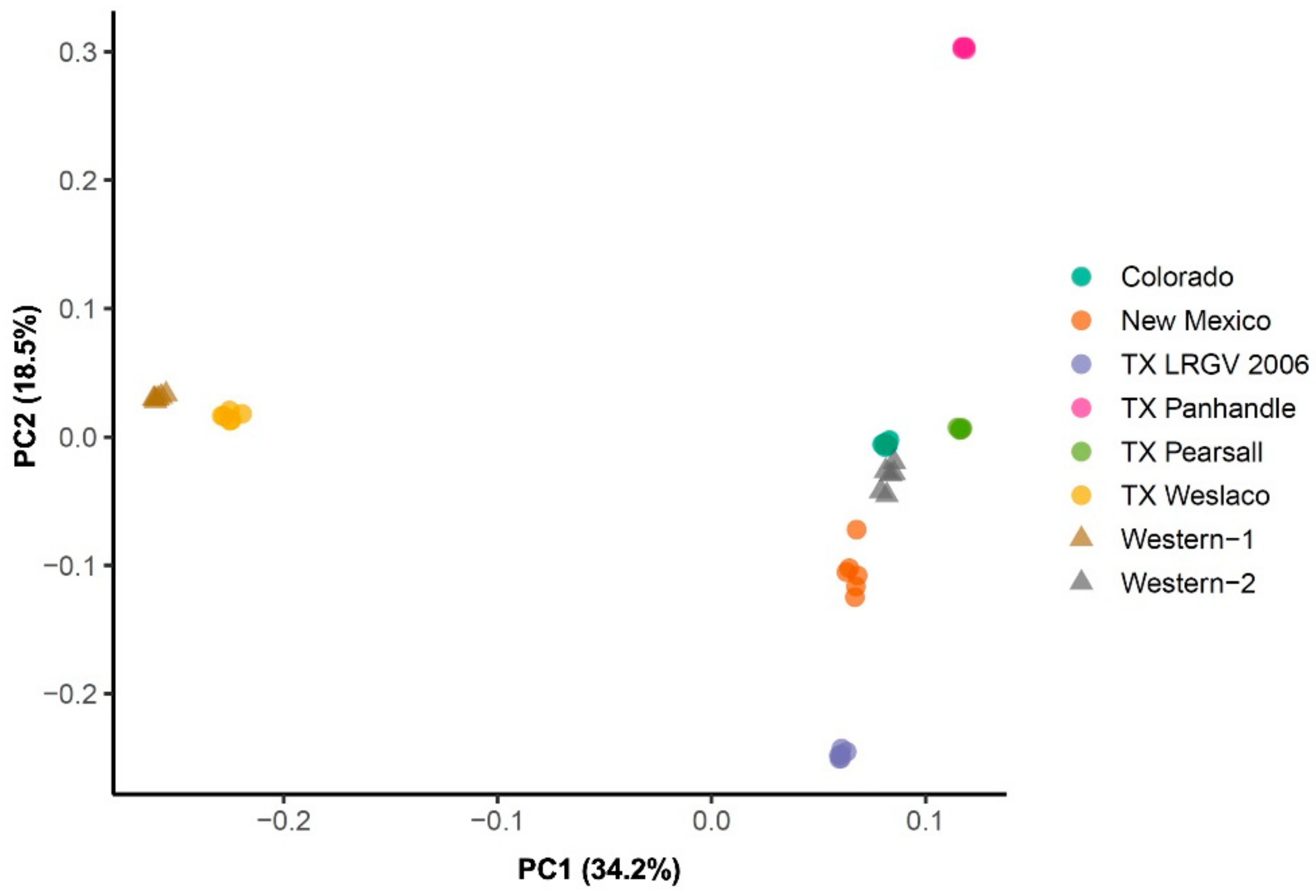
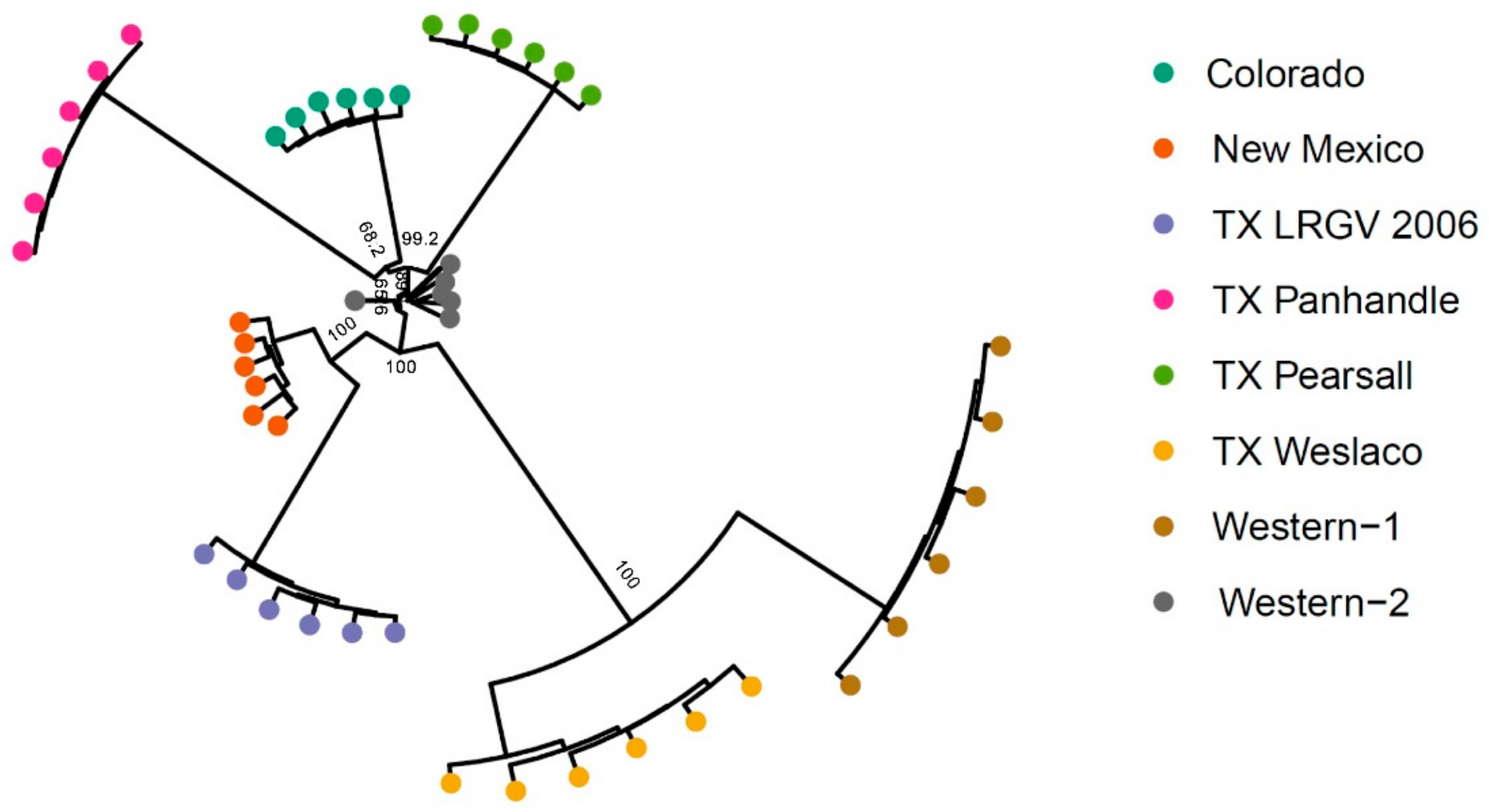
3.2. Population Genetic Structure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perilla-Henao, L.M.; Casteel, C.L. Vector-borne bacterial plant pathogens: Interactions with hemipteran insects and plants. Front. Plant Sci. 2016, 7, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Perring, T.M.; Gruenhagen, N.M.; Farrar, C.A. Management of plant viral diseases through chemical control of insect vectors. Annu. Rev. Entomol. 1999, 44, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Mota-Sanchez, D.; Whalon, M.E.; Holingworth, R.M.; Xue, Q. Documentation of pesticide resistance in arthropods. In Global Pesticide Resistance in Arthropods, 1st ed.; Whalon, M.E., Mota-Sanchez, D., Hollingworth, R.M., Eds.; CABI: Wallingford, UK, 2008; pp. 32–39. [Google Scholar]
- Stillson, P.T.; Bloom, E.H.; Illán, J.G.; Szendrei, Z. A novel plant pathogen management tool for vector management. Pest Manag. Sci. 2020, 76, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Biondi, A.; Desneux, N.; Siscaro, G.; Zappalà, L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: Selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 2012, 87, 803–881. [Google Scholar] [CrossRef]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P.A. Impacts of synthetic and botanical pesticides on beneficial insects. Agric. Sci. 2016, 7, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Pierson, E.A.; Setubal, J.C.; Xu, J.; Levy, J.G.; Zhang, Y.; Li, J.; Rangel, L.T.; Martins, J., Jr. The Candidatus Liberibacter—Host interface: Insights into pathogenesis mechanisms and disease control. Annu. Rev. Phytopathol. 2017, 55, 451–482. [Google Scholar] [CrossRef]
- ffrench-Constant, R.H.; Anthony, N.; Aronstein, K.; Rocheleau, T.; Stilwell, G. Cyclodiene insecticide resistance: From molecular to population genetics. Annu. Rev. Entomol. 2000, 45, 449–466. [Google Scholar] [CrossRef]
- Lietti, M.M.; Botto, E.; Alzogaray, R.A. Insecticide resistance in argentine populations of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Neotrop. Entomol. 2005, 34, 113–119. [Google Scholar] [CrossRef]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef]
