Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
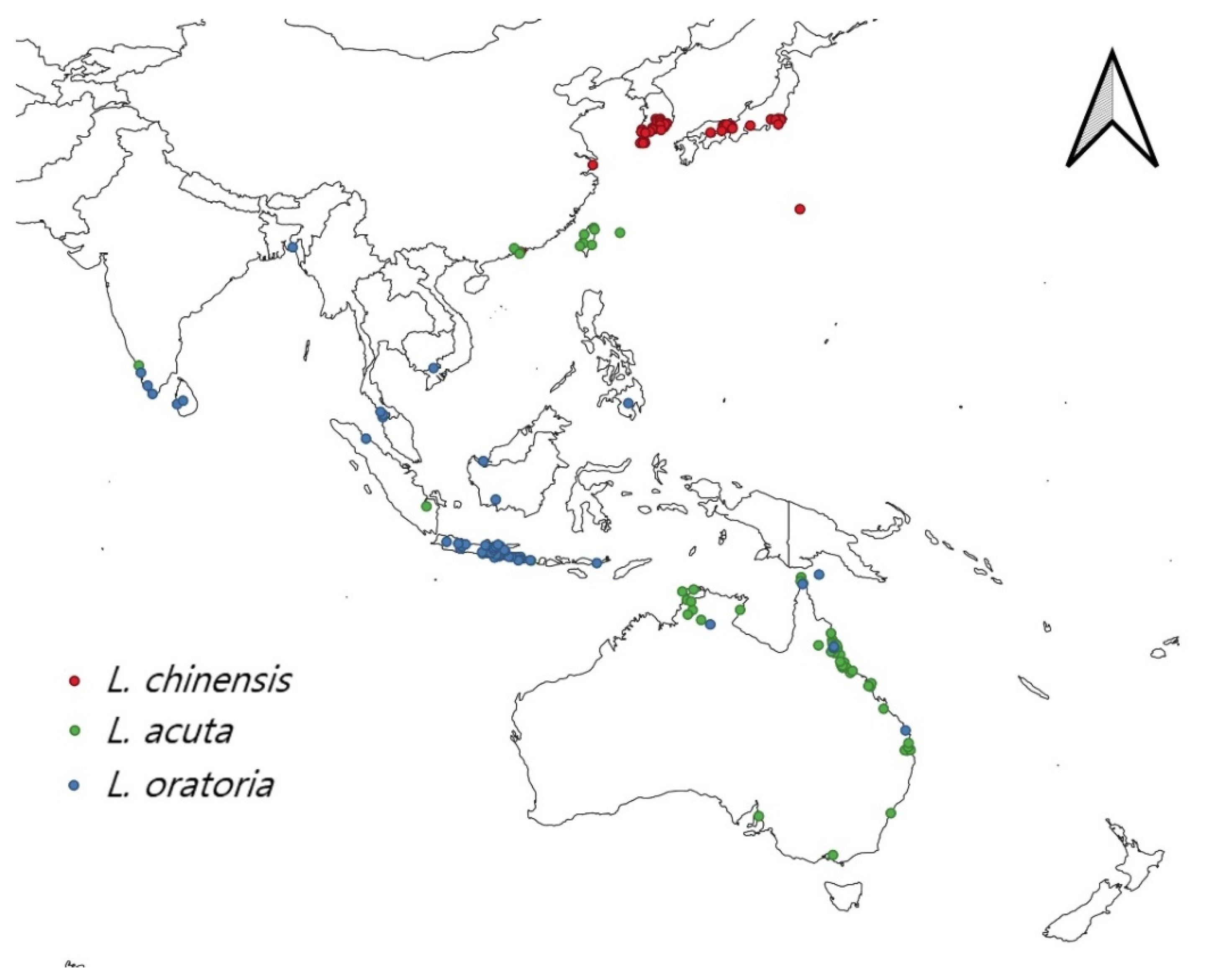
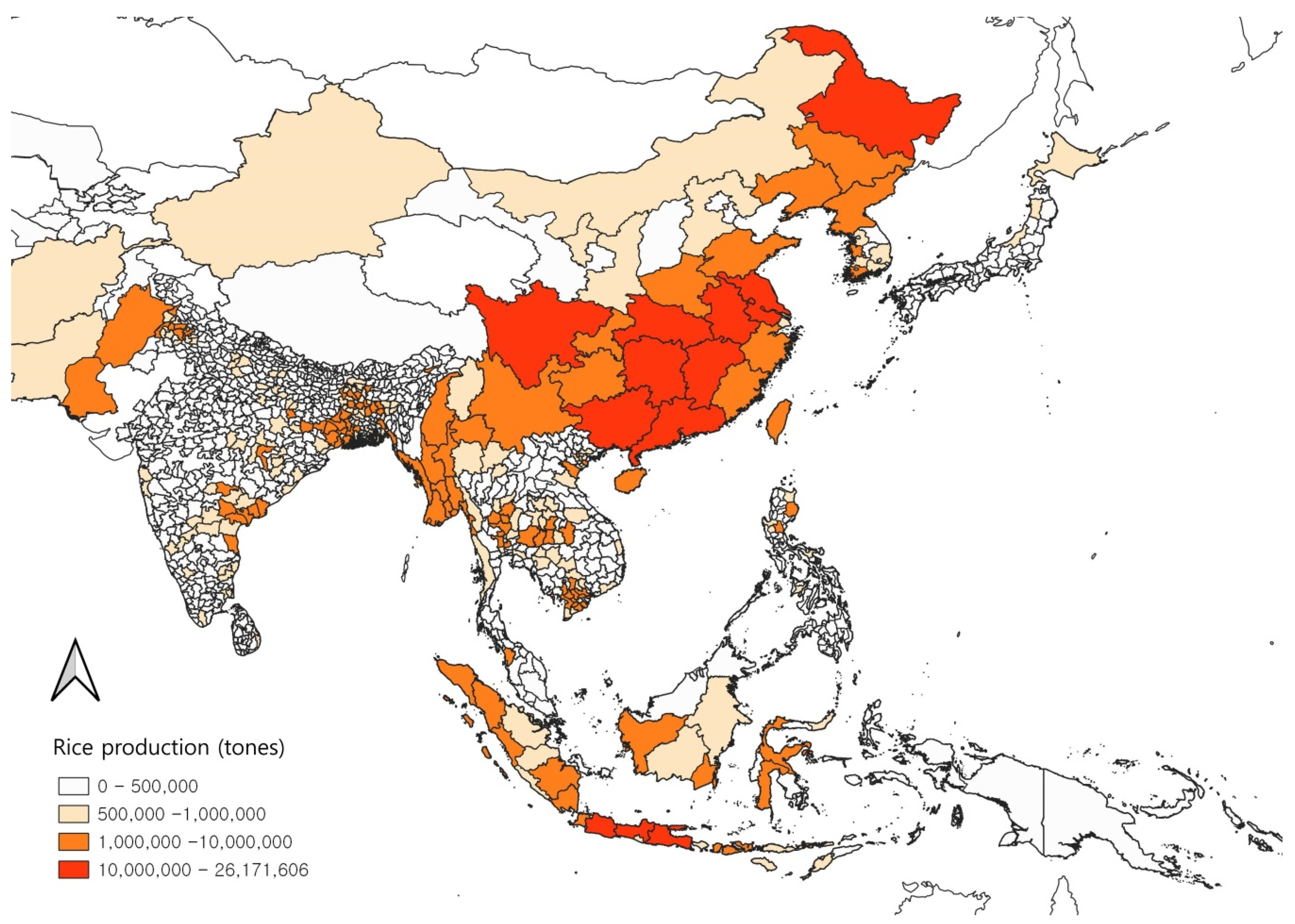
2.1. Distribution Data Acquisition
2.2. Leptocorisa chinensis Distribution
2.3. Leptocorisa acuta Distribution
2.4. Leptocorisa oratoria Distribution
2.5. Final Distribution Data
2.6. Model Variables Selection and Operation
2.7. Climate Change Scenario
2.8. Model Performance Test
3. Results
3.1. The Results of Model Performance Test for the Three Leptocorisa Species
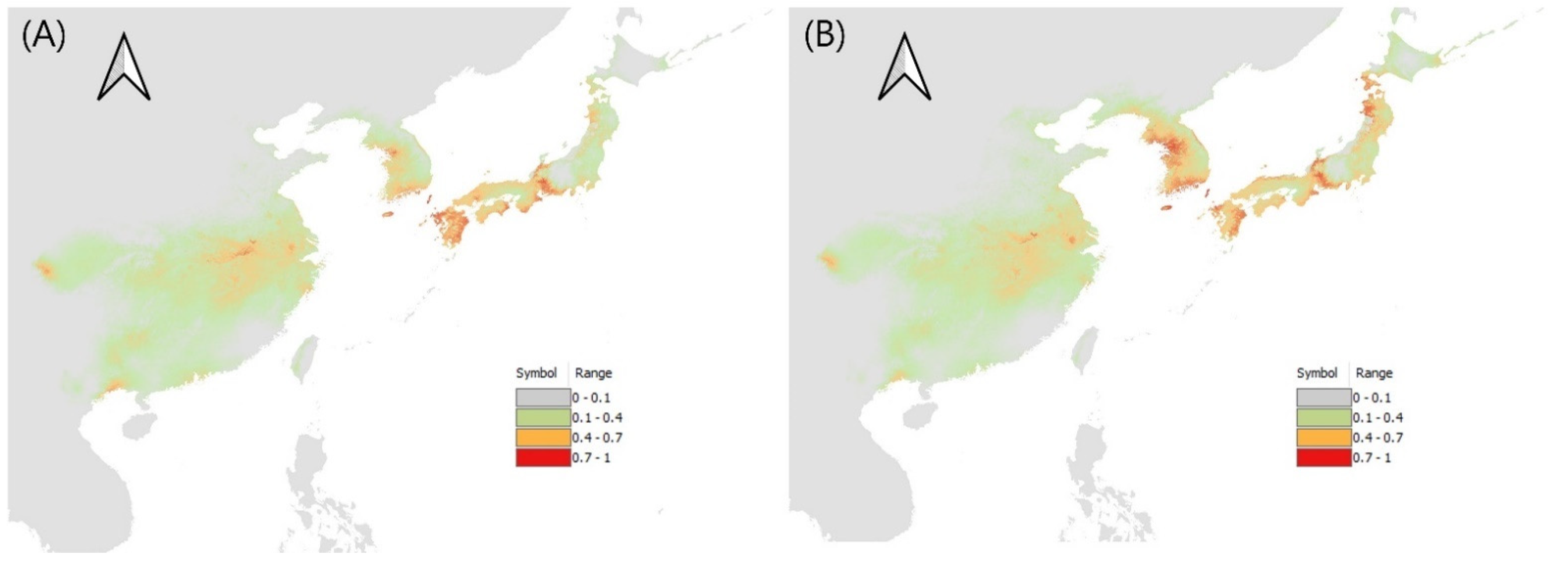
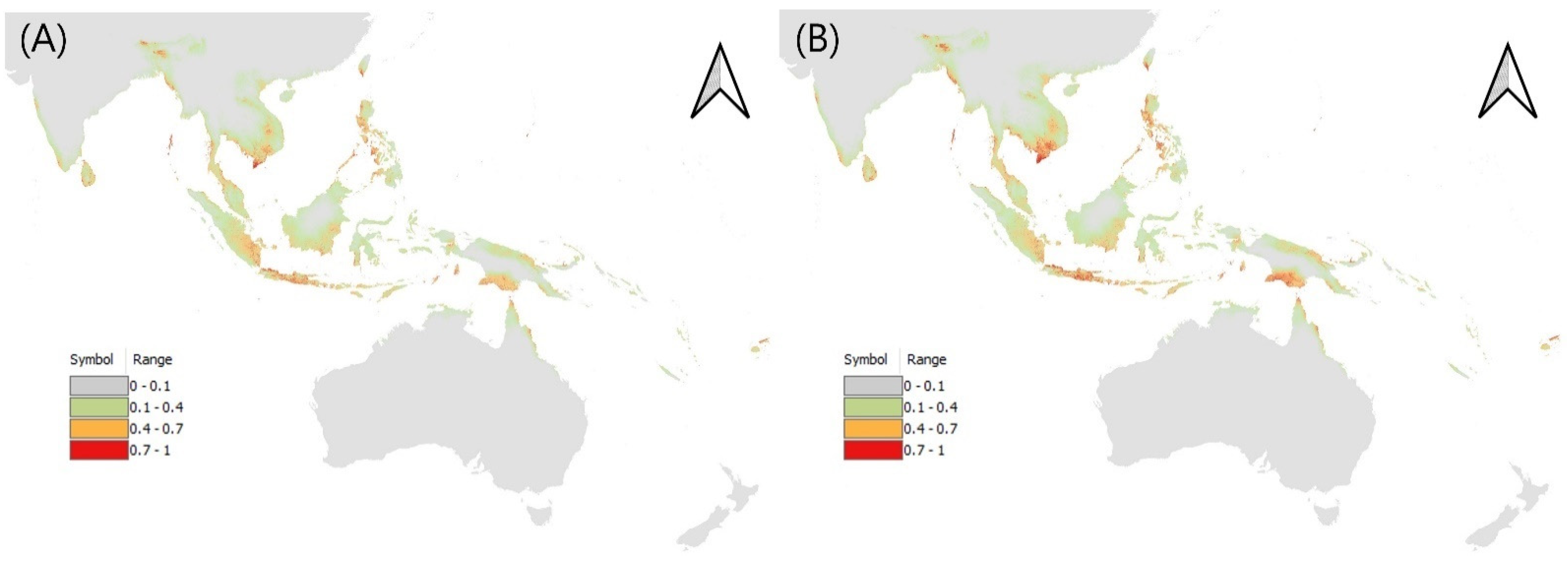
3.2. Potential Distribution of Three Leptocorisa Species in Asia and Oceania
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ane, N.U.; Hussain, M. Diversity of insect pests in major rice growing areas of the world. J. Entomol. Zool. Stud. 2016, 4, 36–41. [Google Scholar]
- Ngalimat, M.S.; Mohd Hata, E.; Zulperi, D.; Ismail, S.I.; Ismail, M.R.; Mohd Zainudin, N.A.I.; Saidi, N.B.; Yusof, M.T. Plant growth-promoting bacteria as an emerging tool to manage bacterial rice pathogens. Microorganisms 2021, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Haefele, S.M.; Nelson, A.; Hijmans, R.J. Soil quality and constraints in global rice production. Geoderma 2014, 235, 250–259. [Google Scholar] [CrossRef]
- Wang, D.; Dowell, F.E.; Lan, Y.; Pasikatan, M.; Maghirang, E. Determining pecky rice kernels using visible and near-infrared spectroscopy. Int. J. Food Prop. 2002, 5, 629–639. [Google Scholar] [CrossRef]
