The Early Season Community of Flower-Visiting Arthropods in a High-Altitude Alpine Environment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
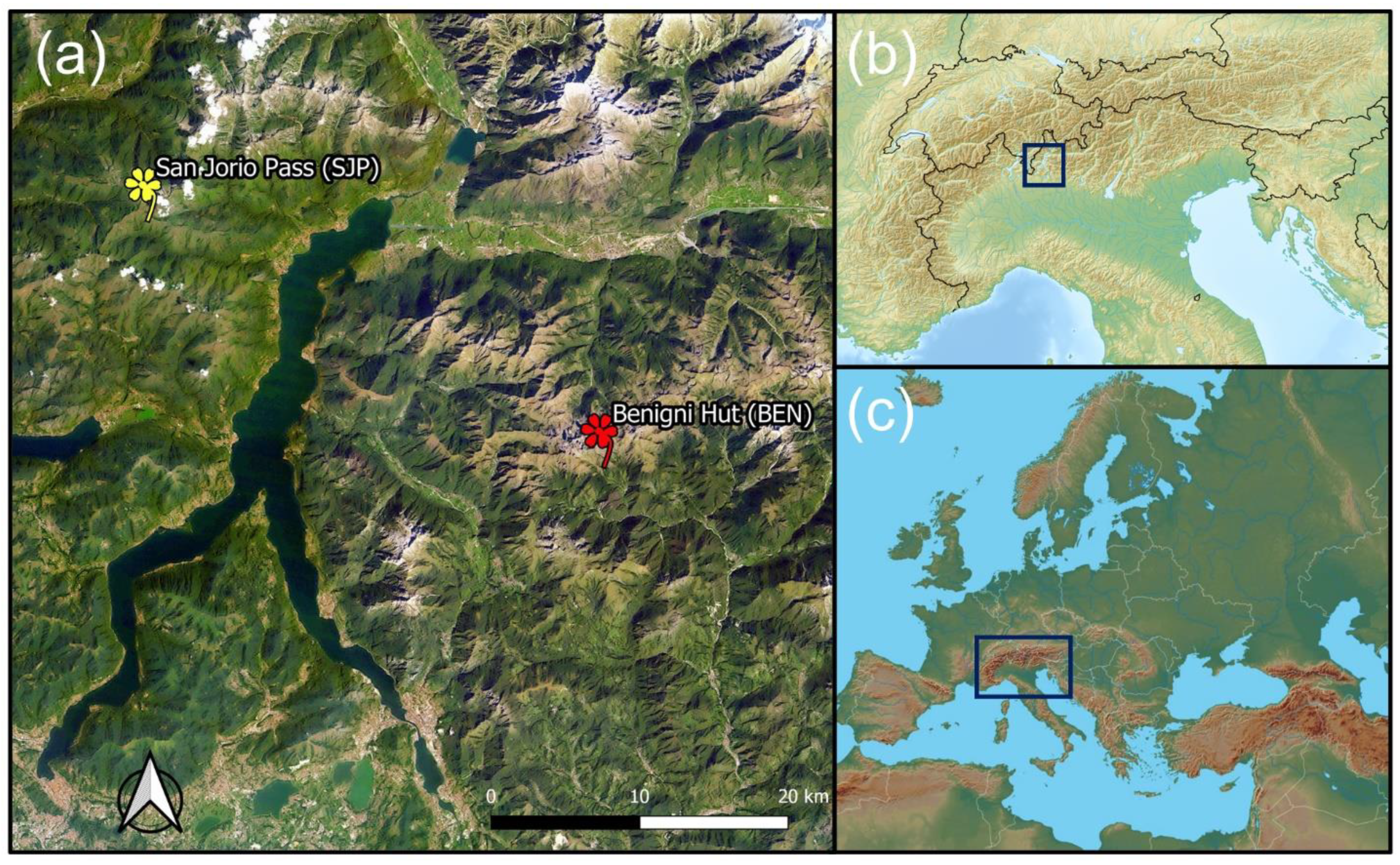
2.2. Study Sites
2.3. Sampling of Flower-Visiting Arthropods
2.4. Identification of Flower-Visiting Arthropods
2.5. Statistical Analyses
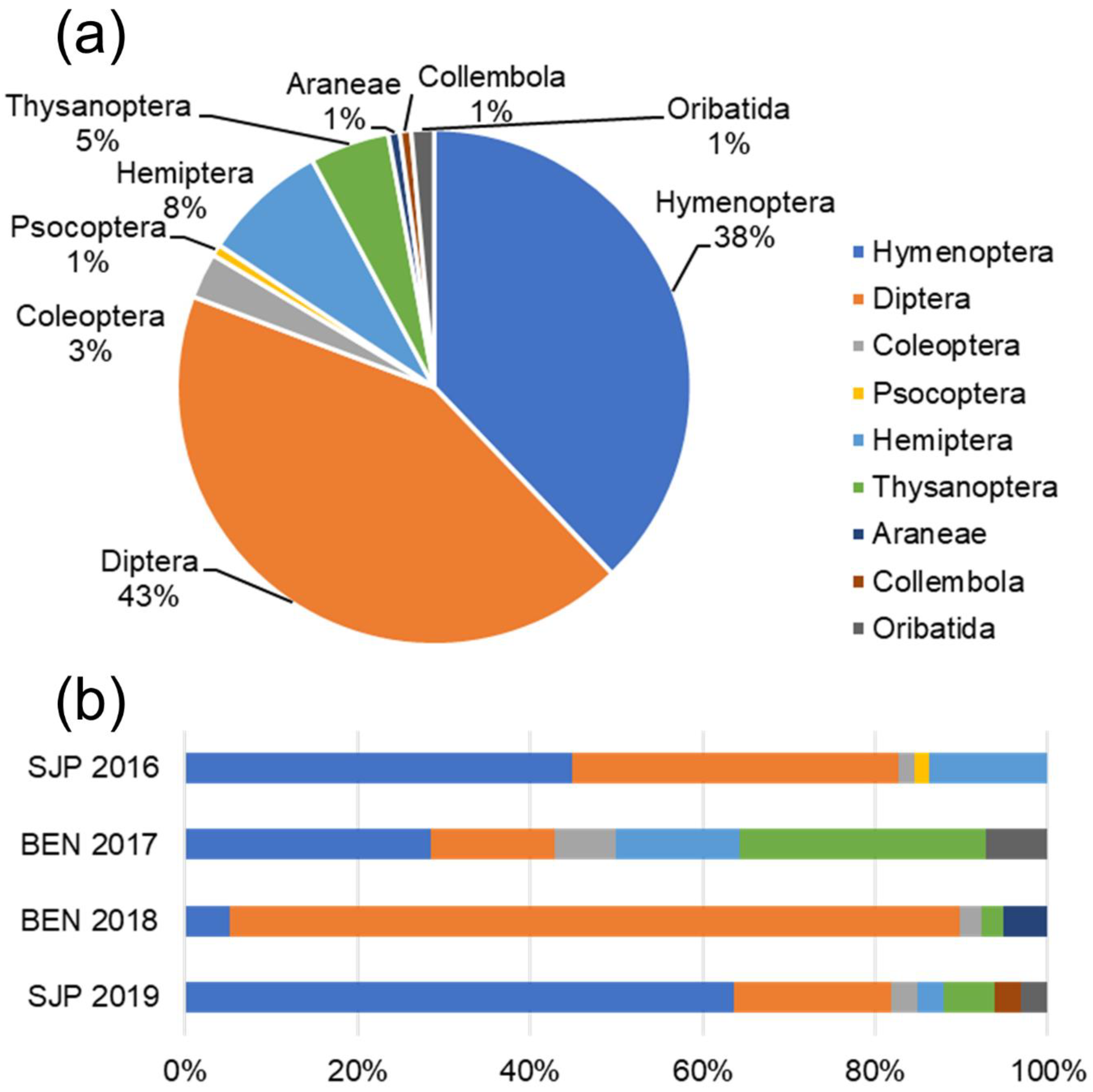
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siquera, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Bálint, M.; Domisch, S.; Engelhardt, C.H.M.; Haase, P.; Lehrian, S.; Sauer, J.; Theissinger, K.; Pauls, S.U.; Nowak, C. Cryptic biodiversity loss linked to global climate change. Nat. Clim. Change 2011, 1, 313–318. [Google Scholar] [CrossRef]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhi, Y.; Franklin, J.; Seddon, N.; Solan, M.; Turner, M.G.; Field, C.B.; Knowlton, N. Climate change and ecosystems: Threats, opportunities and solutions. Philos. Trans. R. Soc. B 2020, 375, 20190104. [Google Scholar] [CrossRef] [Green Version]
- Gottfried, M.; Pauli, H.; Futschik, A.; Akhalkatsi, M.; Barančok, P.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Fernández Calzado, M.R.; et al. Continent-wide response of mountain vegetation to climate change. Nat. Clim. Change 2012, 2, 111–115. [Google Scholar] [CrossRef]
- Seddon, A.W.; Macias-Fauria, M.; Long, P.R.; Benz, D.; Willis, K.J. Sensitivity of global terrestrial ecosystems to climate variability. Nature 2016, 531, 229–232. [Google Scholar] [CrossRef] [Green Version]
- Rudgers, J.A.; Kivlin, S.N.; Whitney, K.D.; Price, M.V.; Waser, N.M.; Harte, J. Responses of high-altitude graminoids and soil fungi to 20 years of experimental warming. Ecology 2014, 95, 1918–1928. [Google Scholar] [CrossRef]
- Morton, E.M.; Rafferty, N.E. Plant-pollinator interactions under climate change: The use of spatial and temporal transplants. Appl. Plant Sci. 2017, 5, 1600133. [Google Scholar] [CrossRef]
- Shah, A.A.; Dillon, M.E.; Hotaling, S.; Woods, H.A. High elevation insect communities face shifting ecological and evolutionary landscapes. Curr. Opin. Insect Sci. 2020, 41, 1–6. [Google Scholar] [CrossRef]
- Inouye, D.W. Effects of climate change on alpine plants and their pollinators. Ann. N. Y. Acad. Sci. 2020, 1469, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Adedoja, O.; Kehinde, T.; Samways, M.J. Asynchrony among insect pollinator groups and flowering plants with elevation. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ohler, L.M.; Lechleitner, M.; Junker, R.R. Microclimatic effects on alpine plant communities and flower-visitor interactions. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Richman, S.K.; Levine, J.M.; Stefan, L.; Johnson, C.A. Asynchronous range shifts drive alpine plant-pollinator interactions and reduce plant fitness. Glob. Change Biol. 2020, 26, 3052–3064. [Google Scholar] [CrossRef] [PubMed]