- Casida, J.E.; Durkin, K.A. Neuroactive insecticides: Targets, selectivity, resistance, and secondary effects. Annu. Rev. Entomol. 2013, 58, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, N. Insecticide resistance in mosquitoes: Impact, mechanisms, and research directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniec, A.; Varela, K.A.; Kiani, M.; Paetzold, L.; Rush, C.M. Incidence of resistance to neonicotinoid insecticides in Bactericera cockerelli across Southwest US. Crop Prot. 2019, 116, 188–195. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. The potato psyllid, Bactericera cockerelli (Sulc) (Hemiptera: Triozidae): Life history, relationship to plant diseases, and management strategies. Terr. Arthropod Rev. 2012, 5, 87–111. [Google Scholar] [CrossRef]
- Munyaneza, J.; Crosslin, J.; Upton, J. Association of Bactericera cockerelli (Homoptera: Psyllidae) with “zebra chip”, a new potato disease in southwestern United States and Mexico. J. Econ. Entomol. 2007, 100, 656–663. [Google Scholar] [CrossRef]
- Brown, J.; Rehman, M.; Rogan, D.; Martin, R.; Idris, A. First report of “Candidatus Liberibacter psyllaurous” (synonym “Ca. L. solanacearum”) associated with ‘tomato vein-greening’and ‘tomato psyllid yellows’ diseases in commercial greenhouses in Arizona. Plant Dis. 2010, 94, 376. [Google Scholar] [CrossRef]
- Butler, C.D.; Trumble, J.T. Identification and impact of natural enemies of Bactericera cockerelli (Hemiptera: Triozidae) in Southern California. J. Econ. Entomol. 2012, 105, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Wen, A.; Johnson, C.; Gudmestad, N.C. Development of a PCR assay for the rapid detection and differentiation of ‘Candidatus Liberibacter solanacearum’ haplotypes and their spatiotemporal distribution in the United States. Am. J. Potato Res. 2013, 90, 229–236. [Google Scholar] [CrossRef]
- Munyaneza, J.E. Zebra chip disease of potato: Biology, epidemiology, and management. Am. J. Potato Res. 2012, 89, 329–350. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Wang, R.; Ren, Y.; McKirdy, S. Potential distribution and the risks of Bactericera cockerelli and its associated plant pathogen Candidatus Liberibacter Solanacearum for global potato production. Insects 2020, 11, 298. [Google Scholar] [CrossRef]
- Swisher, K.D.; Munyaneza, J.E.; Crosslin, J.M. High resolution melting analysis of the cytochrome oxidase I gene identifies three haplotypes of the potato psyllid in the United States. Environ. Entomol. 2012, 41, 1019–1028. [Google Scholar] [CrossRef]
- Swisher, K.D.; Henne, D.C.; Crosslin, J.M. Identification of a fourth haplotype of Bactericera cockerelli (Hemiptera: Triozidae) in the United States. J. Insect Sci. 2014, 14, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, T.; Horton, D.R.; Cooper, W.R.; Swisher, K.D.; Zack, R.S.; Pappu, H.R.; Munyaneza, J.E. Use of electrical penetration graph technology to examine transmission of ‘Candidatus Liberibacter solanacearum’ to potato by three haplotypes of potato psyllid (Bactericera cockerelli; Hemiptera: Triozidae). PLoS ONE 2015, 10, e0138946. [Google Scholar] [CrossRef] [PubMed]
