Gradually Increasing the Temperature Reduces the Diapause Termination Time of Trichogramma dendrolimi While Increasing Parasitoid Performance
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Experiment 1: Diapause Termination Rate
2.3. Experiment 2: Performance of Emerged T. dendrolimi
3. Statistical Analysis
4. Results
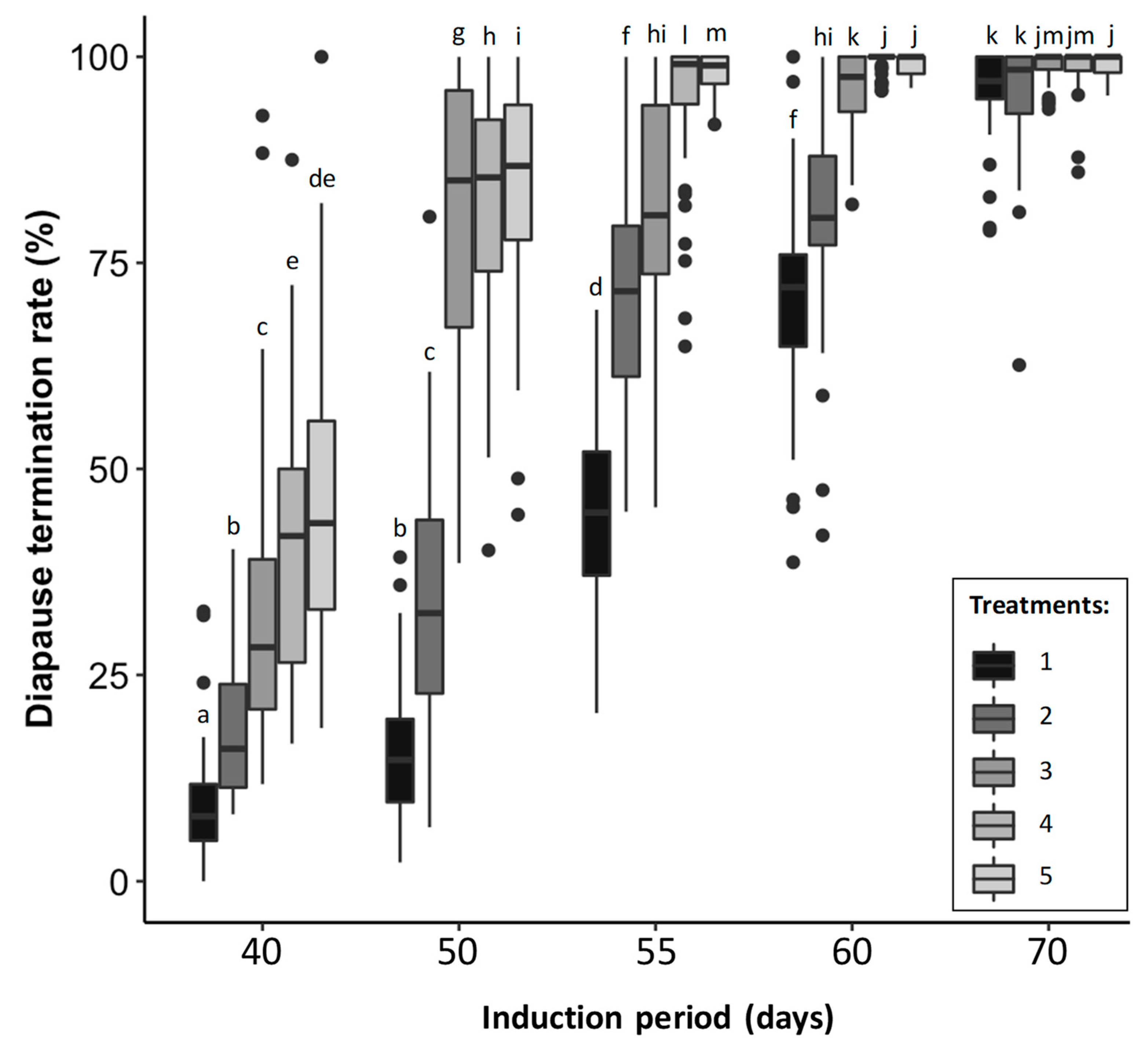
4.1. Experiment 1: Diapause Termination Rate
4.2. Experiment 2: Performance of Emerged Trichgoramma dendrolimi
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hand, S.C.; Denlinger, D.L.; Podrabsky, J.E.; Roy, R. Mechanisms of animal diapause: Recent developments from nematodes, crustaceans, insects, and fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, 1193–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denlinger, D.L. Regulation of Diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Hahn, D.A.; Denlinger, D.L. Energetics of Insect Diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Soria, M.J.; Wäckers, F.; Sanchez, J.A. When natural enemies go to sleep: Diapause induction and termination in the pear psyllid predator Pilophorus gallicus (Hemiptera: Miridae). Pest Manag. Sci. 2019, 75, 3293–3301. [Google Scholar] [CrossRef] [PubMed]
- Poitou, L.; Bras, A.; Pineau, P.; Lorme, P.; Roques, A.; Rousselet, J.; Auger-Rozenberg, M.A.; Laparie, M. Diapause regulation innewly invaded environments: Termination timing allows matching novel climatic constraints in the box tree moth, Cydalima perspectalis (Lepidoptera: Crambidae). Insects 2020, 11, 629. [Google Scholar] [CrossRef]
- van Baaren, J.; Wist, T.; Soroka, J.; Tougeron, K. Host-parasitoid network in extreme conditions: The case of cereal aphids in wheat crops in Saskatchewan, Canada. Entomol. Gen. 2020, 40, 63–77. [Google Scholar] [CrossRef]
- Smith, S.M. Biological control with Trichogramma: Advances, successes, and potential of their use. Annu. Rev. Entomol. 1996, 41, 375–406. [Google Scholar] [CrossRef]
- El-Arnaouty, S.A.; Pizzol, J.; Galal, H.H.; Kortam, M.N.; Afifi, A.I.; Beyssat, V.; Desneux, N.; Biondi, A.; Heikal, I.H. Assessment of two Trichogramma species for the control of Tuta absoluta in North African tomato greenhouses. Afr. Entomol. 2014, 22, 801–809. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Desneux, N. Biological control with Trichogramma in China: History, present status, and perspectives. Annu. Rev. Entomol. 2021, 66, 463–484. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.C.; Du, W.M.; Zang, L.S.; Ruan, C.C.; Zhang, J.J.; Zou, Z.; Monticelli, L.; Harwood, J.; Desneux, N. Multi-parasitism: A promising approach to simultaneously produce Trichogramma chilonis and T. dendrolimi on eggs of Antheraea pernyi. Entomol. Gen. 2021, 41, 627–636. [Google Scholar] [CrossRef]
- Huang, N.; Jaworski, C.C.; Desneux, N.; Zhang, F.; Yang, P.; Wang, S. Long-term and large-scale releases of Trichogramma promote pesticide decrease in maize in northeastern China. Entomol. Gen. 2020, 40, 331–335. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, X.; Monticelli, L.; Zhang, F.; Desneux, N.; Huijie, D.; Ramirez-Romero, R.; Wang, S. Parasitism performance of the parasitoid Trichogramma dendrolimi on the plum fruit moth Grapholitha funebrana. Entomol. Gen. 2020, 40, 385–395. [Google Scholar] [CrossRef]