- Damien, M.; Tougeron, K. Prey–predator phenological mismatch under climate change. Curr. Opin. Insect. Sci. 2019, 35, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Baez, I.; Reitz, S.R.; Funderburk, J.E. Predation by Orius insidiosus (Heteroptera: Anthocoridae) on life stages and species of Frankliniella flower thrips (Thysanoptera: Thripidae) in pepper flowers. Environ. Entomol. 2004, 33, 662–670. [Google Scholar] [CrossRef] [Green Version]
- Abbott, K.R. Bumblebees avoid flowers containing evidence of past predation events. Can. J. Zool. 2006, 84, 1240–1247. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Jones, T.H.; Hails, R.S.; Watt, A.D.; Elston, D.A. Consequences for a host–parasitoid interaction of host-plant aggregation, isolation, and phenology. Ecol. Entomol. 2007, 32, 419–427. [Google Scholar] [CrossRef]
- Kehrli, P.; Bacher, S. Differential effects of flower feeding in insect host–parasitoid system. Basic. Appl. Ecol. 2008, 9, 709–717. [Google Scholar] [CrossRef]
- Thies, C.; Steffan-Dewenter, I.; Tscharntke, T. Interannual landscape changes influence plant–herbivore–parasitoid interactions. Agric. Ecosyst. Environ. 2008, 125, 266–268. [Google Scholar] [CrossRef]
- Lucas-Barbosa, D.; Poelman, E.H.; Aartsma, Y.; Snoeren, T.A.; van Loon, J.J.; Dicke, M. Caught between parasitoids and predators–survival of a specialist herbivore on leaves and flowers of mustard plants. J. Chem. Ecol. 2014, 40, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, G.A.; Dunn, R.R.; Fox, R.; Jongejans, E.; Leather, S.R.; Saunders, M.E.; Shortall, C.R.; Tingley, M.W.; Wagner, D.L. Is the insect apocalypse upon us? How to find out. Biol. Conserv. 2020, 241, 108327. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Väre, H.; Lampinen, R.; Humphries, C.; Williams, P. Taxonomic diversity of vascular plants in the European alpine areas. In Alpine Biodiversity in Europe; Nagy, L., Grabherr, G., Körner, C., Thompson, D.B.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 133–148. [Google Scholar] [CrossRef]
- Schmitt, T. Biogeographical and evolutionary importance of the European high mountain systems. Front. Zool. 2009, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Conti, L.; Schmidt-Kloiber, A.; Grenouillet, G.; Graf, W. A trait-based approach to assess the vulnerability of European aquatic insects to climate change. Hydrobiologia 2014, 721, 297–315. [Google Scholar] [CrossRef] [Green Version]
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publication Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Tordoni, E.; Casolo, V.; Bacaro, G.; Martini, F.; Rossi, A.; Boscutti, F. Climate and landscape heterogeneity drive spatial pattern of endemic plant diversity within local hotspots in South–Eastern Alps. Perspect. Plant Ecol. Evol. Syst. 2020, 43, 125512. [Google Scholar] [CrossRef]
- Menchetti, M.; Talavera, G.; Cini, A.; Salvati, V.; Dincă, V.; Platania, L.; Bonelli, S.; Balletto, E.; Vila, R.; Dapporto, L. Two ways to be endemic. Alps and Apennines are different functional refugia during climatic cycles. Mol. Ecol. 2021, 30, 1297–1310. [Google Scholar] [CrossRef]