- Greenway, G. Economic impact of zebra chip control costs on grower returns in seven US states. Am. J. Potato Res. 2014, 91, 714–719. [Google Scholar] [CrossRef]
- Greenway, G.; Rondon, S. Economic impacts of zebra chip in Idaho, Oregon, and Washington. Am. J. Potato Res. 2018, 95, 362–367. [Google Scholar] [CrossRef]
- Byrne, F.J.; Toscano, N.C. Uptake and persistence of imidacloprid in grapevines treated by chemigation. Crop Prot. 2006, 25, 831–834. [Google Scholar] [CrossRef]
- Prager, S.M.; Vindiola, B.; Kund, G.S.; Byrne, F.J.; Trumble, J.T. Considerations for the use of neonicotinoid pesticides in management of Bactericera cockerelli (Šulk) (Hemiptera: Triozidae). Crop Prot. 2013, 54, 84–91. [Google Scholar] [CrossRef]
- Tomizawa, M.; Casida, J.E. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 247–268. [Google Scholar] [CrossRef] [Green Version]
- Crossley, M.S.; Rondon, S.I.; Schoville, S.D. A comparison of resistance to imidacloprid in Colorado potato beetle (Leptinotarsa decemlineata Say) populations collected in the Northwest and Midwest US. Am. J. Potato Res. 2018, 95, 495–503. [Google Scholar] [CrossRef]
- Liu, D.; Trumble, J.T. Comparative fitness of invasive and native populations of the potato psyllid (Bactericera cockerelli). Entomol. Exp. Appl. 2007, 123, 35–42. [Google Scholar] [CrossRef]
- Prager, S.M.; Trumble, J.T. Psyllids: Biology, ecology, and management. In Sustainable Management of Arthropod Pests of Tomato, 1st ed.; Wakil, W., Brust, G.E., Perring, T.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 163–176. [Google Scholar]
- Rinkevich, F.D.; Du, Y.; Dong, K. Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic. Biochem. Physiol. 2013, 106, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Yang, C.; Yang, Y.; Li, J.; Lin, K.; Li, M.; Qiu, X. Distribution and frequency of G119S mutation in ace-1 gene within Anopheles sinensis populations from Guangxi, China. Malar. J. 2015, 14, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claudianos, C.; Ranson, H.; Johnson, R.; Biswas, S.; Schuler, M.; Berenbaum, M.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [Green Version]