- Ventura Garcia, P.; Wajnberg, E.; Pizzol, J.; Oliveira, M.L. Diapause in the egg parasitoid Trichogramma cordubensis: Role of temperature. J. Insect Physiol. 2002, 48, 349–355. [Google Scholar] [CrossRef]
- Pintureau, B.; Daumal, J. Effects of diapause and host species on some morphometric characters inTrichogramma (Hym.: Trichogrammatidae). Experientia 1995, 51, 67–72. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhang, X.; Zang, L.S.; Du, W.M.; Hou, Y.Y.; Ruan, C.C.; Desneux, N. Advantages of diapause in Trichogramma dendrolimi mass production on eggs of the Chinese silkworm, Antheraea pernyi. Pest Manag. Sci. 2018, 74, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Koštál, V. Eco-physiological phases of insect diapause. J. Insect Physiol. 2006, 52, 113–127. [Google Scholar] [CrossRef]
- Koštál, V.; Mollaei, M.; Schöttner, K. Diapause induction as an interplay between seasonal token stimuli, and modifying and directly limiting factors: Hibernation in Chymomyza costata. Physiol. Entomol. 2016, 41, 344–357. [Google Scholar] [CrossRef]
- Alfaro-Tapia, A.; Alvarez-Baca, J.; Tougeron, K.; Lavandero, B.; Le Lann, C.; van Baaren, J. Overwintering strategies and life-history traits of different populations of Aphidius platensis along a latitudinal gradient in Chile. Entomol. Gen. 2022, 42, 127–145. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhang, X.; Du, W.M.; Ruan, C.C.; Zang, L.S.; Ren, B.Z. Effects of inducing initial stage, temperature and period on diapause induction and diapause termination of Trichogramma dendrolimi. J. Plant. Prot. 2018, 45, 1308–1313. [Google Scholar]
- Zhu, D.F.; Zhang, M.L.; Li, L.Y. A study on the diapause and cold-storage technique. Nat. Enemies Insects 1992, 14, 173–176. [Google Scholar]
- Ma, C.S.; Chen, Y.W. Effects of constant temperature, exposure period, and age on diapause induction in Trichogramma dendrolimi. Biol Control 2006, 36, 267–273. [Google Scholar] [CrossRef]
- Rahimi-Kaldeh, S.; Ashouri, A.; Bandani, A.; Ris, N. Abiotic and biotic factors influence diapause induction in sexual and asexual strains of Trichogramma brassicae (Hym: Trichogrammatidae). Sci. Rep. 2018, 8, 17600. [Google Scholar] [CrossRef] [PubMed]
- Rundle, B.J.; Thomson, L.J.; Hoffmann, A.A. Effects of cold storage on field and laboratory performance of Trichogramma carverae (Hymenoptera: Trichogrammatidae) and the response of three Trichogramma spp. (T. carverae, T. nr. brassicae, and T. funiculatum) to cold. J. Econ. Entomol. 2004, 97, 213–221. [Google Scholar] [CrossRef]
- Zhang, J.J.; Desneux, N.; Benelli, G.; Zang, L.S.; Du, W.M.; Ruan, C.C. Geographic variation of diapause induction rates in Trichogramma dendrolimi (Hymenoptera: Trichogrammatidae) in China. J. Econ. Entomol. 2017, 110, 386–391. [Google Scholar] [CrossRef]