- Theurillat, J.P.; Guisan, A. Potential impact of climate change on vegetation in the European Alps: A review. Clim. Change 2001, 50, 77–109. [Google Scholar] [CrossRef]
- Dainese, M.; Kühn, I.; Bragazza, L. Alien plant species distribution in the European Alps: Influence of species’ climatic requirements. Biol. Invasions 2014, 16, 815–831. [Google Scholar] [CrossRef]
- Vitasse, Y.; Rebetez, M.; Filippa, G.; Cremonese, E.; Klein, G.; Rixen, C. ‘Hearing’ alpine plants growing after snowmelt: Ultrasonic snow sensors provide long-term series of alpine plant phenology. Int. J. Biometeorol. 2017, 61, 349–361. [Google Scholar] [CrossRef]
- Ferré, C.; Caccianiga, M.; Zanzottera, M.; Comolli, R. Soil–plant interactions in a pasture of the Italian Alps. J. Plant Interact. 2020, 15, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Pittarello, M.; Lonati, M.; Enri, S.R.; Lombardi, G. Environmental factors and management intensity affect in different ways plant diversity and pastoral value of alpine pastures. Ecol. Indic. 2020, 115, 106429. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life: Functional Plant Ecology of High Mountain Ecosystems, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Lefebvre, V.; Villemant, C.; Fontaine, C.; Daugeron, C. Altitudinal, temporal and trophic partitioning of flower-visitors in Alpine communities. Sci. Rep. 2018, 8, 4706. [Google Scholar] [CrossRef]
- Zoller, H.; Lenzin, H.; Erhardt, A. Pollination and breeding system of Eritrichium nanum (Boraginaceae). Plant Syst. Evol. 2002, 233, 1–14. [Google Scholar] [CrossRef]
- Rossi, M.; Fisogni, A.; Nepi, M.; Quaranta, M.; Galloni, M. Bouncy versus idles: On the different role of pollinators in the generalist Gentiana lutea L. Flora 2014, 209, 164–171. [Google Scholar] [CrossRef]
- Benadi, G.; Hovestadt, T.; Poethke, H.J.; Blüthgen, N. Specialization and phenological synchrony of plant-pollinator interactions along an altitudinal gradient. J. Anim. Ecol. 2014, 83, 639–650. [Google Scholar] [CrossRef]
- Lefebvre, V.; Fontaine, C.; Villemant, C.; Daugeron, C. Are empidine dance flies major flower visitors in alpine environments? A case study in the Alps, France. Biol. Lett. 2014, 10, 20140742. [Google Scholar] [CrossRef] [Green Version]
- Losapio, G.; Gobbi, M.; Marano, G.; Avesani, D.; Boracchi, P.; Compostella, C.; Pavesi, M.; Schöb, C.; Seppi, R.; Sommaggio, D.; et al. Feedback effects between plant and flower-visiting insect communities along a primary succession gradient. Arthropod Plant Interact. 2016, 10, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Wagner, J.; Lechleitner, M.; Hosp, D. Pollen limitation is not the rule in nival plants: A study from the European Central Alps. Am. J. Bot. 2016, 103, 375–387. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, M.; Avesani, D.; Parolo, G.; Scupola, A.; Zanetti, A.; Bonomi, C. Flower-visiting insects observed on the critically endangered alpine plant species Callianthemum kernerianum Freyn ex A. Kerner (Ranunculaceae). J. Insect Biodivers. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- Lefebvre, V.; Daugeron, C.; Villemant, C.; Fontaine, C. Empidine dance flies pollinate the woodland geranium as effectively as bees. Biol. Lett. 2019, 15, 20190230. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, M.; Melotto, A.; Minici, A.; Eustacchio, E.; Gianfranceschi, L.; Gobbi, M.; Casartelli, M.; Caccianiga, M. Manual Sampling and Video Observations: An Integrated Approach to Studying Flower-Visiting Arthropods in High-Mountain Environments. Insects 2020, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Blaschke, M.; Bässler, C. Altitudinal gradients in biodiversity research: The state of the art and future perspectives under climate change aspects. Waldökol. Landsch. Forsch. Nat. Schutz. 2011, 11, 35–47. [Google Scholar]
- Harry, I.; Höfer, H.; Schielzeth, H.; Assmann, T. Protected habitats of Natura 2000 do not coincide with important diversity hotspots of arthropods in mountain grasslands. Insect Conserv. Divers. 2019, 12, 329–338. [Google Scholar] [CrossRef]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [Green Version]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Seastedt, T.R.; Crossley, D.A. The influence of arthropods on ecosystems. BioScience 1984, 34, 157–161. [Google Scholar] [CrossRef]
- Fontaine, C.; Dajoz, I.; Meriguet, J.; Loreau, M. Functional diversity of plant–pollinator interaction webs enhances the persistence of plant communities. PLoS Biol. 2006, 4, e1. [Google Scholar] [CrossRef]
- Losey, J.E.; Vaughan, M. The economic value of ecological services provided by insects. BioScience 2006, 56, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. Royal Soc. B 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Weisser, W.W.; Siemann, E. The various effects of insects on ecosystem functioning. In Insects and Ecosystem Function; Weisser, W.W., Siemann, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 3–24. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Yang, L.H.; Gratton, C. Insects as drivers of ecosystem processes. Curr. Opin. Insect Sci. 2014, 2, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Molau, U.; Holmgren, B. Micrometeorological impacts on insect activity and plant reproductive success in an alpine environment, Swedish Lapland. Arctic Alpine Res. 1996, 28, 196–202. [Google Scholar] [CrossRef]
- Goodwin, E.K.; Rader, R.; Encinas-Viso, F.; Saunders, M.E. Weather Conditions Affect the Visitation Frequency, Richness and Detectability of Insect Flower Visitors in the Australian Alpine Zone. Environ. Entomol. 2021, 50, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- Mangili, F.; Tampucci, D.; Caccianiga, M. Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana: Androsace brevis (Hegetschw.) Ces. Inf. Bot. Ital. 2014, 46, 97–100. [Google Scholar]
- Rafferty, N.E.; Bertelsen, C.D.; Bronstein, J.L. Later flowering is associated with a compressed flowering season and reduced reproductive output in an early season floral resource. Oikos 2016, 125, 821–828. [Google Scholar] [CrossRef] [Green Version]
