The Influence of Host Aphids on the Performance of Aphelinus asychis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Effects of Aphid Species on Some Life-History Traits of A. asychis
2.3. Effects of Aphid Species on Parasitism of A. asychis on M. pericae
2.4. The Starvation and Thermal Tolerance of A. asychis
2.5. The Body Size Plasticity Analysis of A. asychis
2.6. Statistical Analysis
3. Results
3.1. Effects of Aphid Species on Life-History Parameters of A. asychis
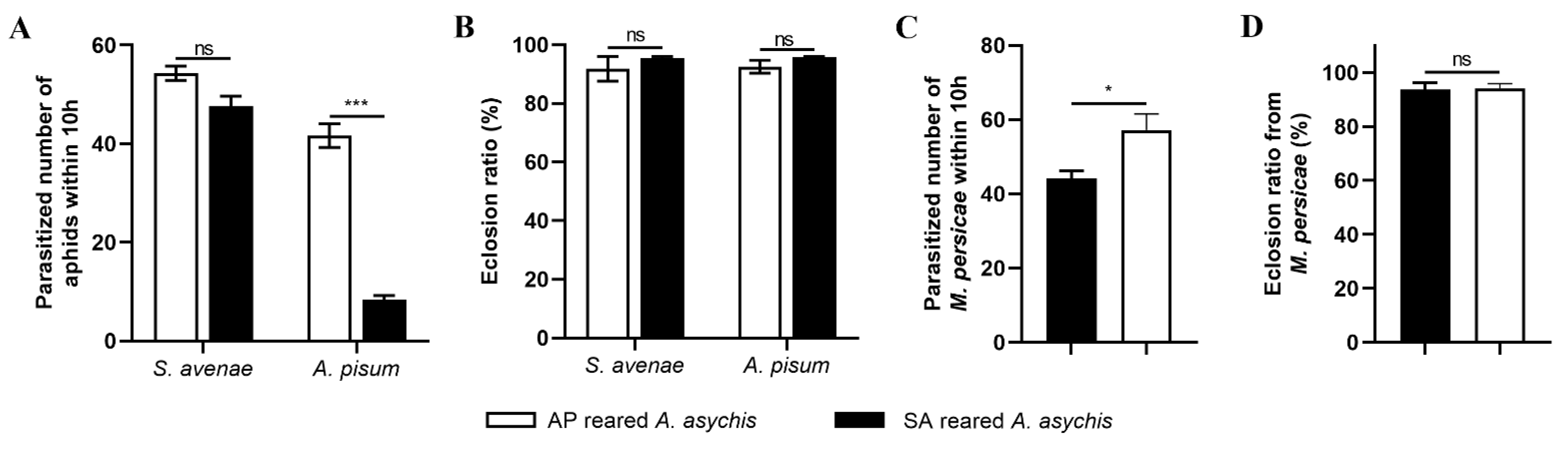
3.2. Effects of Aphid Species on Parasitic Capacity of A. asychis
3.3. Effects of Aphid Species on the Starvation and Thermal Tolerance of A. asychis
3.4. The Body Size Plasticity of A. asychis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Emden, H.F.; Harrington, R. Aphids as Crop Pests; CAB Press: Wallingford, UK, 2007. [Google Scholar]
- Mathers, T.C.; Chen, Y.Z.; Kaithakottil, G.; Legeai, F.; Mugford, S.T.; Baa-Puyoulet, P.; Bretaudeau, A.; Clavijo, B.; Colella, S.; Collin, O.; et al. Rapid transcriptional plasticity of duplicated gene clusters enables a clonally reproducing aphid to colonise diverse plant species. Genome Biol. 2017, 18, 27. [Google Scholar] [PubMed]
- Liu, W.C.; Liu, Z.D.; Huang, C.; Lu, M.H.; Liu, J.; Yang, Q.P. Statistics and analysis of crop yield losses caused by main diseases and insect pests in recent 10 years. Plant Prot. 2016, 42, 1–9. [Google Scholar]
- Bass, C.; Puinean, A.M.; Zimmer, C.T.; Denholm, I.; Field, L.M.; Foster, S.P.; Gutbros, O.; Nauen, R.; Slater, R.; Williamson, M.S. The evolution of insecticide resistance in the peach potato aphid, Myzus persicae. Insect Biochem. Mol. Biol. 2014, 51, 41–51. [Google Scholar] [PubMed]
- Voudouris, C.C.; Williaamson, M.S.; Skouras, P.J.; Kati, A.N.; Sahinoglou, A.J.; Margaritopoulos, J.T. Evolution of imidacloprid resistance in Myzus persicae in Greece and susceptibility data for spirotetramat. Pest Manag. Sci. 2017, 73, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.C.; Peccoud, J. Rapid evolution of aphid pests in agricultural environments. Curr. Opin. Insect Sci. 2018, 26, 17–24. [Google Scholar]
- Kapoor, U.; Srivastava, M.K.; Srivastava, L.P. Toxicological impact of technical imidacloprid on ovarian morphology, hormones and antioxidant enzymes in female rats. Food Chem. Toxicol. 2011, 49, 3086–3089. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar]
- Braak, N.; Neve, R.; Jones, A.K.; Gibbs, M.; Breuker, C.J. The effects of insecticides on butterflies—A review. Environ. Pollut. 2018, 242, 507–518. [Google Scholar]
- Kang, Z.W.; Liu, F.H.; Pang, R.P.; Tian, H.G.; Liu, T.X. Effect of sublethal doses of imidacloprid on the biological performance of aphid endoparasitoid Aphidius gifuensis (Hymenoptera: Aphidiidae) and influence on its related gene expression. Front. Physiol. 2018, 9, 1729. [Google Scholar]
- Samidurai, J.; Subramanian, M.; Venugopal, D. Levels of organochlorine pesticide residues in fresh water fishes of three bird sanctuaries in Tamil Nadu, India. Environ. Sci. Pollut. Res. Int. 2019, 26, 1983–1993. [Google Scholar]
