Reproductive Behaviour of 150-Gy-Treated Female Lobesia botrana (Lepidoptera: Tortricidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Moth Irradiation
2.2. Response of Treated and Untreated Male Moths towards Calling 150 Gy-Treated and Untreated Female Moths
2.3. Female Mating Ability at Different Age at Mating after Irradiation
2.4. Effect of Multiple Mating on the Mating Ability of 150 Gy-treated Female Moths
2.5. Sperm Precedence in Twice-Mated Females
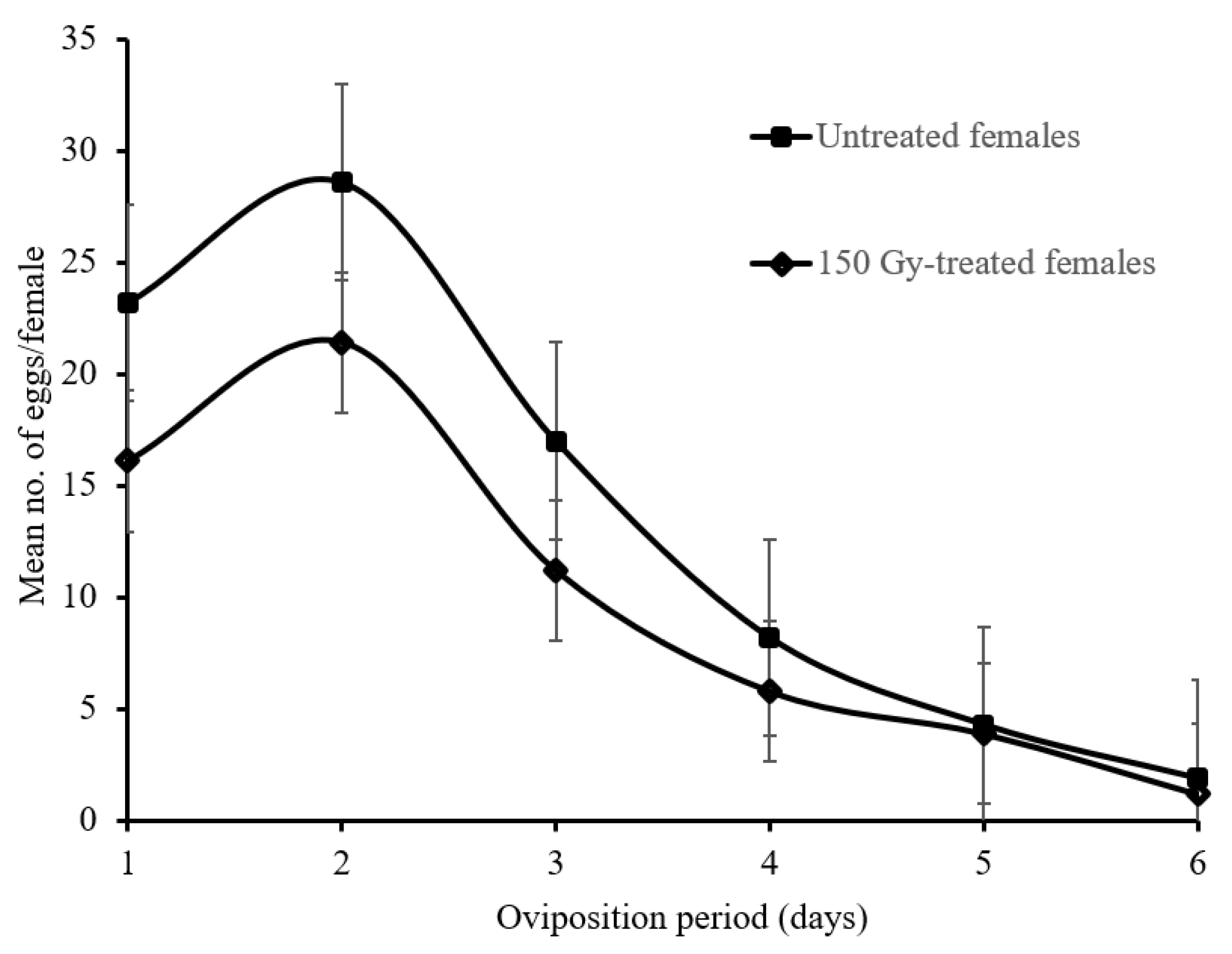
2.6. Temporal Oviposition Dynamics of 150 Gy-Treated and Untreated Female Moths
2.7. Statistical Analysis
3. Results
3.1. Response of Treated and Untreated Male Moths towards Calling 150 Gy-Treated and Untreated Female Moths
3.2. Female Mating Ability at Different Age at Mating after Irradiation
3.3. Effect of Multiple Mating on the Mating Ability of 150 Gy-Treated Female Moths
3.4. Sperm Precedence in Twice-Mated Females
3.5. Temporal Oviposition Dynamics of 150 Gy-Treated and Untreated Female Moths
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiéry, D.; Monceau, K.; Moreau, J. Different emergence phenology of European grapevine moth (Lobesia botrana, Lepidoptera: Tortricidae) on six varieties of grapes. Bull. Entomol. Res. 2014, 104, 277–287. [Google Scholar] [CrossRef]
- Scaramozzino, P.L.; Giovanni, F.D.; Loni, A.; Gisondi, S.; Lucchi, A.; Cerretti, P. Tachinid (Diptera, Tachinidae) parasitoids of Lobesia botrana (Denis & Schiffermüller, 1775) (Lepidoptera, Tortricidae) and other moths. ZooKeys 2020, 934, 111–140. [Google Scholar]
