Effect of Parental Age and Mating Status on Reproductive Performance of Orius laevigatus (Hemiptera: Anthocoridae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Experiments
2.3. Data Analysis
3. Results
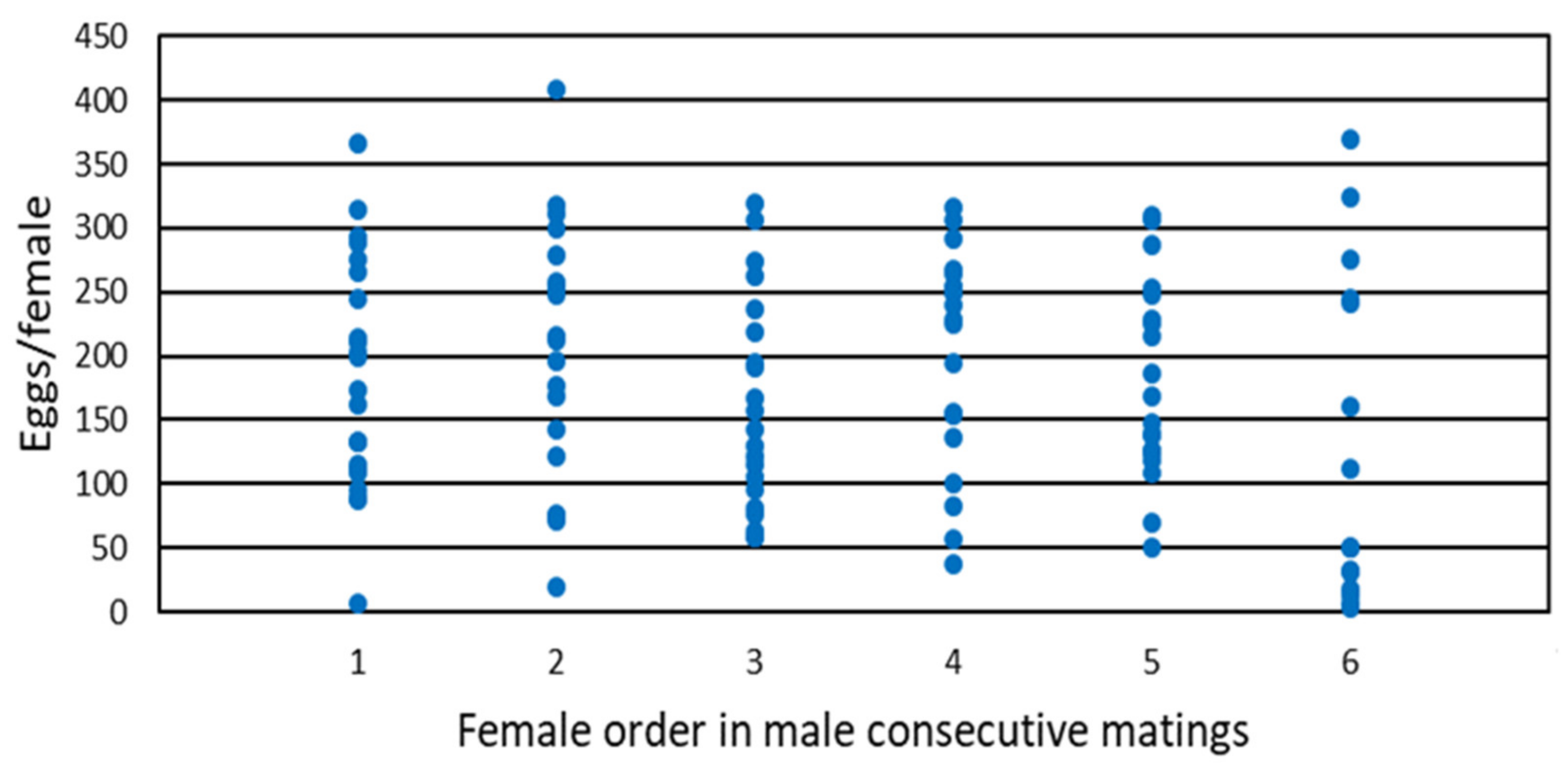
3.1. Multiple Mating Experiment
3.2. Delayed Mating
4. Discussion
4.1. Multiple Mating
4.2. Delayed Mating
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Bielza, P.; Balanza, V.; Cifuentes, D.; Mendoza, J.E. Challenges facing arthropod biological control, Identifying traits for genetic improvement of predators in protected crops. Pest Manag. Sci. 2020, 76, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C. Quality Control and Production of Biological Control Agents: Theory and Testing Procedures; C.A.B.I. Publishing: Wallingford, UK, 2003; 327p. [Google Scholar] [CrossRef]
- Tommasini, M.G.; van Lenteren, J.C.; Burgio, G. Biological traits and predation capacity of four Orius species on two prey species. Bull. Insectol. 2004, 57, 79–93. [Google Scholar]
- Bonte, M.; De Clercq, P. Developmental and reproductive fitness of Orius laevigatus (Hemiptera, Anthocoridae) reared on factitious and artificial diets. J. Econ. Entomol. 2008, 101, 1127–1133. [Google Scholar] [CrossRef]
- Mendoza, J.E.; Balanza, V.; Cifuentes, D.; Bielza, P. Genetic improvement of Orius laevigatus for better fitness feeding on pollen. J. Pest Sci. 2020, 94, 729–742. [Google Scholar] [CrossRef]
- Mendoza, J.E.; Balanza, V.; Cifuentes, D.; Bielza, P. Selection for larger body size in Orius laevigatus, Intraspecific variability and effects on reproductive parameters. Biol. Control 2020, 148, 104310. [Google Scholar] [CrossRef]
- Balanza, V.; Mendoza, J.E.; Bielza, P. Variation in susceptibility and selection for resistance to imidacloprid and thiamethoxam in Mediterranean populations of Orius laevigatus. Entomol. Exp. Appl. 2019, 167, 626–635. [Google Scholar] [CrossRef]
- Balanza, V.; Mendoza, J.E.; Cifuentes, D.; Bielza, P. Selection for resistance to pyrethroids in the predator Orius laevigatus. Pest Manag. Sci. 2021, 77, 2539–2546. [Google Scholar] [CrossRef]
- Balanza, V.; Mendoza, J.E.; Cifuentes, D.; Bielza, P. Genetic improvement of spinosad resistance in the biocontrol agent Orius laevigatus. BioControl 2021, 66, 673–685. [Google Scholar] [CrossRef]
- Leon-Beck, M.; Coll, M. The mating system of the flower bug Orius laevigatus. Biol. Control 2009, 50, 199–203. [Google Scholar] [CrossRef]