- Boivin, G.; Hance, T.; Brodeur, J. Aphid parasitoids in biological control. Can. J. Plant Sci. 2012, 92, 1–12. [Google Scholar] [CrossRef]
- Powell, W.; Pickett, J.A. Manipulation of parasitoids for aphid pest management: Progress and prospects. Pest Manag. Sci. 2003, 59, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.J.; Elliott, E.C. Biological control of cereal aphids in north America and mediating effects of host plant and habitat manipulations. Annu. Rev. Entomol. 2004, 49, 219–242. [Google Scholar] [CrossRef]
- Zang, L.S.; Wang, S.; Zhang, F.; Deesneux, N. Biological control with Trichogramma in China: History, present status and perspectives. Annu. Rev. Entomol. 2021, 66, 24.1–24.22. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; Zhou, S.C.; Wang, Y.; Shi, M.; Chen, X.X.; Huang, J.H. Biocontrol characteristics of the fruit fly pupal parasitoid Trichopria drosophilae (Hymenoptera: Diapriidae) emerging from different hosts. Sci. Rep. 2018, 8, 13323. [Google Scholar] [CrossRef]
- Boycheva Woltering, S.; Romeis, J.; Collatz, J. Influence of the rearing host on biological parameters of Trichopria drosophilae, a potential biological control agent of Drosophila suzukii. Insects 2019, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.K.; Tang, Y.L.; Wei, K.; Wang, X.Y.; Yang, Z.Q.; Zhang, Y.L. Relationships between body size and parasitic fitness and offspring performance of Sclerodermus pupariae Yang et Yao (Hymenoptera: Bethylidae). PLoS ONE 2016, 11, e0156831. [Google Scholar] [CrossRef]
- Pan, M.Z.; Cao, H.H.; Liu, T.X. Effects of winter wheat cultivars on the life history traits and olfactory response of Aphidius gifuensis. BioControl 2014, 59, 539–546. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chi, H.; Liu, T.X. Demography and parasitic effectiveness of Aphelinus asychis reared from Sitobion avenae as a biological control agent of Myzus persicae reared on chili pepper and cabbage. Biol. Control 2016, 92, 111–119. [Google Scholar] [CrossRef]
- Shirley, X.A.; Woolley, J.B.; Hopper, K.R. Revision of the asychis species group of Aphelinus (Hymenoptera: Aphelinidae). J. Hymenopt. Res. 2017, 54, 1–32. [Google Scholar] [CrossRef]
- Brewer, M.J.; Nelson, D.J.; Ahern, R.G.; Donahue, J.D.; Prokrym, D.R. Recovery and range expansion of parasitoids (Hymenoptera: Aphelinidae and Braconidae) released for biological control of Diuraphis noxia (Homoptera: Aphididae) in Wyoming. Environ. Entomol. 2001, 30, 578–588. [Google Scholar] [CrossRef]
- Michels, G.J.; Whitaker-Deerberg, R.L. Recovery of Aphelinus asychis, an imported parasitoid of Russian wheat aphid, in the Texas panhandle. Southwest. Entomol. 1993, 18, 11–17. [Google Scholar]
- Cate, R.H.; Archer, T.L.; Eikenbary, R.D.; Starks, K.J.; Morrison, R.D. Parasitization of the greenbug by Aphelinus asychis and the effect of feeding by the parasitoid on aphid mortality. Environ. Entomol. 1973, 2, 549–554. [Google Scholar] [CrossRef]