- Cox, P.G.; Jahn, G.C.; Mak, S.; Chhorn, N.; Tuy, S. Farmer participatory research for rat management in Cambodia. Int. Rice Res. Notes 1999, 24, 29–31. [Google Scholar]
- Ito, K.; Sugiyama, H.; Salleh, N. Damages in rice plants caused by ear-sucking bugs in the Muda area, West Malaysia. JARQ-Jpn. Agric. Res. Q. 1992, 26, 67–74. [Google Scholar]
- Jahn, G.C.; Domingo, I.; Liberty, M.; Almazan, P.; Pacia, J. Effect of rice bug Leptocorisa oratorius (Hemiptera: Alydidae) on rice yield, grain quality, and seed viability. J. Econ. Entomol. 2004, 97, 1923–1927. [Google Scholar] [CrossRef]
- Mandanayake, M.A.R.; Amarakoon, A.M.; Sirisena, U.G.A.; Hemachandra, K.; Wilson, M.R.; Kahawaththa, U. Occurrence of Leptocorisa acuta (Thunberg) (Hemiptera: Alydidae) in Sri Lanka. Ann. Sri Lanka Dep. Agric. 2014, 16, 323–326. [Google Scholar]
- Mohiuddin, M.S.; Rao, Y.P.; Mohan, S.K.; Verma, J.P. Role of Leptocorisa acuta Thun. in the spread of bacterial blight of rice. Curr. Sci. 1976, 45, 426–427. [Google Scholar]
- Nugaliyadda, L.; Edirisinghe, J.P.; Hidaka, T. Role of weed hosts on the survival of paddy bug, Leptocorisa oratorius (Hemiptera Alydidae). Ann. Sri Lanka Dep. Agric. 2000, 16, 323–326. [Google Scholar]
- Sands, D.P.A. The biology and ecology of Leptocorisa (Hemiptera: Alydidae) in Papua New Guinea; Department of Primary Industry: Port Moresby, Papua New Guinea, 1977; p. 104. [Google Scholar]
- Van den Berg, H.; Soehardi. The influence of the rice bug Leptocorisa oratorius on rice yield. J. Appl. Ecol. 2000, 37, 959–970. [Google Scholar] [CrossRef]
