Differential Expression of Major Royal Jelly Proteins in the Hypopharyngeal Glands of the Honeybee Apis mellifera upon Bacterial Ingestion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybees
2.2. Feeding Experiment
2.3. RNA Extraction
2.4. Northern Blot Analysis
2.5. Statistical Analysis
3. Results
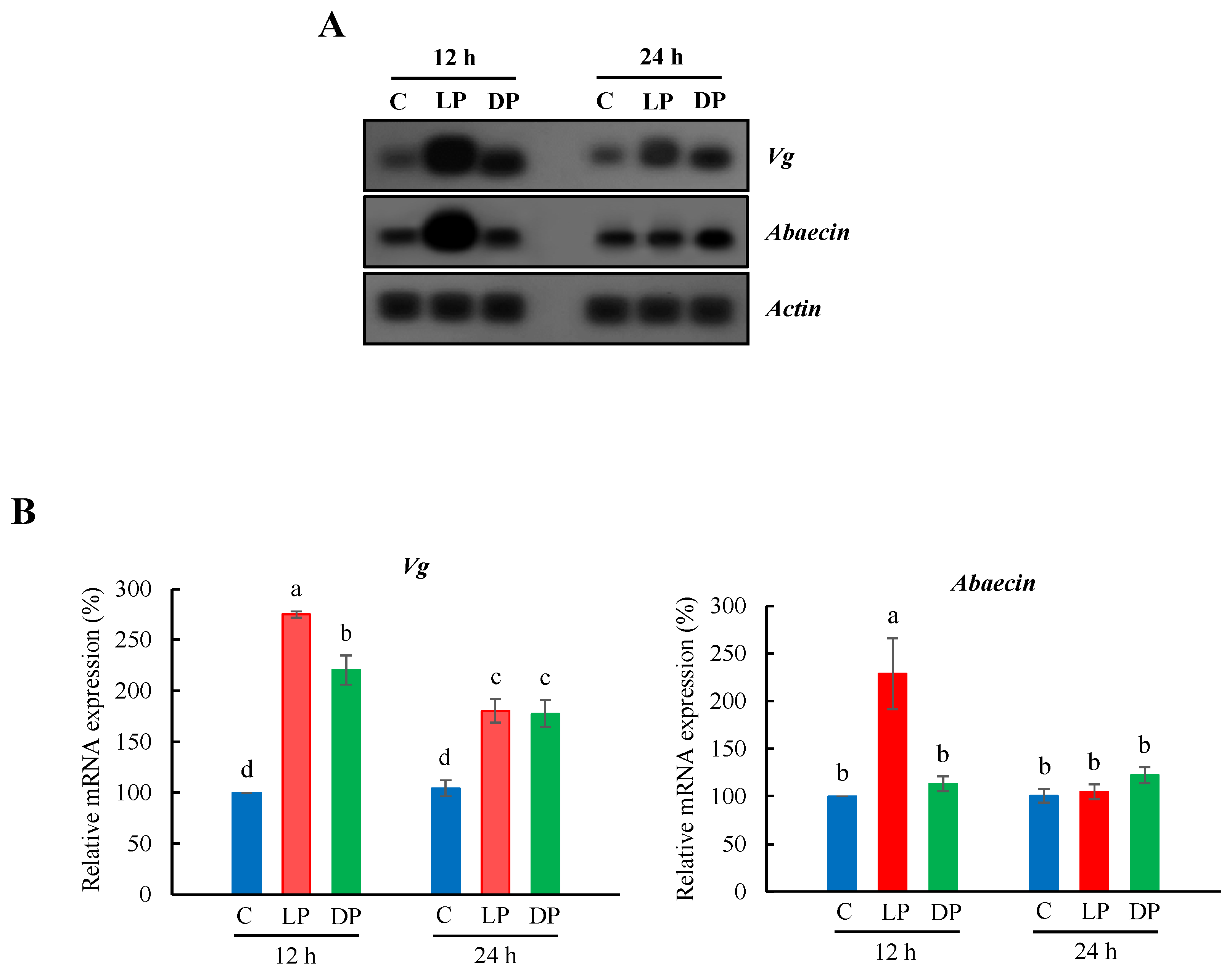
3.1. Expression Profile of Vg and Abaecin in the Fat Body of Nurse Bees
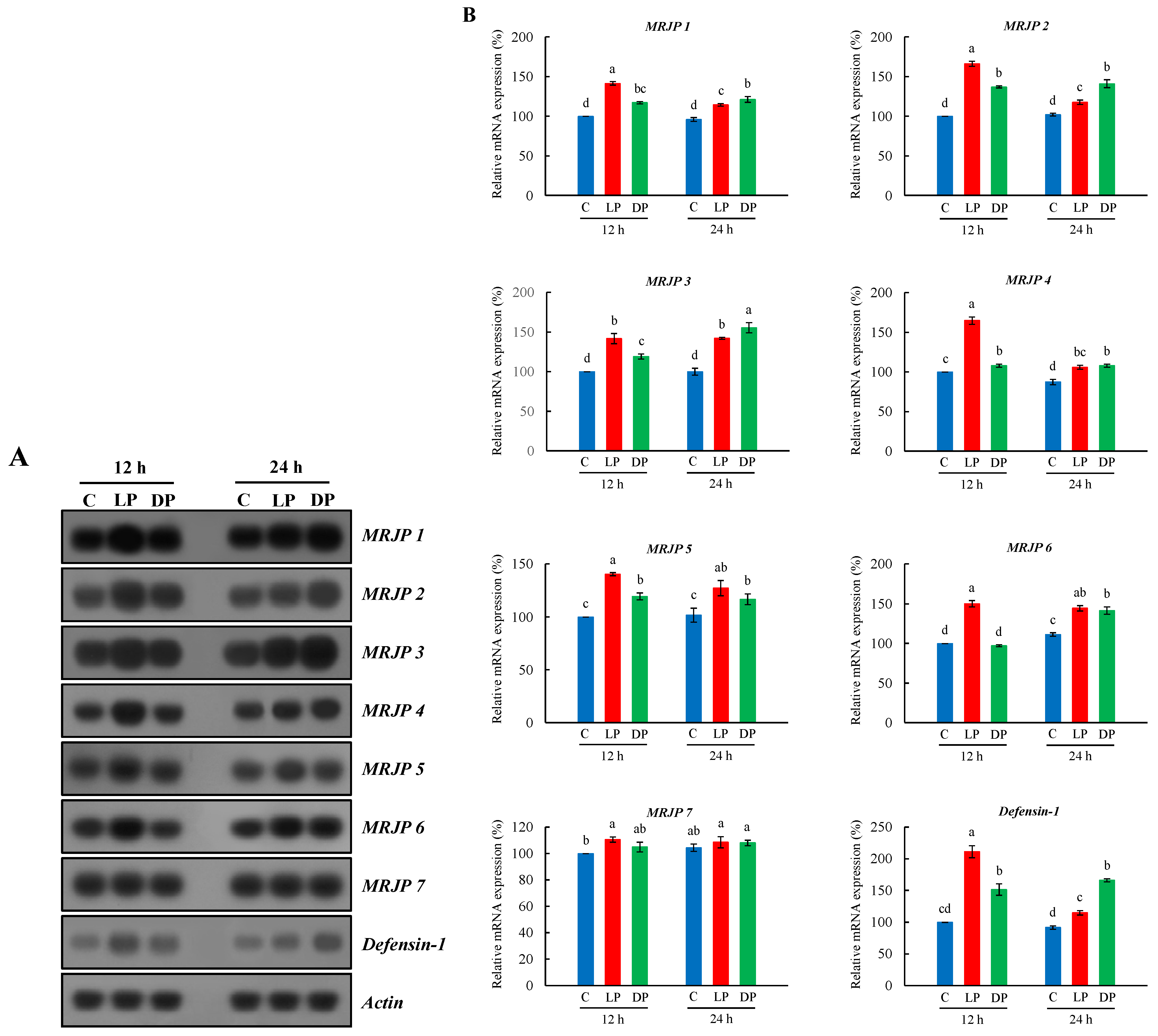
3.2. Expression Profile of MRJPs and Defensin-1 in Hypopharyngeal Glands of Nurse Bees
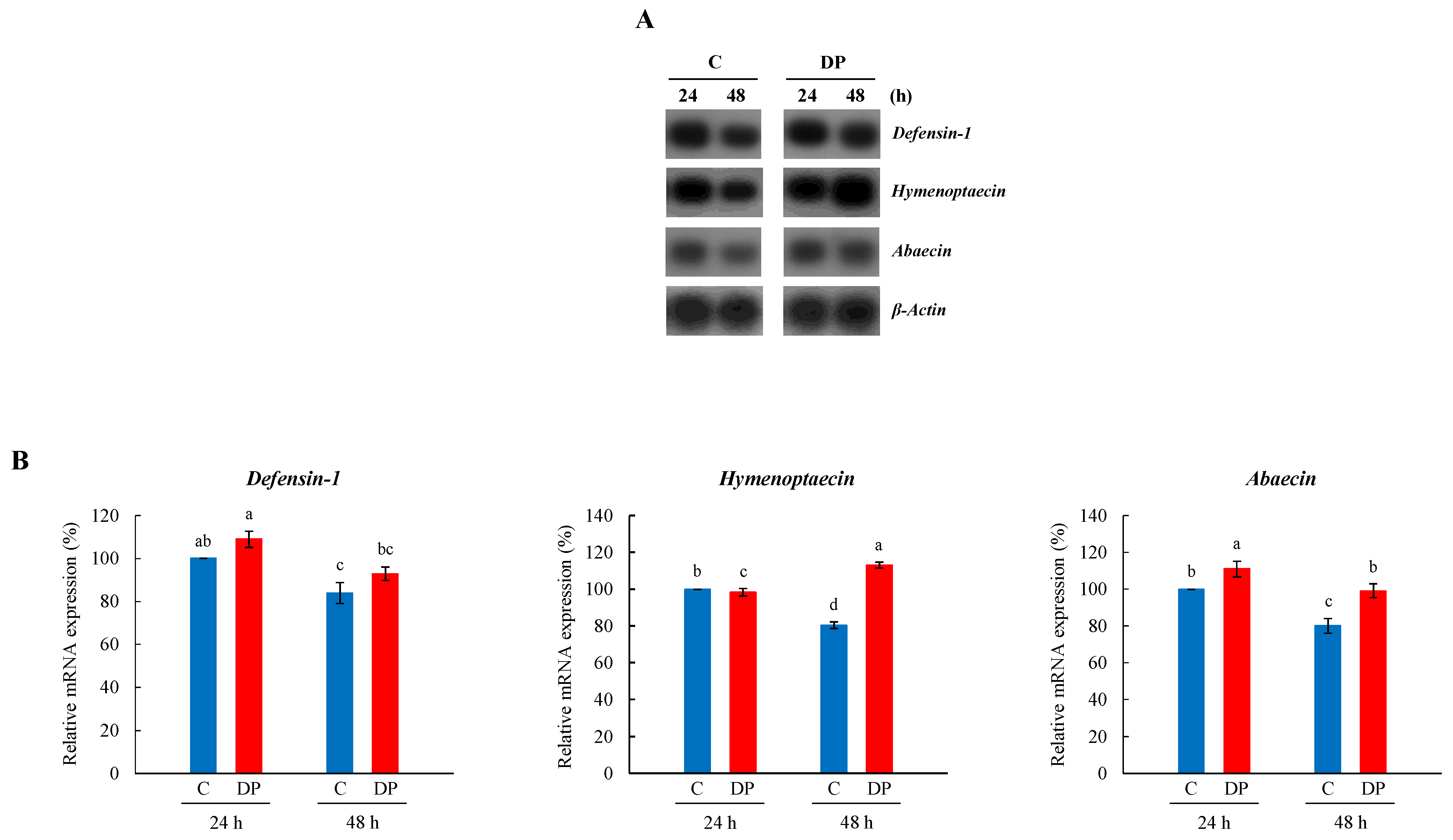
3.3. Expression Profile of Defensin-1, Hymenoptaecin, and Abaecin in Whole Body of Young Larvae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, R.; Donaldio, E.; Giuffrida, M.G.; Fortunato, D.; Conti, A.; Balestreri, E.; Felicioli, R.; Pinzauti, M.; Sabatini, A.G.; Felicioli, A. Towards royal jelly proteome. Proteomics 2005, 5, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Simuth, J. Some properties of the main protein in honeybee (Apis mellifera) royal jelly. Apidologie 2001, 32, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Santos, K.S.; Dos Santos, L.D.; Mendes, M.A.; De Souza, B.M.; Malaspina, O.; Palma, M.S. Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honeybees (Apis mellifera L.). Insect Biochem. Mol. Biol. 2005, 35, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Feng, M.; Li, J. Royal jelly proteome comparison between A. mellifera ligustica and A. cerana cerana. J. Proteome Res. 2010, 9, 2207–2215. [Google Scholar] [CrossRef]
- Han, B.; Li, C.; Zhang, L.; Fang, Y.; Feng, M.; Li, J. Novel royal jelly proteins identified by gel-based and gel-free proteomics. J. Agric. Food Chem. 2011, 59, 10346–10355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, Y.; Li, R.; Feng, M.; Han, B.; Zhou, T.; Li, J. Towards posttranslational modification proteome of royal jelly. J. Proteom. 2012, 75, 5327–5341. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Park, H.G.; Kim, B.Y.; Kim, J.M.; Choi, Y.S.; Yoon, H.J.; Lee, K.S.; Jin, B.R. Upregulation of transferrin and major royal jelly proteins in the spermathecal fluid of mated honeybee (Apis mellifera) queens. Insects 2021, 12, 690. [Google Scholar] [CrossRef] [PubMed]
- Peiren, N.; Vanrobaeys, F.; De Graaf, D.C.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. The protein composition of honeybee venom reconsidered by a proteomic approach. Biochim. Biophys. Acta 2005, 1752, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Peiren, N.; De Graaf, D.C.; Vanrobaeys, F.; Danneels, E.I.; Devreese, B.; Van Beeumen, J.; Jacobs, F.J. Proteomic analysis of the honey bee worker venom gland focusing on the mechanisms of protection against tissue damage. Toxicon 2008, 52, 72–83. [Google Scholar] [CrossRef]
- Blank, S.; Bantleon, F.I.; McIntyre, M.; Oliert, M.; Spiliner, E. The major royal jelly proteins 8 and 9 (Api m 11) are glycosylated components of Apis mellifera venom with allergenic potential beyond carbohydrate-based reactivity. Clin. Exp. Allergy 2012, 42, 976–985. [Google Scholar] [CrossRef]
- Kim, B.Y. Antiapoptotic role of major royal jelly protein 8 of honeybee (Apis mellifera) venom. J. Asia Pac. Entomol. 2021, 24, 666–670. [Google Scholar] [CrossRef]
- Nagai, T.; Inoue, R.; Suzuki, N.; Nagashima, T. Antioxidant properties of enzymatic hydrolysates from royal jelly. J. Med. Food 2006, 9, 363–367. [Google Scholar] [CrossRef] [Green Version]
- Inoue, S.I.; Koya-Miyata, S.; Ushio, S.; Iwak, K.; Ikeda, M.; Kurimoto, M. Royal jelly prolongs the life span of C3H/H3J mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Xin, X.X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.Q.; Shen, L.R. Supplementation with major royal-jelly proteins increases lifespan, feeding, and fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, K.S.; Jung, B.; Choi, Y.S.; Kim, H.K.; Yoon, H.J.; Gui, Z.Z.; Lee, J.; Jin, B.R. Honeybee (Apis cerana) major royal jelly protein 4 exhibits antimicrobial activity. J. Asia Pac. Entomol. 2019, 22, 175–182. [Google Scholar] [CrossRef]