- Kudo, G.; Cooper, E.J. When spring ephemerals fail to meet pollinators: Mechanism of phenological mismatch and its impact on plant reproduction. Proc. Royal Soc. B 2019, 286, 20190573. [Google Scholar] [CrossRef] [Green Version]
- Ettinger, A.K.; Buonaiuto, D.M.; Chamberlain, C.J.; Morales-Castilla, I.; Wolkovich, E.M. Spatial and temporal shifts in photoperiod with climate change. New Phytol. 2021, 230, 462–474. [Google Scholar] [CrossRef]
- European Environment Agency. Biogeographical Regions in Europe. Available online: https://www.eea.europa.eu/data-and-maps/figures/biogeographical-regions-in-europe-2 (accessed on 10 February 2021).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Zeitschrift 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Gibson, R.H.; Knott, B.; Eberlein, T.; Memmott, J. Sampling method influences the structure of plant–pollinator networks. Oikos 2011, 120, 822–831. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987, 19, 11–15. [Google Scholar]
- Bonelli, M.; Messinetti, S.; Spreafico, F. New insights on Troglophilus (Orthoptera Rhaphidophoridae) species distribution in the westernmost area of their main range (Northern Italy). Bull. Insectol. 2019, 72, 103–114. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Breheny, P.; Burchett, W. Visualization of regression models using visreg. R J. 2017, 9, 56–71. [Google Scholar] [CrossRef]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. Int. J. Open Source Softw. Process. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Capinera, J.L. Encyclopedia of Entomology; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Pont, A.C. Observations on anthophilous Muscidae and other Diptera (Insecta) in Abisko National Park, Sweden. J. Natl. Hist. 1993, 27, 631–643. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Romeis, J.; van Rijn, P. Nectar and pollen feeding by insect herbivores and implications for multitrophic interactions. Annu. Rev. Entomol. 2007, 52, 301–323. [Google Scholar] [CrossRef] [Green Version]
- Woodcock, T.S.; Larson, B.M.; Kevan, P.G.; Inouye, D.W.; Lunau, K. Flies and flowers II: Floral attractants and rewards. J. Pollinat. Ecol. 2014, 12, 63–94. [Google Scholar] [CrossRef]
- Inouye, D.W.; Larson, B.M.; Ssymank, A.; Kevan, P.G. Flies and flowers III: Ecology of foraging and pollination. J. Pollinat. Ecol. 2015, 16, 115–133. [Google Scholar] [CrossRef]
- Strathdee, A.T.; Bale, J.S. Life on the edge: Insect ecology in arctic environments. Annu. Rev. Entomol. 1998, 43, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Roháček, J. Sphaeroceridae (Diptera) in burrows of rabbit and fox in central Bohemia (Czech Republic), with description of a new species of Minilimosina Roháček. Entomol. Fenn. 2019, 30, 97–113. [Google Scholar] [CrossRef]
- Roháček, J.; Department of Entomology, Silesian Museum, Opava, Czech Republic. Personal communication, 2020.