- Saour, G. Sterile insect technique and F1 sterility in the European grapevine moth, Lobesia botrana. J. Insect Sci. 2014, 14, 8–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bourtzis, K.; Vreysen, M.J.B. Sterile Insect Technique (SIT) and its applications. Insects 2021, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Marec, F.; Vreysen, M.J.B. Advances and challenges of using the sterile insect technique or the management of pest Lepidoptera. Insects 2019, 10, 371. [Google Scholar] [CrossRef]
- Haff, R.; Ovchinnikova, I.; Liang, P.; Mahoney, N.; Gee, W.; Gomez, J.; Toyofuku, N.; Jackson, E.; Hnasko, R.; Light, D. X-ray-based irradiation of larvae and pupae of the navel orangeworm (Lepidoptera: Pyralidae). J. Econ. Entomol. 2020, 113, 1685–1693. [Google Scholar] [CrossRef]
- Saour, G. Flight ability and dispersal of European grapevine moth gamma-irradiated males (Lepidoptera: Tortricidae). Fla. Entomol. 2016, 99 (Suppl. S1), 73–78. [Google Scholar] [CrossRef][Green Version]
- Makee, H.; Saour, G. Efficiency of inherited sterility technique against Phthorimaea operculella Zeller (Lepidoptera: Gelechiidae) as affected by irradiation of females. J. Veg. Crop. Prod. 2004, 10, 11–22. [Google Scholar] [CrossRef]
- Potgieter, L.; van Vuuren, J.H.; Conlong, D.F. Modelling the effects of the sterile insect technique applied to Eldana saccharina Walker in sugarcane. ORiON 2012, 28, 59–84. [Google Scholar] [CrossRef][Green Version]
- FAO. The Sterile Insect Technique for Use against the Devastating European Grapevine Moth in Chile. 2020. Available online: http://www-naweb.iaea.org/nafa/news/2018-developing-area-wide-SIT-chile.html (accessed on 18 June 2020).