- Jia, Y.J.; Wang, B.; Liu, T.X. Unsuccessful host stinging by Aphelinus asychis (Hymenoptera: Aphelinidae) impacts population parameters of the pea aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2020, 113, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Gerling, D.; Roitberg, B.D.; Mackauer, M. Instar-specific defense of the pea aphid, Acyrthosiphon pisum: Influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera: Aphelinidae). J. Insect Behav. 1990, 3, 501–514. [Google Scholar] [CrossRef]
- Liu, X.; Kang, Z.W.; Yu, X.L.; Li, F.; Liu, T.X.; Li, Q. Role of TRP channels and HSPs in thermal stress response in the aphid parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae). J. Integr. Agric. 2020, 19, 1530–1542. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Z.X.; Kang, Z.W.; Li, H.; Liu, T.X.; Wang, D. Identification and characterization of antioxidant enzyme genes in parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae) and expression profiling analysis under temperature stress. Insects 2022, 13, 447. [Google Scholar] [CrossRef]
- López-Arriaga, F.; Gordillo, V.; Cancino, J.; Montoya, P. Irradiation of early immature Anastrepha ludens stages for the rearing of Doryctobracon areolatus (Hymenoptera: Braconidae), a fruit fly parasitoid. Bull. Entomol. Res. 2020, 110, 630–637. [Google Scholar] [CrossRef]
- Khanzada, M.S.; Wang, S.; Huang, N.X.; Pang, H.; Tan, X.L.; Khanzada, S.R. Optimization of microencapsulated artificial diets for mass rearing of the predacious big eyed bug. Geocoris pallidipennis. Entomol. Gen. 2019, 39, 353–363. [Google Scholar]
- Calvo, F.J.; Knapp, M.; van Houten, Y.M.; Hoogerbrugge, H.; Belda, J.E. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol. 2015, 65, 419–433. [Google Scholar] [CrossRef]
- Cohen, J.E.; Jonsson, T.; Muller, C.B.; Godfray, H.C.J.; Savage, V.M. Body sizes of hosts and parasitoids in individual feeding relationships. Proc. Natl. Acad. Sci. USA 2005, 102, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Song, L.W.; Wen, X.Y.; Zang, L.S.; Ruan, C.C.; Shi, S.S.; Shao, X.W.; Zhang, F. Parasitism and suitability of different egg ages of the Leguminivora glycinivorella (Lepidoptera: Tortricidae) for three indigenous Trichogramma species. J. Econ. Entomol. 2015, 108, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.C.; Wang, Z.C.; Wang, G.R.; Zhong, L.Q.; Zheng, X.S.; Xu, H.X.; Zang, L.S.; Lu, Z.X. The effects of temperature and host age on the fecundity of four Trichogramma species, egg parasitoids of the Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2017, 110, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, J.Y.; Chen, Y.S.; Wang, Q.Z.; Pan, Y.L.; Zhang, J.T.; Liu, X.P. A comparison of fitness-related traits in the coleopteran parasitoid Dastarcus helophoroides (coleoptera: Bothrideridae) reared on two factitious hosts. J. Econ. Entomol. 2020, 13, 2634–2640. [Google Scholar] [CrossRef]
- Ameri, M.; Rasekh, A.; Michaud, J.P. Body size affects host defensive behavior and progeny fitness in a parasitoid wasp, Lysiphlebus fabarum. Entomol. Exp. Appl. 2014, 150, 259–268. [Google Scholar] [CrossRef]