- Ingham, V.A.; Jones, C.M.; Pignatelli, P.; Balabanidou, V.; Vontas, J.; Wagstaff, S.C.; Moore, J.D.; Ranson, H. Dissecting the organ specificity of insecticide resistance candidate genes in Anopheles gambiae: Known and novel candidate genes. BMC Genom. 2014, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Liang, P.; Fang, K.; Chu, D. Silence of inositol 1,4,5-trisphosphate receptor expression decreases cyantraniliprole susceptibility in Bemisia tabaci. Pestic. Biochem. Physiol. 2017, 142, 162–169. [Google Scholar] [CrossRef]
- Fournier-Level, A.; Good, R.T.; Wilcox, S.A.; Rane, R.V.; Schiffer, M.; Chen, W.; Battlay, P.; Perry, T.; Batterham, P.; Hoffmann, A.A.; et al. The spread of resistance to imidacloprid is restricted by thermotolerance in natural populations of Drosophila melanogaster. Nat. Ecol. Evol. 2019, 3, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Glunt, K.D.; Coetzee, M.; Huijben, S.; Koffi, A.A.; Lynch, P.A.; N’Guessan, R.; Oumbouke, W.A.; Sternberg, E.D.; Thomas, M.B. Empirical and theoretical investigation into the potential impacts of insecticide resistance on the effectiveness of insecticide-treated bed nets. Evol. Appl. 2018, 11, 431–441. [Google Scholar] [CrossRef] [Green Version]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef] [Green Version]
- Foll, M.; Gaggiotti, O. A genome-scan method to identify selected loci appropriate for both dominant and codominant markers: A Bayesian perspective. Genetics 2008, 180, 977–993. [Google Scholar] [CrossRef] [Green Version]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. GigaScience 2015, 4, s13742-015. [Google Scholar] [CrossRef] [PubMed]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earl, D.A.; VonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [Green Version]
- Yu, G. Using ggtree to visualize data on tree-like structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef]
- Liu, H.; Miller, M.S.; Swank, D.M.; Kronert, W.A.; Maughan, D.W.; Bernstein, S.I. Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2005, 102, 10522–10527. [Google Scholar] [CrossRef] [Green Version]
- Brown, J.S.; Jackson, S.P. Ubiquitylation, neddylation and the DNA damage response. Open Biol. 2015, 5, 150018. [Google Scholar] [CrossRef] [Green Version]
- George, S.; Palli, S.R. Histone Deacetylase 11 Knockdown blocks larval development and metamorphosis in the red flour beetle, Tribolium castaneum. Front. Genet. 2020, 11, 683–695. [Google Scholar] [CrossRef]