- Park, H.G.; Kim, B.Y.; Park, M.J.; Deng, Y.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antibacterial activity of major royal jelly proteins of the honey (Apis mellifera) royal jelly. J. Asia Pac. Entomol. 2019, 22, 739–741. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, B.Y.; Park, H.G.; Deng, Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Major royal jelly protein 2 acts as an antimicrobial agent and antioxidant in royal jelly. J. Asia Pac. Entomol. 2019, 22, 684–689. [Google Scholar] [CrossRef]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef]
- Tufail, M.; Nagaba, Y.; Elgendy, A.M.; Takeda, M. Regulation of vitellogenin genes in insects. Entomol. Sci. 2014, 17, 269–282. [Google Scholar] [CrossRef]
- Tufail, M.; Takeda, M. Molecular characteristics of insect vitellogenins. J. Insect Physiol. 2008, 54, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Bownes, M. Expression of the genes coding for vitellogenin (yolk protein). Annu. Rev. Entomol. 1986, 31, 507–531. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R. Insect antimicrobial peptides: Structures, properties and gene regulation. Protein Pept. Lett. 2005, 12, 3–11. [Google Scholar] [CrossRef]
- Havukainen, H.; Münch, D.; Baumann, A.; Zhong, S.; Halskau, Ø.; Krogsgaard, M.; Amdam, G.V. Vitellogenin recognizes cell damage through membrane binding and shields living cells from reactive oxygen species. J. Biol. Chem. 2013, 288, 28369–28381. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.K.; Pakkianathan, B.C.; Kumar, M.; Prasad, T.; Kannan, M.; König, S.; Krishnan, M. Vitellogenin from the silkworm, Bombyx mori: An effective anti-bacterial agent. PLoS ONE 2013, 8, e73005. [Google Scholar] [CrossRef] [Green Version]