- Vieira, L.J.P.; Franco, G.M.; Sampaio, M.V. Host Preference and fitness of Lysiphlebus testaceipes (Hymenoptera: Braconidae) in different instars of the aphid Schizaphis graminum. Neotrop. Entomol. 2019, 48, 391–398. [Google Scholar] [CrossRef]
- Henry, L.; Roitberg, B.D.; Gillespie, D.R. Host-range evolution in Aphidius parasitoids: Fidelity, virulence and fitness trade-offs on an ancestral host. Evolution 2008, 62, 689–699. [Google Scholar] [CrossRef]
- Wang, X.G.; Kacar, G.; Biondi, A.; Daane, K.M. Life-history and host preference of Trichopria drosophilae, a pupal parasitoid of spotted wing. Drosophila. BioControl 2016, 61, 387–397. [Google Scholar] [CrossRef]
- Sagarra, L.A.; Vincent, C.; Stewart, R.K. Body size as an indicator of parasitoid quality in male and female Anagyrus kamali (Hymenoptera: Encyrtidae). Bull. Entomol. Res. 2001, 91, 363–367. [Google Scholar] [CrossRef]
- Jervis, M.A.; Ferns, P.N.; Heimpel, G.E. Body size and the timing of egg production in parasitoid wasps: A comparative analysis. Oikos 2003, 102, 164–172. [Google Scholar] [CrossRef]
- Dai, P.; Ruan, C.C.; Zang, L.S.; Wan, F.H.; Liu, L.Z. Effects of rearing host species on the host-feeding capacity and parasitism of the whitefly parasitoid Encarsia formosa. J. Insect Sci. 2014, 14, 118. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.Z.; Liu, T.X. Suitability of three aphid species for Aphidius gifuensis (Hymenoptera: Braconidae): Parasitoid performance varies with hosts of origin. Biol. Control 2014, 69, 90–96. [Google Scholar] [CrossRef]
- He, Y.Y.; Liu, Y.C.; Wang, K.; Zhang, Y.J.; Wu, Q.J.; Wang, S.L. Development and fitness of the parasitoid, Encarsia formosa (Hymenoptera: Aphelinidae), on the B and Q of the sweetpotato whitefly (Hemiptera: Aleyrodidae). J. Econ. Entomol. 2019, 112, 2597–2603. [Google Scholar] [CrossRef]
- Albittar, L.; Ismail, M.; Bragard, C.; Hance, T. Host plants and aphid hosts influence the selection behaviour of three aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae). Eur. J. Entomol. 2016, 113, 516–522. [Google Scholar] [CrossRef]
- Hopkinson, J.; Zalucki, M.P.; Murray, D.A.H. Host selection and parasitism behavior of Lysiphlebus testaceipes: Role of plant, aphid species and instar. Biol. Control 2013, 64, 283–290. [Google Scholar] [CrossRef]
- Ali, M.Y.; Lu, Z.Z.; Ali, A.; Amir, M.B.; Ahmed, M.A.; Shahid, S.; Liu, T.X.; Pan, M.Z. Effects of plant-mediated differences in aphid size on suitability of its parasitoid, Aphelinus varipes (Hymenoptera: Aphelinidae). J. Econ. Entomol. 2022, 115, 74–80. [Google Scholar]
- Gergs, A.; Jager, T. Body size-mediated starvation resistance in an insect predator. J. Anim. Ecol. 2014, 83, 758–768. [Google Scholar] [CrossRef]
- Kang, Z.W.; Liu, F.H.; Liu, X.; Yu, W.B.; Tan, X.L.; Zhang, S.Z.; Tian, H.G.; Liu, T.X. The potential coordination of the heat-shock proteins and antioxidant enzyme genes of Aphidius gifuensis in response to thermal stress. Front. Physiol. 2017, 8, 976. [Google Scholar] [CrossRef]
- Ismail, M.; Vernon, P.; Hance, T.; Pierre, J.; van Baaren, J. What are the possible benefits of small size for energy-constrained ectotherms in cold stress conditions? Oikos 2012, 121, 2072–2080. [Google Scholar] [CrossRef]