- Jiang, X.F.; Huang, S.H.; Luo, L.Z.; Liu, Y.; Zhang, L. Diapause termination, post-diapause development and reproduction in the beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae). J. Insect Physiol. 2010, 56, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Wang, Z.J.; Clarke, A.R.; Pereira, R.; Desneux, N.; Niu, C.Y. Pupal diapause development and termination is driven by low temperature chilling in Bactrocera minax. J. Pest Sci. 2013, 86, 429–436. [Google Scholar] [CrossRef]
- Wu, S.; Kostromytska, O.S.; Xue, F.; Koppenhöfer, A.M. Chilling effect on termination of reproductive diapause in Listronotus maculicollis (Coleoptera: Curculionidae). J. Insect Physiol. 2018, 104, 25–32. [Google Scholar] [CrossRef]
- Zhou, S.X.; Lu, X.; Zhang, G.H.; Li, L.J.; Ding, Y. Research on the induction and termination of diapause in Trichogramma dendrolimi. Chinese J. Applied Entomol. 2014, 51, 45–52. [Google Scholar]
- Yang, Y.P.; Zhao, L.Q.; Zhu, Q.S. Thermal response of diapause in the egg of Teleogryllus emma. J. Cent. South For. Univ. 2006, 26, 38–42. [Google Scholar]
- Pang, X.F.; Hou, R.H.; Bao, H.L. Method to construct the natural life table of Nilaparvata lugens. J. S. Chin. Agric. Univ. 1992, 13, 1–5. [Google Scholar]
- Chi, H.; You, M.; Atlhan, R.; Smith, C.L.; Liu, T.X. Age-Stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 103–124. [Google Scholar] [CrossRef]
- Pinto, J.D. Novel taxa of Trichogramma from the new world tropics and Australia (Hymenoptera: Trichogrammatidae). J. N. Y. Entomol. Soc. 1992, 100, 621–633. [Google Scholar]
- Wang, Y.; Zou, Z.P.; Hou, Y.Y.; Yang, X.B.; Wang, S.; Dai, H.J.; Xu, Y.Y.; Zang, L.S. Manually-extracted unfertilized eggs of Chinese oak silkworm, Antheraea pernyi, enhance mass production of Trichogramma parasitoids. Entomol. Gen. 2020, 40, 397–406. [Google Scholar] [CrossRef]
- Morris, R.F. Predictive population equations based on key factors. Mem. Entomol. Soc. Can. 1963, 95, 16–21. [Google Scholar] [CrossRef]
- Pitcher, S.A.; Hoffmann, M.P.; Gardner, J.; Wright, M.G.; Kuhar, T.P. Cold storage of Trichogramma ostriniae reared on Sitotroga cerealella eggs. BioControl 2002, 47, 525–535. [Google Scholar] [CrossRef]
- Hiroyoshi, S.; Reddy, G.V.P.; Mitsuhashi, J. Effects of photoperiod, temperature and aging on adult diapause termination and post-diapause development in female Asian comma butterflies, Polygonia c-aureum Linnaeus (Lepidoptera: Nymphalidae). J. Comp. Physiol. A 2018, 204, 849–858. [Google Scholar] [CrossRef]
- Emery, S.E.; Nicholas, J.M. Effects of temperature and other environmental factors on the post-diapause development of walnut husk fly Rhagoletis completa (Diptera: Tephritidae). Physiol. Entomol. 2019, 44, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Moraiti, C.A.; Nakas, C.T.; Papadopoulos, N.T. Diapause termination of Rhagoletis cerasi pupae is regulated by local adaptation and phenotypic plasticity: Escape in time through bet-hedging strategies. J. Evol. Biol. 2014, 27, 43–54. [Google Scholar] [CrossRef]