- Salmela, H.; Amdam, G.V.; Freitak, D. Transfer of immunity from mother to offspring is mediated via egg-yolk protein vitellogenin. PLoS Pathog. 2015, 11, e1005015. [Google Scholar] [CrossRef] [Green Version]
- Salmela, H.; Sundström, L. Vitellogenin in inflammation and immunity in social insects. Inflamm. Cell Signal. 2017, 4, e1506. [Google Scholar]
- Park, H.G.; Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Lee, K.Y.; Wan, H.; Li, J.; Jin, B.R. Honeybee (Apis cerana) vitellogenin acts as an antimicrobial and antioxidant agent in the body and venom. Dev. Comp. Immunol. 2018, 85, 51–60. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef] [Green Version]
- Seehuus, S.C.; Norberg, K.; Krekling, T.; Fondrk, K.; Amdam, G.V. Immunogold localization of vitellogenin in the ovaries, hypopharyngeal glands and head fat bodies of honeybee workers, Apis mellifera. J. Insect Sci. 2007, 7, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harwood, G.; Amdam, G.; Freitak, D. The role of vitellogenin in the transfer of immune elicitors from gut to hypopharyngeal glands in honey bees (Apis mellifera). J. Insect Physiol. 2019, 112, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Harwood, G.; Salmela, H.; Freitak, D.; Amdam, G. Social immunity in honey bees: Royal jelly as a vehicle in transferring bacterial pathogen fragments between nestmates. J. Exp. Biol. 2021, 224, jeb231076. [Google Scholar] [CrossRef] [PubMed]
- López, J.H.; Schuehly, W.; Crailsheim, K.; Riessberger-Gallé, U. Trans-generational immune priming in honeybees. Proc. R. Soc. B 2014, 281, 20140454. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Lee, M.Y.; Hong, I.P.; Woo, S.O.; Sim, H.S.; Byuon, G.H.; Thapa, R.; Lee, M.L. Detection of American foulbrood in Apis cerana and Rachymeria ornatipes. Kor. J. Apic. 2013, 28, 205–210. [Google Scholar]
- Lee, K.S.; Kim, B.Y.; Yoon, H.J.; Choi, Y.S.; Jin, B.R. Secapin, a bee venom peptide, exhibits anti-fibrinolytic, anti-elastolytic, and anti-microbial activities. Dev. Comp. Immunol. 2016, 63, 27–35. [Google Scholar] [CrossRef]