- Stringer, L.D.; Sullivan, N.J.; Sullivan, T.E.S.; Mitchell, V.J.; Manning, L.-A.M.; Mas, F.; Hood-Nowotny, R.C.; Suckling, D.M. Attractiveness and competitiveness of irradiated light brown apple moths. Entomol. Exp. Appl. 2013, 148, 203–212. [Google Scholar] [CrossRef]
- Ikegawa, Y.; Ito, K.; Himuro, C.; Honma, A. Sterile males and females can synergistically suppress wild pests targeted by sterile insect technique. J. Theor. Biol. 2021, 530, 110878. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vila, L.M.; Facundo, J.G.; Rodríguez-Molina, M.C.; Stockel, J. Heritable variation for female remating in Lobesia botrana, a usually monandrous moth. Anim. Behav. 2002, 64, 899–907. [Google Scholar] [CrossRef]
- Lucchi, A.; Sambado, P.; Royo, A.B.J.; Bagnoli, B.; Benelli, G. Lobesia botrana males mainly fly at dusk: Video camera-assisted pheromone traps and implications for mating disruption. J. Pest Sci. 2018, 91, 1327–1334. [Google Scholar] [CrossRef]
- Guerfali, M.M.; Chevrier, C. Determinant factors for sperm transfer and sperm storage within Ceratitis capitata (Diptera: Tephritidae) and impact on Sterile Insect Technique. J. Radiat. Res. Appl. Sci. 2020, 13, 792–807. [Google Scholar] [CrossRef]
- Thiéry, D.; Moreau, J. Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 2005, 143, 548–557. [Google Scholar] [CrossRef]
- Ueno, H.; Ito, Y. Sperm precedence in Eysarcoris lewisi Distant (Heteroptera: Pentatomidae) in relation to duration between oviposition and the last copulation. Appl. Entomol. Zool. 1992, 27, 421–426. [Google Scholar] [CrossRef][Green Version]
- Abacus Concepts. StatView, Version 4.02; Abacus Concepts: Piscataway, NJ, USA, 1994. [Google Scholar]
- Makee, H.; Saour, G. Factors influencing mating success, mating frequency, and fecundity in Phthorimaea operculella (Lepidoptera: Gelechiidae). Environ. Entomol. 2001, 30, 31–36. [Google Scholar] [CrossRef]
- Makee, H.; Idris, I.; Hussian, K. Factors influencing mating incidence and reproduction in codling moth Cydia pomonella L. (Lepidoptera: Tortricidae). Adv. Hort. Sci. 2012, 26, 180–186. [Google Scholar]
- Suckling, D.M.; Hackett, J.K.; Chhagan, A.; Barrington, A.; El-Sayed, A.M. Effect of irradiation on female painted apple moth Teia anartoides (Lep., Lymantriidae) sterility and attractiveness to males. J. Appl. Entomol. 2006, 130, 167–170. [Google Scholar] [CrossRef]
- Stepien, T.L.; Zmurchok, C.; Hengenius, J.B.; Rivera, R.M.C.; D’Orsogna, M.R.; Lindsay, A.E. Moth mating: Modeling female pheromone calling and male navigational strategies to optimize reproductive success. Appl. Sci. 2020, 10, 6543. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Stockel, J. Delayed mating reduces reproductive output of female European grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). Bull Entomol. Res. 2002, 92, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Lalanne-Cassou, B.; Percy, J.; MacDonald, J.A. Ultrastructure of sex pheromone gland cells in Lobesia botrana Den & Schiff. (Lepidoptera: Olethreutidae). Can. J. Zool. 1977, 55, 672–680. [Google Scholar]