- Milonas, P.G.; Savopoulou-Soultani, M. Diapause induction and termination in the parasitoid Colpoclypeus florus (Hymenoptera: Eulophidae): Role of photoperiod and temperature. Ann. Entomol. Soci. Am. 2000, 93, 512–518. [Google Scholar] [CrossRef]
- Lehmann, P.; Pruisscher, P.; Posledovich, D.; Carlsson, M.; Käkelä, R.; Tang, P.; Nylin, S.; Wheat, C.W.; Wiklund, C.; Gotthard, K. Energy and lipid metabolism during direct and diapause development in a pierid butterfly. J. Exp. Biol. 2016, 219, 3049–3060. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.H.; Shiao, S.F.; Okuyama, T. Development of insects under fluctuating temperature: A review and case study. J. Appl. Entomol. 2015, 139, 592–599. [Google Scholar] [CrossRef]
- Kambule, I.N.; Hanrahan, S.A.; Duncan, F.D. Metabolic rate in diapause and nondiapause brown locust eggs correlated with embryonic development. Physiol. Entomol. 2011, 36, 299–308. [Google Scholar] [CrossRef]
- Ragland, G.J.; Fuller, J.; Feder, J.L.; Hahn, D.A. Biphasic metabolic rate trajectory of pupal diapause termination and post-diapause development in a tephritid fly. J. Insect. Physiol. 2009, 55, 344–350. [Google Scholar] [CrossRef]
- Delava, E.; Fleury, F.; Gibert, P. Effects of daily fluctuating temperatures on the Drosophila–Leptopilina boulardi parasitoid association. J. Therm. Biol. 2016, 60, 95–102. [Google Scholar] [CrossRef]
- Castellanos, N.L.; Bueno, A.F.; Haddi, K.; Silveira, E.C.; Rodrigues, H.S.; Hirose, E.; Smagghe, G.; Oliveira, E.E. The Fitness and economic benefits of rearing the parasitoid Telenomus podisi under fluctuating temperature regime. Neotrop. Entomol. 2019, 48, 934–948. [Google Scholar] [CrossRef]
- Torres, J.B.; Musolin, D.L.; Zanuncio, J.C. Thermal requirements and parasitism Capacity of Trissolcus brochymenae (Ashmead) (Hymenoptera: Scelionidae) under constant and fluctuating temperatures, and assessment of development in field conditions. Biocontrol Sci. Techn. 2002, 12, 583–593. [Google Scholar] [CrossRef]
- Colinet, H.; Hance, T.; Vernon, P. Water relations, fat reserves, survival, and longevity of a cold-exposed parasitic wasp Aphidius colemani (Hymenoptera: Aphidiinae). Environ. Entomol. 2006, 35, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Colombari, F.; Tonina, L.; Battisti, A.; Mori, N. Performance of Trichopria drosophilae (Hymenoptera: Diapriidae), a generalist parasitoid of Drosophila suzukii (Diptera: Drosophilidae), at low temperature. J. Insect Sci. 2020, 20, 9. [Google Scholar] [CrossRef]
- Wang, X.G.; Serrato, M.A.; Son, Y.; Walton, V.M.; Hogg, B.N.; Daane, K.M. Thermal performance of two indigenous pupal parasitoids attacking the invasive Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2018, 47, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.J.; Bauer, L.S.; Van Driesche, R.; Schmude, J.M.; Petrice, T.; Chandler, J.L.; Elkinton, J. Effects of extreme low winter temperatures on the overwintering survival of the introduced larval parasitoids Spathius galinae and Tetrastichus planipennisi: Implications for biological control of emerald ash borer in north America. J. Econ. Entomol. 2020, 113, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Renault, D.; Salin, C.; Vannier, G.; Vernon, P. Survival at low temperatures in insects: What is the ecological significance of the supercooling point? Cryo. Letters 2002, 23, 217–228. [Google Scholar] [PubMed]