- Brødsgaard, C.J.; Ritter, W.; Hansen, H. Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 1998, 29, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Schmehl, D.R.; Tome, H.V.V.; Mortensen, A.N.; Martins, G.F.; Ellis, J.D. Protocol for the in vitro rearing of honey bee (Apis mellifera L.) workers. J. Apic. Res. 2016, 55, 113–129. [Google Scholar] [CrossRef] [Green Version]
- Haydak, M.H. Honey bee nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- López-Uribe, M.M.; Fitzgerald, A.; Simone-Finstrom, M. Inducible versus constitutive social immunity: Examining effects of colony infection on glucose oxidase and defensin-1 production in honeybees. R. Soc. Open Sci. 2017, 4, 170224. [Google Scholar] [CrossRef] [Green Version]
- Erler, S.; Popp, M.; Lattorff, M.G. Dynamics of immune system gene expression upon bacterial challenge and wounding in a social insect (Bombus terrestris). PLoS ONE 2011, 6, e18126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Kim, B.-Y.; Kim, J.-M.; Choi, Y.-S.; Lee, M.-Y.; Lee, K.-S.; Jin, B.-R. Differential Expression of Major Royal Jelly Proteins in the Hypopharyngeal Glands of the Honeybee Apis mellifera upon Bacterial Ingestion. Insects 2022, 13, 334. https://doi.org/10.3390/insects13040334
Kim Y-H, Kim B-Y, Kim J-M, Choi Y-S, Lee M-Y, Lee K-S, Jin B-R. Differential Expression of Major Royal Jelly Proteins in the Hypopharyngeal Glands of the Honeybee Apis mellifera upon Bacterial Ingestion. Insects. 2022; 13(4):334. https://doi.org/10.3390/insects13040334
Chicago/Turabian StyleKim, Yun-Hui, Bo-Yeon Kim, Jin-Myung Kim, Yong-Soo Choi, Man-Young Lee, Kwang-Sik Lee, and Byung-Rae Jin. 2022. "Differential Expression of Major Royal Jelly Proteins in the Hypopharyngeal Glands of the Honeybee Apis mellifera upon Bacterial Ingestion" Insects 13, no. 4: 334. https://doi.org/10.3390/insects13040334