- Ahmad, I. Studies on the Taxonomy of the Alydidae with Special Reference to the Subfamily Leptocoisinae (Heteroptera). Ph.D. Thesis, University of London, London, UK, 1963. [Google Scholar]
- Aukema, B.; Rieger, C. (Eds.) Catalogue of the Heteroptera of the Palaearctic Region. Volume Pentatomomorpha II; The Netherlands Entomological Society: Wageningen, The Netherlands, 2006; ISBN 978-90-71912-28-3. [Google Scholar]
- Litsinger, J.A.; Barrion, A.T.; Canapi, B.L.; Libetario, E.M.; Pantua, P.C.; Cruz, C.G.; Apostol, R.F.; Lumaban, M.D.; Bandong, J.P.; Macatula, R.F. Leptocorisa rice seed bugs (Hemiptera: Alydidae) in Asia: A review. Philipp. Entomol. 2015, 29, 1–103. [Google Scholar]
- Soti, A.; Bhandari, S.; Acharya, A.; Shrestha, S. Screening of early maturing rice varieties against rice earhead bug, Leptocorisa oratorius Fabricius (Hemiptera: Alydidae) in IRD Center Jhapa, Nepal. J. Entomol. Zool. Stud. 2020, 8, 1363–1366. [Google Scholar]
- Siwi, S.S.; Van Doesburg, P.H. Leptocorisa Latreille in Indonesia (Heteroptera, Coreidae, Alydinae). Zool. Meded. 1984, 58, 117–129. [Google Scholar]
- Jalaeian, M.; Golizadeh, A.; Sarafrazi, A.; Naimi, B. Inferring climatic controls of rice stem borers’ spatial distributions using maximum entropy modelling. J. Appl. Entomol. 2018, 142, 388–396. [Google Scholar] [CrossRef]
- Lestina, J.; Cook, M.; Kumar, S.; Morisette, J.; Ode, P.J.; Peairs, F. MODIS imagery improves pest risk assessment: A case study of wheat stem sawfly (Cephus cinctus, Hymenoptera: Cephidae) in Colorado, USA. Environ. Entomol. 