- Colinet, H.; Renault, D.; Hance, T.; Vernon, P. The impact of fluctuating thermal regimes on the survival of a cold-exposed parasitic wasp, Aphidius colemani. Physiol. Entomol. 2006, 31, 234–240. [Google Scholar] [CrossRef]
- Tougeron, K.; Brodeur, J.; Le Lann, C.; van Baaren, J. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, M.; Izadi, H. Cold acclimation of Trogoderma granarium Everts is iightly linked to regulation of enzyme activity, energy content, and ion concentration. Front. Physiol. 2018, 9, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellers, J.; Van Alphen, J.J.M. A trade-off between diapause duration and fitness in female parasitoids. Ecol. Entomol. 2002, 27, 279–284. [Google Scholar] [CrossRef]
- Saunders, D.S. Larval diapause duration and fat metabolism in three geographical strains of the blow fly, Calliphora vicina. J. Insect Physiol. 2000, 46, 509–517. [Google Scholar] [CrossRef]
- Poras, M. Influence de la photoperiode et de la temperature sur quelques aspects de la diapause imaginale chez les femelles de Tetrix undulata (Sow.) (Orthoptere: Tetrigidae). Ann. Zool. Ecol. Anim. Genus 1976, 8, 373–380. [Google Scholar]
- Sáringer, G.; Szentkirályi, F. Contribution to the knowledge of the diapause of Grapholitha funebrana Treitschke (Lepid., Tortricidae). J. Appl. Entomol. 1980, 90, 493–505. [Google Scholar] [CrossRef]
- Wang, X.P.; Xue, F.S.; Hua, A.; Ge, F. Effects of diapause duration on future reproduction in the cabbage beetle, Colaphellus bowringi: Positive or negative? Physiol. Entomol. 2006, 31, 190–196. [Google Scholar] [CrossRef]
- Fantinou, A.A.; Perdikis, D.C.; Zota, K.F. Reproductive responses to photoperiod and temperature by diapausing and nondiapausing populations of Sesamia nonagrioides Lef. (Lepidoptera−Noctuidae). Physiol. Entomol. 2004, 29, 169–175. [Google Scholar] [CrossRef]
- Ferro, D.N.; Tuttle, A.F.; Weber, D.C. Ovipositional and flight behavior of overwintered colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1991, 20, 1309–1314. [Google Scholar] [CrossRef]
- Jansson, R.K.; Zitzman, A.E., Jr.; Lashomb, J.H. Effects of food plant and diapause on adult survival and fecundity of colorado potato beetle (Coleoptera: Chrysomelidae). Environ. Entomol. 1989, 18, 291–297. [Google Scholar] [CrossRef]
- Hu, D.F.; Chen, Q.X.; Liu, W.H.; Han, S.; Zhang, M.L.; Li, L.Y. A preliminary report on the effect of diapaused and long-term cold-stored Trichogramma to control sugarcane borers in the field. Nat. Enemy Insects 1992, 18, 291–297. [Google Scholar]
- Li, Y.N.; Liu, Y.B.; Xie, X.Q.; Zhang, J.N.; Li, W.L. The modulation of trehalose metabolism by 20-Hydroxyecdysone in Antheraea pernyi (Lepidoptera: Saturniidae) during its diapause termination and post-termination period. J. Insect Sci. 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ruan, C.C.; Zhang, J.J.; Sun, G.Z. Changes of glycerine protein and carbohydrate during diapause and breaking diapause of Trichogramma dendrolimi. J. Jilin Agricul. Univ. 2011, 33, 367–370,375. [Google Scholar]