- Schiestl, F.P.; Glaser, F. Specific ant-pollination in an alpine orchid and the role of floral scent in attracting pollinating ants. Alp. Bot. 2012, 122, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lebas, C.; Galkowski, C.; Blatrix, R.; Wegnez, P. Guida alle Formiche d’Europa; Ricca Editore: Rome, Italy, 2019. [Google Scholar]
- Guariento, E.; Martini, J.; Fiedler, K. Bait visitation by Formica lemani (Hymenoptera: Fomicidae) indicates shortage of carbohydrates in alpine grasslands. Eur. J. Entomol. 2018, 115, 217–222. [Google Scholar] [CrossRef]
- Guariento, E.; Rabl, D.; Ballini, S.; Fiedler, K. Wood ants (Formicidae, Insecta) at the alpine tree–line ecotone: Negative and positive associations with other arthropods. Gredleriana 2018, 18, 103–120. [Google Scholar]
- Jervis, M.A.; Kidd, N.A.C.; Fitton, M.G.; Huddleston, T.; Dawah, H.A. Flower-visiting by hymenopteran parasitoids. J. Nat. Hist. 1993, 27, 67–105. [Google Scholar] [CrossRef]
- Zemenick, A.T.; Kula, R.R.; Russo, L.; Tooker, J. A network approach reveals parasitoid wasps to be generalized nectar foragers. Arthropod-Plant Interact. 2019, 13, 239–251. [Google Scholar] [CrossRef]
- Žikić, V.; Lazarević, M.; Milošević, D. Host range patterning of parasitoid wasps Aphidiinae (Hymenoptera: Braconidae). Zool. Anz. 2017, 268, 75–83. [Google Scholar] [CrossRef]
- Gobbi, M.; Isaia, M.; De Bernardi, F. Arthropod colonisation of a debris-covered glacier. Holocene 2011, 21, 343–349. [Google Scholar] [CrossRef]
- Kirk, W.D.J. Pollen-feeding in thrips (Insecta: Thysanoptera). J. Zool. 1984, 204, 107–117. [Google Scholar] [CrossRef]
- Kirk, W.D. Aggregation and mating of thrips in flowers of Calystegia sepium. Ecol. Entomol. 1985, 10, 433–440. [Google Scholar] [CrossRef]
- McFarlane, D.J.; Rafter, M.A.; Booth, D.T.; Walter, G.H. Behavioral responses of a tiny insect, the flower thrips Frankliniella schultzei Trybom (Thysanoptera, Thripidae), to atmospheric pressure change. J. Insect. Behav. 2015, 28, 473–481. [Google Scholar] [CrossRef]
- Vaello, T.; Pineda, A.; Marcos-García, M. Role of thrips omnivory and their aggregation pheromone on multitrophic interactions between sweet pepper plants, aphids, and hoverflies. Front. Ecol. Evol. 2019, 6, 240. [Google Scholar] [CrossRef] [Green Version]
- Müller, H. The fertilisers of alpine flowers. Nature 1880, 21, 275. [Google Scholar] [CrossRef] [Green Version]
- Sieber, Y.; Holderegger, R.; Waser, N.M.; Thomas, V.F.; Braun, S.; Erhardt, A.; Reyer, H.U.; Wirth, L.R. Do alpine plants facilitate each other’s pollination? Experiments at a small spatial scale. Acta Oecol. 2011, 37, 369–374. [Google Scholar] [CrossRef] [Green Version]
- McCabe, L.M.; Cobb, N.S. From Bees to Flies: Global Shift in Pollinator Communities Along Elevation Gradients. Front. Ecol. Evol. 2021, 8, 518. [Google Scholar] [CrossRef]
- Bezzi, M. Studi sulla Ditterofauna nivale delle Alpi italiane. Mem. Soc. Entomol. Ital. 1918, 9, 1–164. [Google Scholar]
- Orford, K.A.; Vaughan, I.P.; Memmott, J. The forgotten flies: The importance of non-syrphid Diptera as pollinators. Proc. R. Soc. B 2015, 282, 20142934. [Google Scholar] [CrossRef] [Green Version]