- Tasin, M.; Bäckman, A.-C.; Bengtsson, M.; Varela, N.; Ioriatti, C.; Witzgall, P. Wind tunnel attraction of grapevine moth females, Lobesia botrana, to natural and artificial grape odour. Chemoecology 2006, 16, 87–92. [Google Scholar] [CrossRef]
- Wang, X.P.; Fang, Y.L.; Zhang, Z.N. Effects of delayed mating on the fecundity and longevity of females of diamondback moth, Plutella xylostella. Insect Sci. 2011, 18, 305–310. [Google Scholar] [CrossRef]
- Makee, H.; Saour, G. Noninherited sterility in irradiated Phthorimaea operculella females. J. Appl. Entomol. 2003, 127, 489–493. [Google Scholar] [CrossRef]
- Zheng, X.-L.; Junyan, L.; Wen, L.; He, X.Z.; Wang, Q. Mating delay reduces reproductive performance but not longevity in a monandrous moth. J. Insect Sci. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Walker, P.W.; Allen, G.R. Mating frequency and reproductive success in an income breeding moth, Mnesampela private. Entomol. Exp. Et App. 2010, 136, 290–300. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Yu, J.-F.; Lu, Q.; Xu, J.; Ye, H. Female and male moths display different reproductive behavior when facing new versus previous mates. PLoS ONE 2014, 9, e109564. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Q. Mechanisms of last male precedence in a moth: Sperm displacement at ejaculation and storage sites. Behav. Ecol. 2010, 21, 714–721. [Google Scholar] [CrossRef]
- Milonas, P.G.; Partsinevelos, G.K.; Andow, D.A. Effect of male mating history and age on remating by female European corn borer. PLoS ONE 2017, 12, e0175512. [Google Scholar] [CrossRef]
- Thorburn, D.-M.J.; Knell, R.J.; Parrett, J.M. Sperm morph and remating frequency in the Indian meal moth, Plodia interpunctella. Biol. Lett. 2018, 14, 20180304. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, Q. Ejaculate economics: An experimental test in a moth. Biol. Lett. 2014, 10, 20131031. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.K.; Khan, Z.; Rao, D.K.; Zarin, M. Flight activity and mating behavior of irradiated Spodoptera litura (Lepidoptera: Noctuidae) males and their F1 progeny for use of inherited sterility in pest management approaches. Fla. Entomol. 2016, 99 (Suppl. S1), 119–130. [Google Scholar] [CrossRef]
- Saour, G.; Makee, H. Effect of gamma irradiation on sperm utilization in twice- mated female Phthorimaea operculella Zeller (Lep., Gelechiidae). J. Appl. Entomol. 1999, 123, 513–517. [Google Scholar] [CrossRef]
- Suckling, D.M.; Conlong, D.E.; Carpenter, J.E.; Bloem, K.A.; Rendon, P.; Vreysen, M.J.B. Global range expansion of pest Lepidoptera requires socially acceptable solutions. Biol. Invasions 2017, 19, 1107–1119. [Google Scholar] [CrossRef]
- Chapman, R.F.; Simpson, S.J.; Douglas, A.E. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Li, H.-L.; Wang, X.-Y.; Zheng, X.-L.; Lu, W. Research progress on oviposition-related genes in insects. J. Insect Sci. 2020, 20, 36. [Google Scholar] [CrossRef]
- Wainwright, C.; Sascha, J.Y.; Wilson, D.; Elliott, M.; Jukes, A.; Rosemary, C.R. Phenology of the diamondback moth (Plutella xylostella) in the UK and provision of decision support for Brassica growers. Insects 2020, 11, 118. [Google Scholar] [CrossRef]