- Tauszig, S.; Jouanguy, E.; Hoffmann, J.A.; Imler, J.-L. Toll-related receptors and the control of antimicrobial peptide expression in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 97, 10520–10525. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, A.; Habineza, P.; Wang, X.; Xiao, R.; Ji, T.; Hou, Y.; Shi, Z. Spätzle homolog-mediated Toll-like pathway regulates innate immune responses to maintain the homeostasis of gut microbiota in the red palm weevil, Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Front. Microbiol. 2020, 11, 846–857. [Google Scholar] [CrossRef]
- Jiang, D.; Du, X.; Wang, C.; Zhang, S.; Wang, G. CD109 antigen-like gene is induced by ecdysone signaling and involved in the cellular immunity of Helicoverpa armigera. Biosci. Biotechnol. Biochem. 2020, 84, 1183–1190. [Google Scholar] [CrossRef]
- Vézilier, J.; Nicot, A.; De Lorgeril, J.; Gandon, S.; Rivero, A. The impact of insecticide resistance on Culex pipiens immunity. Evol. Appl. 2013, 6, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Lawrie, R.D.; Mitchell, R.D., III; Deguenon, J.M.; Ponnusamy, L.; Reisig, D.; Pozo-Valdivia, A.D.; Kurtz, R.W.; Roe, R.M. Multiple known mechanisms and a possible role of an enhanced immune system in Bt-resistance in a field population of the bollworm, Helicoverpa zea: Differences in gene expression with RNAseq. Int. J. Mol. Sci. 2020, 21, 6528. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.; Zhongyuan, Z.; Wang, J.; Wong, C.O.; Karagas, N.E.; Roessner, U.; Rupasinghe, T.; Venkatachalam, K.; Perry, T.; Bellen, H.J.; et al. Low doses of the neonicotinoid insecticide imidacloprid induce ROS triggering neurological and metabolic impairments in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 25840–25850. [Google Scholar] [CrossRef] [PubMed]
- Werck-Reichhart, D.; Feyereisen, R. Cytochromes P450: A success story. Genome Biol. 2000, 1, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 2006, 34, 1252–1255. [Google Scholar] [CrossRef] [Green Version]
- Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry, 1st ed.; Gilbert, L.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 236–316. [Google Scholar]
- Feyereisen, R.; Dermauw, W.; Van Leeuwen, T. Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic. Biochem. Physiol. 2015, 121, 61–77. [Google Scholar] [CrossRef]
- Corbel, V.; N’guessan, R.; Brengues, C.; Chandre, F.; Djogbenou, L.; Martin, T.; Akogbeto, M.; Hougard, J.M.; Rowland, M. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007, 101, 207–216. [Google Scholar] [CrossRef]
- de Little, S.C.; Edwards, O.; van Rooyen, A.R.; Weeks, A.; Umina, P.A. Discovery of metabolic resistance to neonicotinoids in green peach aphids (Myzus persicae) in Australia. Pest Manag. Sci. 2017, 73, 1611–1617. [Google Scholar] [CrossRef]