- Tougeron, K.; Brodeur, J.; Le Lann, C.; van Baaren, J. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Scaven, V.L.; Rafferty, N.E. Physiological effects of climate warming on flowering plants and insect pollinators and potential consequences for their interactions. Curr. Zool. 2013, 59, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundberg, H. Effects of weather on foraging-flights of bumblebees (Hymenoptera, Apidae) in a subalpine/alpine area. Ecography 1980, 3, 104–110. [Google Scholar] [CrossRef]
- Totland, Ø. Influence of climate, time of day and season, and flower density on insect flower visitation in alpine Norway. Arctic Alpine Res. 1994, 26, 66–71. [Google Scholar] [CrossRef]
- Vicens, N.; Bosch, J. Weather-dependent pollinator activity in an apple orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environ. Entomol. 2000, 29, 413–420. [Google Scholar] [CrossRef]
- Wikström, L.; Milberg, P.; Bergman, K.O. Monitoring of butterflies in semi-natural grasslands: Diurnal variation and weather effects. J. Insect Conserv. 2009, 13, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Polatto, L.P.; Chaud-Netto, J.; Alves-Junior, V.V. Influence of abiotic factors and floral resource availability on daily foraging activity of bees. J. Insect Behav. 2014, 27, 593–612. [Google Scholar] [CrossRef]
- Hennessy, G.; Harris, C.; Pirot, L.; Lefter, A.; Goulson, D.; Ratnieks, F.L. Wind slows play: Increasing wind speed reduces flower visiting rate in honey bees. Anim. Behav. 2021, 178, 87–93. [Google Scholar] [CrossRef]
- Horák, J.; Rada, P.; Lettenmaier, L.; Andreas, M.; Bogusch, P.; Jaworski, T. Importance of meteorological and land use parameters for insect diversity in agricultural landscapes. Sci. Total Environ. 2021, 791, 148159. [Google Scholar] [CrossRef]
- Ohashi, K.; Yahara, T. Effects of variation in flower number on pollinator visits in Cirsium purpuratum (Asteraceae). Am. J. B 1998, 85, 219–224. [Google Scholar] [CrossRef]
- Akter, A.; Biella, P.; Klecka, J. Effects of small-scale clustering of flowers on pollinator foraging behaviour and flower visitation rate. PLoS ONE 2017, 12, e0187976. [Google Scholar] [CrossRef] [Green Version]
- Kuppler, J.; Wieland, J.; Junker, R.R.; Ayasse, M. Drought-induced reduction in flower size and abundance correlates with reduced flower visits by bumble bees. AoB Plants 2021, 13, plab001. [Google Scholar] [CrossRef]
- Cherry, M.J.; Barton, B.T. Effects of wind on predator-prey interactions. Food Webs 2017, 13, 92–97. [Google Scholar] [CrossRef]
- MacKay, W.P.; MacKay, E.E. Diurnal foraging patterns of Pogonomyrmex harvester ants (Hymenoptera: Formicidae). Southwest. Nat. 1989, 34, 213–218. [Google Scholar] [CrossRef]
- Herrera, C.M. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer-flowering Mediterranean shrub. Oikos 1990, 58, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Balducci, M.G.; Van der Niet, T.; Johnson, S.D. Diel scent and nectar rhythms of an African orchid in relation to bimodal activity patterns of hawkmoth pollinators. Ann. Bot. 2020, 126, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ren, Z.X.; Trunschke, J.; Kuppler, J.; Zhao, Y.H.; Knop, E.; Wang, H. Bimodal activity of diurnal flower visitation at high elevation. Ecol. Evol. 2021, 11, 13487–13500. [Google Scholar] [CrossRef]
- Kehoe, R.; Frago, E.; Sanders, D. Cascading extinctions as a hidden driver of insect decline. Ecol. Entomol. 2021, 46, 743–756. [Google Scholar] [CrossRef]