- Bonte, M.; De Clercq, P. Influence of male age and diet on reproductive potential of Orius laevigatus (Hemiptera: Anthocoridae). Ann. Entomol. Soc. Am. 2010, 103, 597–602. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Alcazar, A.; Lacasa, A.; Llamas, A.; Bielza, P. Integrated pest management strategies in sweet pepper plastic houses in the Southeast of Spain. IOBC/WPRS Bull. 2000, 23, 21–30. [Google Scholar]
- Vacacela Ajila, H.E.; Michaud, J.P.; Abdelwahab, A.H.; Kuchta, S.V.; Stowe, H.E. How efficient is fertilization by traumatic insemination in Orius insidiosus (Hemiptera: Anthocoridae)? J. Econ. Entomol. 2019, 112, 1618–1622. [Google Scholar] [CrossRef]
- Arakawa, T.; Taniai, K.; Maeda, T. The mating systems of three species of minute pirate bug, Orius sauteri, O. minutus, and O. strigicollis. Entomol. Exp. Appl. 2018, 167, 141–151. [Google Scholar] [CrossRef]
- Mendoza, J.E.; Balanza, V.; Rodríguez-Gómez, A.; Cifuentes, D.; Bielza, P. Enhanced biocontrol services in artificially selected strains of Orius laevigatus. J. Pest Sci 2022, in press, published online. [Google Scholar] [CrossRef]
- Musolin, D.L.; Tsytsulina, K.; Ito, K. Photoperiodic and temperature control of reproductive diapause induction in the predatory bug Orius strigicollis (Heteroptera: Anthocoridae) and its implications for biological control. Biol. Control 2004, 31, 91–98. [Google Scholar] [CrossRef]
- Musolin, D.L.; Ito, K. Photoperiodic and temperature control of nymphal development and induction of reproductive diapause in two predatory Orius bugs: Interspecific and geographic differences. Physiol. Entomol. 2008, 33, 291–301. [Google Scholar] [CrossRef]
- Lüpold, s.; Manier, M.K.; Ala-Honkola, O.; Belote, J.M.; Pitnick, S. Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity, and age. Behav. Ecol. 2011, 22, 184–191. [Google Scholar] [CrossRef]
- Gromko, M.H.; Newport, M.E.; Kortier, M.G. Sperm dependence of female receptivity to remating in Drosophila melanogaster. Evolution 1984, 38, 1237–1282. [Google Scholar] [CrossRef]
- Rankin, S.M.; TeBrugge, V.A.; Murray, J.A.; Schuler, A.M.; Tobe, S.S. Effects of selected neuropeptides, mating status and castration on male reproductive tract movements and immunolocalization of neuropeptides in earwigs. Comp. Biochem. Physiol. A 2009, 152, 83–90. [Google Scholar] [CrossRef]