2016, 45, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, H.; Zhao, W.; Zhao, Q. Niche shifts and the potential distribution of Phenacoccus solenopsis (Hemiptera: Pseudococcidae) under climate change. PLoS ONE 2017, 12, e0180913. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS predicting species distributions working group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Negrini, M.; Fidelis, E.G.; Picanço, M.C.; Ramos, R.S. Mapping of the Steneotarsonemus spinki invasion risk in suitable areas for rice (Oryza sativa) cultivation using MaxEnt. Exp. Appl. Acarol. 2020, 80, 445–461. [Google Scholar] [CrossRef]
- Qi, G.; Gao, Y.; Huang, D. Historical invasion, expansion process and the potential geographic distributions for the rice water weevil, Lissorhoptrus oryzophilus in China based on MAXENT. Acta Phytophylacica Sin. 2012, 39, 129–136. [Google Scholar]
- Venter, T.S.; Robertson, M.P.; Saccaggi, D.L.; Faulkner, K.T. The wheat curl mite (Aceria tosichella, Prostigmata: Eriophyidae) could establish in South Africa. Afr. Zool. 2021, 56, 17–24. [Google Scholar] [CrossRef]
- Laborte, A.G.; Gutierrez, M.A.; Balanza, J.G.; Saito, K.; Zwart, S.J.; Boschetti, M.; Murty, M.V.R.; Villano, L.; Aunario, J.K.; Reinke, R.; et al. RiceAtlas, a spatial database of global rice calendars and production. Sci. Data 2017, 4, 170074. [Google Scholar] [CrossRef] [PubMed]
- Centre for Agriculture and Bioscience International (CABI). Invasive Species Compendium International. Available online: https://www.cabi.org/isc (accessed on 10 March 2021).