- Guzman, L.M.; Chamberlain, S.A.; Elle, E. Network robustness and structure depend on the phenological characteristics of plants and pollinators. Ecol. Evol. 2021, 11, 13321–13334. [Google Scholar] [CrossRef]
- Kellermann, V.; van Heerwaarden, B. Terrestrial insects and climate change: Adaptive responses in key traits. Physiol. Entomol. 2019, 44, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2011, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome coxidase subunit I from diverse metazoan invertebrates. Mol. Marine Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- De Jong, Y.; Verbeek, M.; Michelsen, V.; de Place Bjørn, P.; Los, W.; Steeman, F.; Bailly, N.; Basire, C.; Chylarecki, P.; Stloukal, E. Fauna Europaea–all European animal species on the web. Biodivers. Data J. 2014, 2, e4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Year | Site | Day | Time Windows | N of Sampling Sessions | h of Timed Observations per Year | N of Flowers at Anthesis (per Plant) |
|---|---|---|---|---|---|---|
| 2016 | SJP | 25 May 2016 | 11.30–12.30 | 8 | 16 | 69 (A); 109 (B) |
| 13.30–14.30 | ||||||
| 15.30–16.30 | ||||||
| 17.30–18.30 | ||||||
| 26 May 2016 | 11.30–12.30 | 8 | ||||
| 13.30–14.30 | ||||||
| 15.30–16.30 | ||||||
| 17.30–18.30 | ||||||
| 2017 | BEN | 31 May 2017 | 11.00–12.00 | 8 | 16 | 37 (C); 25 (E) |
| 12.30–13.30 | ||||||
| 14.00–15.00 | ||||||
| 15.30–16.30 | ||||||
| 1 June 2017 | 11.00–12.00 | 8 | ||||
| 12.30–13.30 | ||||||
| 14.00–15.00 | ||||||
| 15.30–16.30 | ||||||
| 2018 | BEN | 9 June 2018 | 13.00–14.00 | 8 | 16 | 33 (F); 36 (G) |
| 14.30–15.30 | ||||||
| 16.00–17.00 | ||||||
| 17.30–18.30 | ||||||
| 10 June 2018 | 10.15–11.15 | 8 | ||||
| 11.45–12.45 | ||||||
| 13.15–14.15 | ||||||
| 14.45–15.45 | ||||||
| 2019 | SJP | 4 June 2019 | 10.30–11.30 | 6 | 6 | 20 (H); 25 (I) |
| 12.00–13.00 | ||||||
| 15.00–16.00 |
| SJP 2016 | BEN 2017 | BEN 2018 | SJP 2019 | Total | |
|---|---|---|---|---|---|
| N of sampled specimens | 58 | 14 | 35 | 33 | 140 |
| Captures per hour (mean ± SEM) | 3.6 ± 0.5 | 0.9 ± 0.3 | 2.2 ± 0.6 | 5.5 ± 1.3 | 2.6 ± 0.3 |
| N of orders | 5 | 6 | 5 | 7 | 9 |
| N of families | 17 | 9 | 9 | 17 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonelli, M.; Eustacchio, E.; Avesani, D.; Michelsen, V.; Falaschi, M.; Caccianiga, M.; Gobbi, M.; Casartelli, M. The Early Season Community of Flower-Visiting Arthropods in a High-Altitude Alpine Environment. Insects 2022, 13, 393. https://doi.org/10.3390/insects13040393
Bonelli M, Eustacchio E, Avesani D, Michelsen V, Falaschi M, Caccianiga M, Gobbi M, Casartelli M. The Early Season Community of Flower-Visiting Arthropods in a High-Altitude Alpine Environment. Insects. 2022; 13(4):393. https://doi.org/10.3390/insects13040393
Chicago/Turabian StyleBonelli, Marco, Elena Eustacchio, Daniele Avesani, Verner Michelsen, Mattia Falaschi, Marco Caccianiga, Mauro Gobbi, and Morena Casartelli. 2022. "The Early Season Community of Flower-Visiting Arthropods in a High-Altitude Alpine Environment" Insects 13, no. 4: 393. https://doi.org/10.3390/insects13040393
APA StyleBonelli, M., Eustacchio, E., Avesani, D., Michelsen, V., Falaschi, M., Caccianiga, M., Gobbi, M., & Casartelli, M. (2022). The Early Season Community of Flower-Visiting Arthropods in a High-Altitude Alpine Environment. Insects, 13(4), 393. https://doi.org/10.3390/insects13040393