- Boyd, D.W., Jr.; Alverson, D.R. Effects of temperature and water source on development of Deraeocoris nebulosus (Uhler) (Hemiptera: Miridae), a predacious plant bug. J. Entomol. Sci. 2004, 39, 202–213. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Rodríguez-Molina, M.C.; Stockel, J. Delayed mating reduces reproductive output of female European grapevine moth, Lobesia botrana (Lepidoptera: Tortricidae). Bull. Entomol. Res. 2004, 92, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.P.; Shirk, P.D. Ovarian Development in Predacious Orius pumilo: Relationship to Diet, Mating, and Juvenile Hormone. Ann. Entomol. Soc. Am. 2010, 103, 971–978. [Google Scholar] [CrossRef]
- Price, D.K.; Hansen, T.F. How does offspring quality change with age in male Drosophila melanogaster? Behav. Genet. 1998, 28, 395–402. [Google Scholar] [CrossRef]
- Jones, T.M.; Balmford, A.; Quinnell, R.J. Adaptative female choice for middle-aged mates in a lekking sandfly. Proc. R. Soc. Lond. B. 2000, 267, 681–686. [Google Scholar] [CrossRef]
- Jones, T.M.; Edgar, M.A. The role of male age sperm age and mating history on fecundity and fertilization success in the hide beetle. Proc. R. Lond. B Biol. 2004, 271, 1311–1318. [Google Scholar] [CrossRef]
- Thornhill, R.; Alcock, J. The Evolution of Insect Mating Systems; Harvard University Press: Cambridge, MA, USA, 1983; 547p. [Google Scholar] [CrossRef]
- Simmons, L.W.; Kotiaho, J.S. The effects of reproduction on courtship, fertility and longevity within and between alternative male mating tactics of the horned beetle, Onthophagus binodis. J. Evol. Biol. 2007, 20, 488–495. [Google Scholar] [CrossRef]
| Female Order | Pre-Mating Time (s) | Mating Time (s) | Total Fecundity (Eggs/Female) | First 10-Day Fecundity (Eggs/Female) | Fertility (Eggs Hatched) | Female Longevity (Days) |
|---|---|---|---|---|---|---|
| 1 | 4.8 ± 0.6 b | 270.0 ± 9.9 a | 185.9 ± 19.4 a | 58.4 ± 3.4 ab | 0.90 ±0.01 a | 47.± 5.3 bc |
| 2 | 9.4 ± 3.7 b | 184.3 ± 12.6 b | 202.6 ± 23.3 a | 65.2 ± 4.2 a | 0.89 ± 0.02 a | 47.7 ± 5.0 bc |
| 3 | 20.0 ± 4.9 b | 165.6 ± 12.7 bc | 160.7 ± 18.1 a | 59.0 ± 2.5 ab | 0.88 ± 0.02 a | 38.9 ± 4.7 c |
| 4 | 28.4 ± 17.9 b | 142.9 ± 12.9 cd | 203.2 ± 18.7 a | 60.5 ± 3.7 ab | 0.85 ± 0.03 a | 51.0 ± 6.2 abc |
| 5 | 67.8 ± 32.3 a | 130.9 ± 13.5 d | 178.8 ± 17.1 a | 50.9 ± 2.7 b | 0.89 ± 0.02 a | 57.6 ± 5.5 ab |