- Global Biodiversity Information Facility (GBIF). Occurrence Download. Available online: https://www.gbif.org (accessed on 10 March 2021).
- Kim, H.; Baek, S.; Lee, J.H. Temperature-dependent development and oviposition models of Leptocorisa chinensis Dallas (Hemiptera: Alydidae). J. Asia-Pac. Entomol. 2018, 21, 244–251. [Google Scholar] [CrossRef]
- Korean Natural History Research Information System (NARIS). Leptocorisa chinensis Distribution Data. Available online: http://www.naris.go.kr (accessed on 10 March 2021).
- Ryu, J.; Kim, Y.K.; Suh, S.J.; Choi, K.S. The Insect database in Dokdo, Korea: An updated version in 2020. Biodivers. Data J. 2021, 9, e62011. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Ecology (NIE). National Ecosystem Survey Animal and Plant Data. Available online: https://www.nie-ecobank.kr (accessed on 12 March 2021).
- Tsunoda, T.; Moriya, S. Measurement of flight speed and estimation of flight distance of the bean bug, Riptortus pedestris (Fabricius) (Heteroptera: Alydidae) and the rice bug, Leptocorisa chinensis Dallas (Heteroptera: Alydidae) with a speed sensor and flight mills. Appl. Entomol. Zool. 2008, 43, 451–456. [Google Scholar] [CrossRef]
- Ahmad, I. Leptocorisinae (Heteroptera: Alydidae) of the world. Bull. Br. Mus. (Nat. Hist.) Entomol. 1965, 5, 1–156. [Google Scholar] [CrossRef]
- Hasegawa, H. Distribution and taxonomy of rice bugs in Southeast Asia. In Proceedings of the Tropical Agriculture Research Series: Proceedings of the Symposium on Tropical Agriculture Researches, Tokyo, Japan, 19–24 July 1971; pp. 229–234. [Google Scholar]
- Karenina, T.; Herlinda, S.; Irsan, C.; Pujiastuti, Y.; Hasbi; Suparman; Lakitan, B.; Hamidson, H.; Umayah, A. Community structure of arboreal and soil-dwelling arthropods in three different rice planting indexes in freshwater swamps of South Sumatra, Indonesia. Biodivers. J. Biol. Divers. 2020, 21, 4839–4849. [Google Scholar] [CrossRef]
- Commonwealth Institute of Entomology (CIE). Leptocorisa acuta. [Distribution map]. Available online: https://doi.org/10.1079/dmpp20056600225 (accessed on 15 March 2021).
- National Institute of Biological Resources (NIBR). National Species List of Korea. Available online: http://kbr.go.kr (accessed on 15 March 2021).
- Siregar, A.Z.; Yurnaliza, Y. Control of pests in saline paddy of Percut, Northern Sumatera. In Proceedings of the Third Workshop on Multidisciplinary and Its Applications (WMA-3 2019), Medan, Indonesia, 11–14 December 2019; pp. 1–9. [Google Scholar]
- Morshed, M.N.; Uddin, M.E.; Hera, M.H.R.; Sultana, N. Effect of temperature, rainfall and relative humidity on seasonal incidence of major rice insect pests. Int. J. Biosci. 2020, 17, 92–102. [Google Scholar] [CrossRef]
- Balleras, G.D.; Endonela, L.E. Aboveground Arthropod Composition, Abundance and Guild Structure in Upland Rice Agro-ecosystem at Matalam, North Cotabato, Philippines. Int. Peer Rev. J. 2014, 9, 61–76. [Google Scholar] [CrossRef]
- Bambaradeniya, C.N.B.; Edirisinghe, J.P. Composition, structure and dynamics of arthropod communities in a rice agro-ecosystem. Ceylon J. Sci. 2009, 37, 23–48. [Google Scholar] [CrossRef]
- Prakash, R.; Kunal, G. Evaluation of efficacy of insecticides and bio pesticide against paddy Earhead bug, Leptocorisa oratorius F. J. Pharmacogn. Phytochem. 2020, 9, 2021–2023. [Google Scholar] [CrossRef]
- Lee, S.C.; Alvenda, M.E.; Bonman, J.M.; Heinrichs, E.A. Insects and pathogens associated with rice grain discoloration and their relationship in the Philippines. Korean J. Appl. Entomol. 1986, 25, 107–112. [Google Scholar]
- Brown, J.L. SDM toolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- McQuillan, M.A.; Rice, A.M. Differential effects of climate and species interactions on range limits at a hybrid zone: Potential direct and indirect impacts of climate change. Ecol. Evol. 2015, 5, 5120–5137. [Google Scholar] [CrossRef] [PubMed]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- International Steering Committee for Global Mapping (ISCGM). Land Cover Download. Available online: https://globalmaps.github.io (accessed on 11 May 2022).