- Kishani Farahani, H.; Ashouri, A.; Zibaee, A.; Abroon, P.; Alford, L. The effect of host nutritional quality on multiple components of Trichogramma brassicae fitness. Bull. Entomol. Res. 2016, 106, 633–641. [Google Scholar] [CrossRef]
- Kant, R.; Minor, M.A.; Trewick, S.A.; Sandanayaka, W.R.M. Body size and fitness relation in male and female Diaeretiella rapae. BioControl 2012, 57, 759–766. [Google Scholar] [CrossRef]
- López, O.P.; Hénaut, Y.; Cancino, J.; Lambin, M.; Cruz-López, L.; Rojas, J.C. Is host size an indicator of quality in the mass-reared parasitoid Diachasmimorpha longicaudata (Hymenoptera: Braconidae)? Fla. Entomol. 2009, 92, 441–449. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.; Ratkowsky, D.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- McCalla, K.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.; Hoddle, M.S. The influence of temperature variation on life history parameters and thermal performance curves of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the Asian citrus psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef] [PubMed]


| Host | Body Length (mm) | Hind Tibia Length (mm) | Developmental Times (Days) | Sex Ratio | ||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | |||
| A. pisum | 1.22 ± 0.02 a | 0.93 ± 0.02 a | 0.43 ± 0.01 a | 0.36 ± 0.01 a | 12.70 ± 0.14 b | 0.56 ± 0.06 a |
| S. avenae | 1.06 ± 0.02 b | 0.83 ± 0.02 b | 0.32 ± 0.01 b | 0.32 ± 0.01 b | 16.00 ± 0.15 a | 0.62 ± 0.04 a |
| Host Generation | Body Length (mm) | Hind Tibia Length (mm) | Developmental Time (Days) | ||
|---|---|---|---|---|---|
| Female | Male | Female | Male | ||
| F0 (Parental) | 1.06 ± 0.02 c | 0.83 ± 0.02 b | 0.32 ± 0.01 c | 0.32 ± 0.01 c | 16.00 ± 0.15 a |
| F1 | 1.13 ± 0.02 b | 0.82 ± 0.03 b | 0.39 ± 0.01 b | 0.28 ± 0.01 b | 12.03 ± 0.19 c |
| F2 | 1.15 ± 0.02 ab | 0.92 ± 0.03 ab | 0.41 ± 0.1 ab | 0.35 ± 0.01 a | 12.77 ± 0.10 b |
| >F10 * | 1.22 ± 0.02 a | 0.93 ± 0.02 a | 0.43 ± 0.01 a | 0.36 ± 0.01 a | 12.70 ± 0.14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-X.; Ji, M.-Q.; Zhang, C.; Yang, Y.-B.; Chen, Z.-Z.; Zhao, H.-P.; Xu, Y.-Y.; Kang, Z.-W. The Influence of Host Aphids on the Performance of Aphelinus asychis. Insects 2022, 13, 795. https://doi.org/10.3390/insects13090795
Li Z-X, Ji M-Q, Zhang C, Yang Y-B, Chen Z-Z, Zhao H-P, Xu Y-Y, Kang Z-W. The Influence of Host Aphids on the Performance of Aphelinus asychis. Insects. 2022; 13(9):795. https://doi.org/10.3390/insects13090795
Chicago/Turabian StyleLi, Zhen-Xiang, Meng-Qi Ji, Chi Zhang, Yi-Bing Yang, Zhen-Zhen Chen, Hai-Peng Zhao, Yong-Yu Xu, and Zhi-Wei Kang. 2022. "The Influence of Host Aphids on the Performance of Aphelinus asychis" Insects 13, no. 9: 795. https://doi.org/10.3390/insects13090795
APA StyleLi, Z.-X., Ji, M.-Q., Zhang, C., Yang, Y.-B., Chen, Z.-Z., Zhao, H.-P., Xu, Y.-Y., & Kang, Z.-W. (2022). The Influence of Host Aphids on the Performance of Aphelinus asychis. Insects, 13(9), 795. https://doi.org/10.3390/insects13090795