| 6 | 54.1 ± 25.5 ab | 115.7 ± 9.9 d | 128.9 ± 33.2 a | 39.1 ± 6.2 c | 0.91 ± 0.02 a | 64.2 ± 6.8 a |
| Female Age (Days) | Pre-Mating Time (s) | Mating Time (s) | Total Fecundity (Eggs/Female) | First 10-Day Fecundity (Eggs/Female) | Fertility (Eggs Hatched) | Female Longevity (Days) |
|---|---|---|---|---|---|---|
| 0 | 4.8 ± 0.6 b | 270.0 ± 9.9 a | 185.9 ± 19.4 a | 58.4 ± 3.4 a | 0.90 ± 0.01 b | 47.1 ± 5.3 b |
| 7 | 21.8 ± 7.6 a | 184.7 ± 18.0 b | 156.4 ± 17.5 a | 46.3 ± 4.3 b | 0.91 ± 0.02 ab | 59.8 ± 4.4 a |
| 14 | 16.6 ± 4.3 a | 198.6 ± 22.2 b | 175.8 ± 16.1 a | 50.3 ± 3.3 b | 0.93 ± 0.01 a | 60.8 ± 3.8 a |
| Male Age (Days) | Pre-Mating Time (s) | Mating Time (s) | Total Fecundity (Eggs/Female) | First 10-Day Fecundity (Eggs/Female) | Fertility (Eggs Hatched) | Female Longevity (Days) |
|---|---|---|---|---|---|---|
| 0 | 4.8 ± 0.6 a | 270.0 ± 9.9 b | 185.9 ± 19.4 b | 58.4 ± 3.4 b | 0.90 ± 0.01 a | 47.1 ± 5.3 a |
| 7 | 7.4 ± 1.3 a | 304.3 ± 12.8 a | 222.1 ± 12.6 a | 60.8 ± 2.0 a | 0.89 ± 0.01 ab | 43.9 ± 4.0 ab |
| 14 | 9.5 ± 2.5 a | 354.5 ± 27.4 a | 202.3 ± 27.4 ab | 68.6 ± 6.6 a | 0.86 ± 0.03 b | 39.8 ± 5.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Gómez, A.; Balanza, V.; Donate, A.; Abelaira, A.B.; Reche, M.d.C.; Sánchez-Martínez, I.; Bielza, P. Effect of Parental Age and Mating Status on Reproductive Performance of Orius laevigatus (Hemiptera: Anthocoridae). Insects 2022, 13, 827. https://doi.org/10.3390/insects13090827
Rodríguez-Gómez A, Balanza V, Donate A, Abelaira AB, Reche MdC, Sánchez-Martínez I, Bielza P. Effect of Parental Age and Mating Status on Reproductive Performance of Orius laevigatus (Hemiptera: Anthocoridae). Insects. 2022; 13(9):827. https://doi.org/10.3390/insects13090827
Chicago/Turabian StyleRodríguez-Gómez, Amador, Virginia Balanza, Alberto Donate, Ana Belén Abelaira, María del Carmen Reche, Isabel Sánchez-Martínez, and Pablo Bielza. 2022. "Effect of Parental Age and Mating Status on Reproductive Performance of Orius laevigatus (Hemiptera: Anthocoridae)" Insects 13, no. 9: 827. https://doi.org/10.3390/insects13090827
APA StyleRodríguez-Gómez, A., Balanza, V., Donate, A., Abelaira, A. B., Reche, M. d. C., Sánchez-Martínez, I., & Bielza, P. (2022). Effect of Parental Age and Mating Status on Reproductive Performance of Orius laevigatus (Hemiptera: Anthocoridae). Insects, 13(9), 827. https://doi.org/10.3390/insects13090827