- Huercha; Song, R.; Ma, Y.; Hu, Z.; Li, Y.; Li, M.; Wu, L.; Li, C.; Dao, E.; Fan, X.; et al. MaxEnt Modeling of Dermacentor marginatus (Acari: Ixodidae) Distribution in Xinjiang, China. J. Med. Entomol. 2020, 57, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Mudereri, B.T.; Mukanga, C.; Mupfiga, E.T.; Gwatirisa, C.; Kimathi, E.; Chitata, T. Analysis of potentially suitable habitat within migration connections of an intra-African migrant-the Blue Swallow (Hirundo atrocaerulea). Ecol. Inf. 2020, 57, 101082. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S-PLUS; Springer Science & Business Media: Berlin, Germany, 2013; ISBN 978-038-721-706-2. [Google Scholar]
- Kramer-Schadt, S.; Niedballa, J.; Pilgrim, J.D.; Schröder, B.; Lindenborn, J.; Reinfelder, V.; Stillfried, M.; Heckmann, I.; Scharf, A.K.; Augeri, D.M.; et al. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013, 19, 1366–1379. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Tatebe, H.; Ogura, T.; Nitta, T.; Komuro, Y.; Ogochi, K.; Takemura, T.; Sudo, K.; Sekiguchi, M.; Abe, M.; Saito, F.; et al. Description and basic evaluation of simulated mean state, internal variability, and climate sensitivity in MIROC6. Geosci. Model Dev. 2019, 12, 2727–2765. [Google Scholar] [CrossRef]
- van Vuuren, D.P.; Carter, T.R. Climate and socio-economic scenarios for climate change research and assessment: Reconciling the new with the old. Clim. Chang. 2014, 122, 415–429. [Google Scholar] [CrossRef]
- Lee, W.H.; Jung, J.M.; Lee, H.S.; Lee, J.H.; Jung, S. Evaluating the invasion risk of longhorn crazy ants (Paratrechina longicornis) in South Korea using spatial distribution model. J. Asia-Pac. Entomol. 2021, 24, 279–287. [Google Scholar] [CrossRef]
- Kumar, S.; Graham, J.; West, A.M.; Evangelista, P.H. Using district-level occurrences in MaxEnt for predicting the invasion potential of an exotic insect pest in India. Comput. Electron. Agric. 2014, 103, 55–62. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions (MPB-49); Princeton University Press: Princeton, NJ, USA, 2011; ISBN 978-069-113-688-2. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Ben Rais Lasram, F.; Guilhaumon, F.; Albouy, C.; Somot, S.; Thuiller, W.; Mouillot, D. The Mediterranean Sea as a ‘cul-de-sac’for endemic fishes facing climate change. Glob. Chang. Biol. 2010, 16, 3233–3245. [Google Scholar] [CrossRef]
- Lin, C.T.; Chiu, C.A. The Relic Trochodendron aralioides Siebold & Zucc. (Trochodendraceae) in Taiwan: Ensemble distribution modeling and climate change impacts. Forests 2019, 10, 7. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecol. 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Svenning, J.C.; Normand, S.; Kageyama, M. Glacial refugia of temperate trees in Europe: Insights from species distribution modelling. J. Ecol. 2008, 96, 1117–1127. [Google Scholar] [CrossRef]
- van Proosdij, A.S.; Sosef, M.S.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, W.H. Methodological analysis of bioclimatic variable selection in species distribution modeling with application to agricultural pests (Metcalfa pruinosa and Spodoptera litura). Comput. Electron. Agric. 2021, 190, 106430. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Barbet-Massin, M.; Bellard, C. Without quality presence–absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Insights into the area under the receiver operating characteristic curve (AUC) as a discrimination measure in species distribution modelling. Glob. Ecol. Biogeogr. 2012, 21, 498–507. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A. Threshold-dependence as a desirable attribute for discrimination assessment: Implications for the evaluation of species distribution models. Biodivers. Conserv. 2014, 23, 369–385. [Google Scholar] [CrossRef]