- Pizzol, J.; Pintureau, B.; Khoualdia, O.; Desneux, N. Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hymenoptera: Trichogrammatidae). J. Pest Sci. 2010, 83, 447–452. [Google Scholar] [CrossRef]
- Tabone, E.; Bardon, C.; Desneux, N.; Wajnberg, E. Parasitism of different Trichogramma species and strains on Plutella xylostella L. on greenhouse cauliflower. J. Pest Sci. 2010, 83, 251–256. [Google Scholar] [CrossRef]
- Kenis, M.; Benelli, G.; Biondi, A.; Calatayud, P.A.; Day, R.; Desneux, N.; Harrison, R.D.; Kriticos, D.; Rwomushana, I.; van den Berg, J.; et al. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol. Gen. 2022, in press. [Google Scholar] [CrossRef]
- Han, P.; Lavoir, A.V.; Rodriguez-Saona, C.; Desneux, N. Bottom-up forces in agroecosystems and their potential impact on arthropod pest management. Annu. Rev. Entomol. 2022, 67, 239–259. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]

| Induction | Treatment | Buffering | Development |
|---|---|---|---|
| 3 °C for 40, 50, 55, 60, 70 (d), respectively, then divided into five treatments | 1 | -- | 25 °C until to adults |
| 2 | 10 °C (2d) | 25 °C until to adults | |
| 3 | 10 °C (5d) | 25 °C until to adults | |
| 4 | 10 °C (2d)-20-15 °C (2d) | 25 °C until to adults | |
| 5 | 10 °C (2d)-20-15 °C (2d) | 25-20 °C until to adults |
| Index | T1 | T2 | T3 | T4 | T5 | CK |
|---|---|---|---|---|---|---|
| Sp | 0.797 | 0.841 | 0.850 | 0.870 | 0.924 | 0.877 |
| FPF | 168.556 | 171.578 | 171.067 | 168.600 | 181.289 | 147.822 |
| P♀ | 0.826 | 0.854 | 0.840 | 0.843 | 0.844 | 0.844 |
| I | 111.1 a | 123.3 b | 122.1 b | 123.6 b | 141.5 c | 109.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; He, B.; Monticelli, L.S.; Du, W.; Ruan, C.; Desneux, N.; Zhang, J. Gradually Increasing the Temperature Reduces the Diapause Termination Time of Trichogramma dendrolimi While Increasing Parasitoid Performance. Insects 2022, 13, 720. https://doi.org/10.3390/insects13080720
Zhang X, He B, Monticelli LS, Du W, Ruan C, Desneux N, Zhang J. Gradually Increasing the Temperature Reduces the Diapause Termination Time of Trichogramma dendrolimi While Increasing Parasitoid Performance. Insects. 2022; 13(8):720. https://doi.org/10.3390/insects13080720
Chicago/Turabian StyleZhang, Xue, Bingxin He, Lucie S. Monticelli, Wenmei Du, Changchun Ruan, Nicolas Desneux, and Junjie Zhang. 2022. "Gradually Increasing the Temperature Reduces the Diapause Termination Time of Trichogramma dendrolimi While Increasing Parasitoid Performance" Insects 13, no. 8: 720. https://doi.org/10.3390/insects13080720
APA StyleZhang, X., He, B., Monticelli, L. S., Du, W., Ruan, C., Desneux, N., & Zhang, J. (2022). Gradually Increasing the Temperature Reduces the Diapause Termination Time of Trichogramma dendrolimi While Increasing Parasitoid Performance. Insects, 13(8), 720. https://doi.org/10.3390/insects13080720