- Weetman, D.; Wilding, C.S.; Neafsey, D.E.; Müller, P.; Ochomo, E.; Isaacs, A.T.; Steen, K.; Rippon, E.J.; Morgan, J.C.; Mawejje, H.D.; et al. Candidate-gene based GWAS identifies reproducible DNA markers for metabolic pyrethroid resistance from standing genetic variation in East African Anopheles gambiae. Sci. Rep. 2018, 8, 2920–2931. [Google Scholar] [CrossRef]
- Traverso, L.; Lavore, A.; Sierra, I.; Palacio, V.; Martinez-Barnetche, J.; Latorre-Estivalis, J.M.; Mougabure-Cueto, G.; Francini, F.; Lorenzo, M.G.; Rodríguez, M.H.; et al. Comparative and functional triatomine genomics reveals reductions and expansions in insecticide resistance-related gene families. PLoS Negl. Trop. Dis. 2017, 11, e0005313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Umina, P.A.; Rašić, G.; Bell, N.; Fang, J.; Lord, A.; Hoffmann, A.A. Origin of resistance to pyrethroids in the redlegged earth mite (Halotydeus destructor) in Australia: Repeated local evolution and migration. Pest Manag. Sci. 2020, 76, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Epstein, B.; Kelley, J.L.; Zheng, Q.; Bergland, A.O.; Castillo Carrillo, C.I.; Jensen, A.S.; Dahan, J.; Karasev, A.V.; Snyder, W.E. Using NextRAD sequencing to infer movement of herbivores among host plants. PLoS ONE 2017, 12, e0177742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Meier, A.R.; Epstein, B.; Bergland, A.O.; Castillo Carrillo, C.I.; Cooper, W.R.; Cruzado, R.K.; Horton, D.R.; Jensen, A.S.; Kelley, J.L.; et al. Host plants and Wolbachia shape the population genetics of sympatric herbivore populations. Evol. Appl. 2020, 13, 2740–2753. [Google Scholar] [CrossRef]
- Toews, D.P.; Brelsford, A. The biogeography of mitochondrial and nuclear discordance in animals. Mol. Ecol. 2012, 21, 3907–3930. [Google Scholar] [CrossRef]


| Pop ID | Haplotype | Original Collection Location | Collection Time | Resistance to Insecticide |
|---|---|---|---|---|
| Colorado | Central | Wray, CO | 2015 | Resistant |
| New Mexico | Central | Farmington, NM | 2015 | Resistant |
| TX LRGV | Central | Weslaco, TX | 2006 | Susceptible |
| TX Panhandle | Central | Dalhart, TX | 2016 | Resistant |
| TX Pearsall | Central | Pearsall, TX | 2014 | Resistant |
| TX Weslaco | Central | Weslaco, TX | 2015 | Resistant |
| Western-1 | Western | Unknown | 2013 | Resistant |
| Western-2 | Western | Unknown | 2015 | Resistant |
| SeqName | Description | e-Value | Number of Variant Sites |
|---|---|---|---|
| CL_2391 | Diaphorina citri protein spaetzle 4-like | 1.98 × 10−23 | 2 |
| CL_3747 | Diaphorina citri DDB1- and CUL4-associated factor 5 | 1.55 × 10−5 | 4 |