- Litsinger, J.A.; Taylo, L.D.; Cadapan, E.P. Host plant range of the rice bug Leptocorisa oratorius (Fabricius) (Hemiptera: Alydidae). J. Plant Prot. Trop. 1993, 10, 119–130. [Google Scholar]
- Rattanapun, W. Biology of rice bug Leptocorisa oratorius (Fabricius) (Hemiptera: Alydidae), population change and alternative host plants. Commun. Agric. Appl. Biol. Sci. 2013, 78, 193–197. [Google Scholar]
- Reji, G.; Chander, S. A degree-day simulation model for the population dynamics of the rice bug, Leptocorisa acuta (Thunb.). J. Appl. Entomol. 2008, 132, 646–653. [Google Scholar] [CrossRef]
- Reji, G.; Chander, S.; Singh, V.S.; Satish, D.G. Determination of threshold of development and thermal constant of rice gundhi bug, Leptocorisa acuta. In Proceedings of the National Symposium on Frontier Areas of Entomological Research, New Delhi, India, 5–7 November 2003; pp. 22–23. [Google Scholar]
- Cobblah, M.A.; Den Hollander, J. Specific differences in immature stages, oviposition sites and hatching patterns in two rice pests, Leptocorisa oratorius (Fabricius) and L. acuta (Thunberg) (Heteroptera: Alydidae). Int. J. Trop. Insect Sci. 1992, 13, 1–6. [Google Scholar] [CrossRef]
- Takeuchi, H.; Watanabe, T.; Ishizaki, M.; Oku, S.; Suzuki, Y. The relationship between developmental stages of rice spikelets and the incidence of the rice bugs Leptocorisa chinensis, Lagynotomus elongatus, and Stenotus rubrovittatus in rice fields. Appl. Entomol. Zool. 2005, 40, 351–357. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Kwak, Y. Relationship between the geographical features of methane sources of SCIAMACHY and vegetation index of MODIS. In Proceedings of the 2014 IEEE Geoscience and Remote Sensing Symposium, Quebec City, QC, Canada, 13–18 July 2014; pp. 4986–4989. [Google Scholar]
- Yamashita, K.I.; Takabayashi, J.; Miura, K. Temperature and photoperiodic effects on induction and termination of diapause in female Leptocorisa chinensis (Hemiptera: Alydidae). Ann. Entomol. Soc. Am. 2010, 103, 366–370. [Google Scholar] [CrossRef]
- Biswas, D.; Thakur, N.A.; Gogoi, J.; Nakambam, S. Study on the biodiversity of insects in apple in mid hills of Meghalaya. J. Entomol. Zool. Stud. 2020, 8, 818–823. [Google Scholar]
- Devi, N.S.; Ray, D.C. Field evaluation of some safer pesticides and bio-pesticides against Leptocorisa acuta (Thunb.) on rice in Cachar district of Assam, NE India. Environ. Ecol. 2010, 28, 2579–2583. [Google Scholar]
- Loc, N.T.; Chi, V.T.B.; Hung, P.Q. Efficacy of some new isolates of Metarhizium anisopliae and Beauveria bassiana against rice earhead bug, Leptocorisa acuta. Omonrice 2005, 13, 69–75. [Google Scholar]
- International Rice Research Institute. Workshop on Research Priorities in Tidal Swamp Rice; International Rice Research Institute: Manila, Philippines, 1984; pp. 167–173. ISBN 971-104-102-2. [Google Scholar]
- Sugimoto, A.; Nugaliyadde, L. Damage of rice grains caused by the rice bug, Leptocorisa oratorius (Fabricius) (Heteroptera: Alydidae). Jpn. Int. Res. Cent. Agric. Sci. J. 1995, 2, 13–17. [Google Scholar]
- Suzuki, Y. The point of insect pest control in rice. Jpn. Agric. Technol. 2001, 45, 24–27. [Google Scholar]
- Takeuchi, H.; Watanabe, T. Mortality factors of eggs of Leptocorisa chinensis (Hemiptera: Alydidae) in rice fields. J. Econ. Entomol. 2006, 99, 366–372. [Google Scholar] [CrossRef]
- KOSIS (Korean Statistical Information System). Paddy Rice Production by City and County. Available online: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1ET0034&conn_path=I2 (accessed on 21 June 2021).
- Statistics Japan. Prefecture Comparisons of Rice Production. Available online: https://stats-japan.com/t/kiji/10665 (accessed on 21 June 2021).