- Idris, I.; Hussian, K.; Alali, N.; Ikhtiar, A. Irreversible fertility of irradiated Phthorimaea operculella (Lepidoptera: Gelechiidae) female. J. Bio Innov. 2019, 8, 517–531. [Google Scholar]
- Vreysen, M.J.B.; Hendrichs, J.; Enkerlin, W.R. The sterile insect technique as a component of sustainable area-wide integrated pest management of selected horticulture insect pests. J. Fruit Ornam. Plant Res. 2006, 14, 107–130. [Google Scholar]

| Male Compartment | Female Compartment |
|---|---|
| Combination 1 Untreated ♂ + 150 Gy-treated ♂ (n = 25 for each) | Untreated ♀ (n = 5) |
| Combination 2 Untreated ♂ + 150 Gy-treated ♂ (n = 25 for each) | 150 Gy-treated ♀ (n = 5) |
| Type of Female/Male Tested * | % of Males That Flew into Females Compartment on the Indicated Day | % of Non-Flying Males | |||
|---|---|---|---|---|---|
| 1st Day | 2nd Day | 3rd Day | 4th Day | ||
| Untreated female/ | |||||
| 150 Gy-treated male | 18.1 ± 3.8 B,b | 43.3 ± 4.6 A,a | 14.6 ± 4.8 B,a | 8.9 ± 3.6 C,a | 15.1 ± 6.7 B,b |
| Untreated male | 23.4 ± 3.2 B,a | 47.4 ± 2.7 A,a | 12.7 ± 4.7 C,a | 6.0 ± 3.3 D,a | 10.5 ± 4.2 C,c |
| Treated female/ | |||||
| 150 Gy-treated male | 13.2 ± 3.0 C,c | 38.2 ± 2.0 A,b | 16.0 ± 2.5 C,a | 5.6 ± 3.2 D,a | 27.0 ± 4.5 B,a |
| Untreated male | 20.0 ± 2.5 B,a | 44.6 ± 4.8 A,a | 14.2 ± 3.8 C,a | 6.7 ± 2.1 D,a | 14.5 ± 3.0 C,b |
| Dose (Gy) | No. of Females | Mating Ability (%) | No. of Mating | No. of Mated Females (%) | Mean No. of Eggs/Female (±SD) | Mean % Fertility (±SD) |
|---|---|---|---|---|---|---|
| 150 | 45 | 27/45 (60.0) b | 1 | 18 (66.7) b | 54.1 ± 9.0 b | 0 |
| 2 | 7(25.9) cd | 56.8 ± 6.4 b | 0 | |||
| 3 | 2(7.4) e | 55.5 ± 6.4 b | 0 | |||
| 0 | 45 | 43/45 (95.6) a | 1 | 26 (60.4) a | 84.4 ± 6.4 a | 82.2 ± 2.6 a |
| 2 | 10 (23.3) cd | 83.4 ± 5.8 a | 80.1 ± 1.9 a | |||
| 3 | 7 (16.3) cde | 81.3 ± 4.4 a | 79.9 ± 3.1 a |
| Mating Sequence | Mean % Egg Hatch | Twice-Mated Females (%) | P2 Value |
|---|---|---|---|
| 0.97 | |||
| Untreated–Untreated | 79.7 ± 0.60 a | 25.0 ab | |
| Untreated–Treated | 41.2 ± 1.7 b | 30.0 a | |
| Treated–Untreated | 78.7 ± 2.3 a | 20.0 b | |
| Treated–Treated | 40.7 ± 2.4 b | 25.0 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saour, G.; Hashem, A.; Jassem, I. Reproductive Behaviour of 150-Gy-Treated Female Lobesia botrana (Lepidoptera: Tortricidae). Insects 2022, 13, 600. https://doi.org/10.3390/insects13070600
Saour G, Hashem A, Jassem I. Reproductive Behaviour of 150-Gy-Treated Female Lobesia botrana (Lepidoptera: Tortricidae). Insects. 2022; 13(7):600. https://doi.org/10.3390/insects13070600
Chicago/Turabian StyleSaour, George, Ali Hashem, and Iyad Jassem. 2022. "Reproductive Behaviour of 150-Gy-Treated Female Lobesia botrana (Lepidoptera: Tortricidae)" Insects 13, no. 7: 600. https://doi.org/10.3390/insects13070600
APA StyleSaour, G., Hashem, A., & Jassem, I. (2022). Reproductive Behaviour of 150-Gy-Treated Female Lobesia botrana (Lepidoptera: Tortricidae). Insects, 13(7), 600. https://doi.org/10.3390/insects13070600