| CL_4398 | Diaphorina citri histone deacetylase 11 | 1.57 × 10−4 | 1 |
| CL_8229 | Diaphorina citri paramyosin | 1.36 × 10−11 | 1 |
| CL_9321 | Acyrthosiphon pisum CD109 antigen | 9.81 × 10−20 | 2 |
| CL_28618 | Cephus cinctus inositol 1,4,5-trisphosphate receptor | 9.22 × 10−14 | 1 |
| CL_29918 | Diaphorina citri neurogenic locus Notch protein | 3.17 × 10−39 | 1 |
| CL_51462 | Diaphorina citri non-lysosomal glucosylceramidase-like | 4.66 × 10−12 | 1 |
| CL_60403 | Diaphorina citri angiotensin-converting enzyme 2-like | 7.22 × 10−9 | 1 |
| CL_27124 | Candidatus Liberibacter solanacearum CLso-ZC1 | 1.09 × 10−37 | 1 |
| CL_57538 | Wolbachia endosymbiont of Culex molestus DNA methylase-like protein | 1.04 × 10−45 | 2 |
| CL_58510 | Candidatus Liberibacter solanacearum CLso-ZC1 | 4.30 × 10−50 | 1 |
| Pop ID | Private | %Polymorphic_Loci | Obs_Het | Exp_Het |
|---|---|---|---|---|
| Colorado | 118 | 2.78 × 10−2 | 1.8 × 10−4 | 1.1 × 10−4 |
| New Mexico | 72 | 3.12 × 10−2 | 1.9 × 10−4 | 1.2 × 10−4 |
| TX LRGV | 161 | 2.81 × 10−2 | 1.7 × 10−4 | 1.1 × 10−4 |
| TX Panhandle | 356 | 2.35 × 10−2 | 1.6 × 10−4 | 9.0 × 10−5 |
| TX Pearsall | 235 | 2.55 × 10−2 | 1.7 × 10−4 | 1.0 × 10−4 |
| TX Weslaco | 167 | 3.60 × 10−2 | 2.5 × 10−4 | 1.5 × 10−4 |
| Western-1 | 249 | 3.32 × 10−2 | 2.3 × 10−4 | 1.4 × 10−4 |
| Western-2 | 29 | 3.15 × 10−2 | 1.6 × 10−4 | 1.1 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, M.; Fu, Z.; Szczepaniec, A. ddRAD Sequencing Identifies Pesticide Resistance-Related Loci and Reveals New Insights into Genetic Structure of Bactericera cockerelli as a Plant Pathogen Vector. Insects 2022, 13, 257. https://doi.org/10.3390/insects13030257
Kiani M, Fu Z, Szczepaniec A. ddRAD Sequencing Identifies Pesticide Resistance-Related Loci and Reveals New Insights into Genetic Structure of Bactericera cockerelli as a Plant Pathogen Vector. Insects. 2022; 13(3):257. https://doi.org/10.3390/insects13030257
Chicago/Turabian StyleKiani, Mahnaz, Zhen Fu, and Adrianna Szczepaniec. 2022. "ddRAD Sequencing Identifies Pesticide Resistance-Related Loci and Reveals New Insights into Genetic Structure of Bactericera cockerelli as a Plant Pathogen Vector" Insects 13, no. 3: 257. https://doi.org/10.3390/insects13030257
APA StyleKiani, M., Fu, Z., & Szczepaniec, A. (2022). ddRAD Sequencing Identifies Pesticide Resistance-Related Loci and Reveals New Insights into Genetic Structure of Bactericera cockerelli as a Plant Pathogen Vector. Insects, 13(3), 257. https://doi.org/10.3390/insects13030257