- Huang, S.; Wang, L.; Liu, L.; Fu, Q.; Zhu, D. Nonchemical pest control in China rice: A review. Agron. Sustain. Dev. 2014, 34, 275–291. [Google Scholar] [CrossRef]
- Hoesain, M.; Prastowo, S.; Suharto, S.; Pradana, A.P.; Asyiah, I.N.; Alfarizy, F.K.; Adiwena, M. Combination of plant growth-promoting bacteria and botanical pesticide increases organic red rice yield and reduces the Leptocorisa acuta population. Biodivers. J. Biol. Divers. 2021, 22, 1686–1694. [Google Scholar] [CrossRef]
- Kay, I.R.; Brown, J.D.; Mayer, R.J. Insecticidal control of Eysarcoris trimaculatus (Distant) (Heteroptera: Pentatomidae) and Leptocorisa acuta (Thunberg) (heteroptera: Alydidae) on rice in north Queensland, Australia. Crop Prot. 1993, 12, 310–314. [Google Scholar] [CrossRef]
- Lee, J.G.; Hong, S.S.; Kim, J.Y.; Park, K.Y.; Lim, J.W.; Lee, J.H. Occurrence of stink bugs and pecky rice damage by stink bugs in paddy fields in Gyeonggi-do, Korea. Korean J. Appl. Entomol. 2009, 48, 37–44. [Google Scholar] [CrossRef][Green Version]
- Maulina, F. Parasitoid as a biological control agent of rice bug (Leptocorisa oratorius Fabricius): Effort Towards Food Security. In Proceedings of the 3rd International conference on Ssecurity in Food, Renewable Resources, and Natural Medicines, Sumatra Barat, Indonesia, 25–26 September 2019; p. 20. [Google Scholar]
- Palayukan, P.A.; Sjam, S.; Rosmana, A.; Dewi, V.S. Application of the combination of Calontropis gigantea L. and Crescentia cujete L. against Schirpophaga innotata and Leptocorisa acuta Thunb. and predator in paddy plants. IOP Conf. Ser. Earth Environ. Sci. 2021, 807, 022087. [Google Scholar] [CrossRef]
- Sankar, S.H.; Rani, O.R. Pathogenicity and field efficacy of the entomopathogenic fungus, Lecanicillium saksenae Kushwaha, Kurihara and Sukarno in the management of rice bug, Leptocorisa acuta Thunberg. J. Biol. Control 2018, 32, 230–238. [Google Scholar] [CrossRef]
- Sugimoto, A.; Nugaliyadde, L. Relation between population density of the rice bug Leptocorisa oratorius Fabricius (Heteroptera: Alydidae), and damage of rice grains. Jpn. Int. Res. Cent. Agric. Sci. J. 1995, 2, 59–64. [Google Scholar]
- Sugiura, K.; Oi, T.; Tanaka, T.; Hamagashira, A.; Ouk, R.; Nakamura, M.; Ide, Y.; Tsuda, K.; Ito, A.; Yamauchi, A. Resistance factors of pecky rice incidence caused by the rice stink bugs (Leptocorisa chinensis, Nezara viridula) in rice line CRR-99-95W. Plant Prod. Sci. 2022, 25, 172–182. [Google Scholar] [CrossRef]


| Variable Code a | Description | Percentage Contribution | ||
|---|---|---|---|---|
| L. chinensis | L. acuta | L. oratoria | ||
| Bio2 | Mean diurnal range b | - | 1.9 | - |
| Bio3 | Isothermality c | 18.8 | 4.8 | - |
| Bio5 | Maximum temperature of the warmest month | 0 | 1.7 | - |
| Bio6 | Minimum temperature of the coldest month | 30.1 | 24.8 | 38.5 |
| Bio7 | Temperature annual range (Bio5–Bio6) | - | - | 6.5 |
| Bio8 | Mean temperature of the wettest quarter | 7.9 | 0.2 | - |
| Bio12 | Annual precipitation | 5 | - | - |
| Bio13 | Precipitation of wettest month | - | - | 39.9 |
| Bio17 | Precipitation of the driest quarter | 0 | 1.8 | 7 |
| Bio18 | Precipitation of the warmest quarter | 10.6 | 44.5 | 1.2 |
| Bio19 | Precipitation of the coldest quarter | - | 0.2 | 0.6 |
| Elevation | Altitude data | 3.3 | 6.9 | 0.4 |
| Land cover | Land covers with 20 classifications | 24.3 | 13.2 | 6.0 |
| Measure | L. chinensis | L. acuta | L. oratoria |
|---|---|---|---|
| Test AUC | 0.993 | 0.980 | 0.980 |
| TSS | 0.958 | 0.915 | 0.905 |
| OR10% | 0.160 | 0.170 | 0.187 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.H.; Kim, S.-H.; Yoon, S.; Jung, S.; Kim, D.H.; Lee, W.-H. Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model. Insects 2022, 13, 750. https://doi.org/10.3390/insects13080750
Hwang JH, Kim S-H, Yoon S, Jung S, Kim DH, Lee W-H. Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model. Insects. 2022; 13(8):750. https://doi.org/10.3390/insects13080750
Chicago/Turabian StyleHwang, Jeong Ho, Se-Hyun Kim, Sunhee Yoon, Sunghoon Jung, Dong Hee Kim, and Wang-Hee Lee. 2022. "Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model" Insects 13, no. 8: 750. https://doi.org/10.3390/insects13080750
APA StyleHwang, J. H., Kim, S.-H., Yoon, S., Jung, S., Kim, D. H., & Lee, W.-H. (2022). Evaluation of Spatial Distribution of Three Major Leptocorisa (Hemiptera: Alydidae) Pests Using MaxEnt Model. Insects, 13(8), 750. https://doi.org/10.3390/insects13080750






