Enemy-Risk Effects in Parasitoid-Exposed Diamondback Moth Larvae: Potential Mediation of the Interaction by Host Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Insects
2.2. Experimental Design
2.3. Statistical Analyses
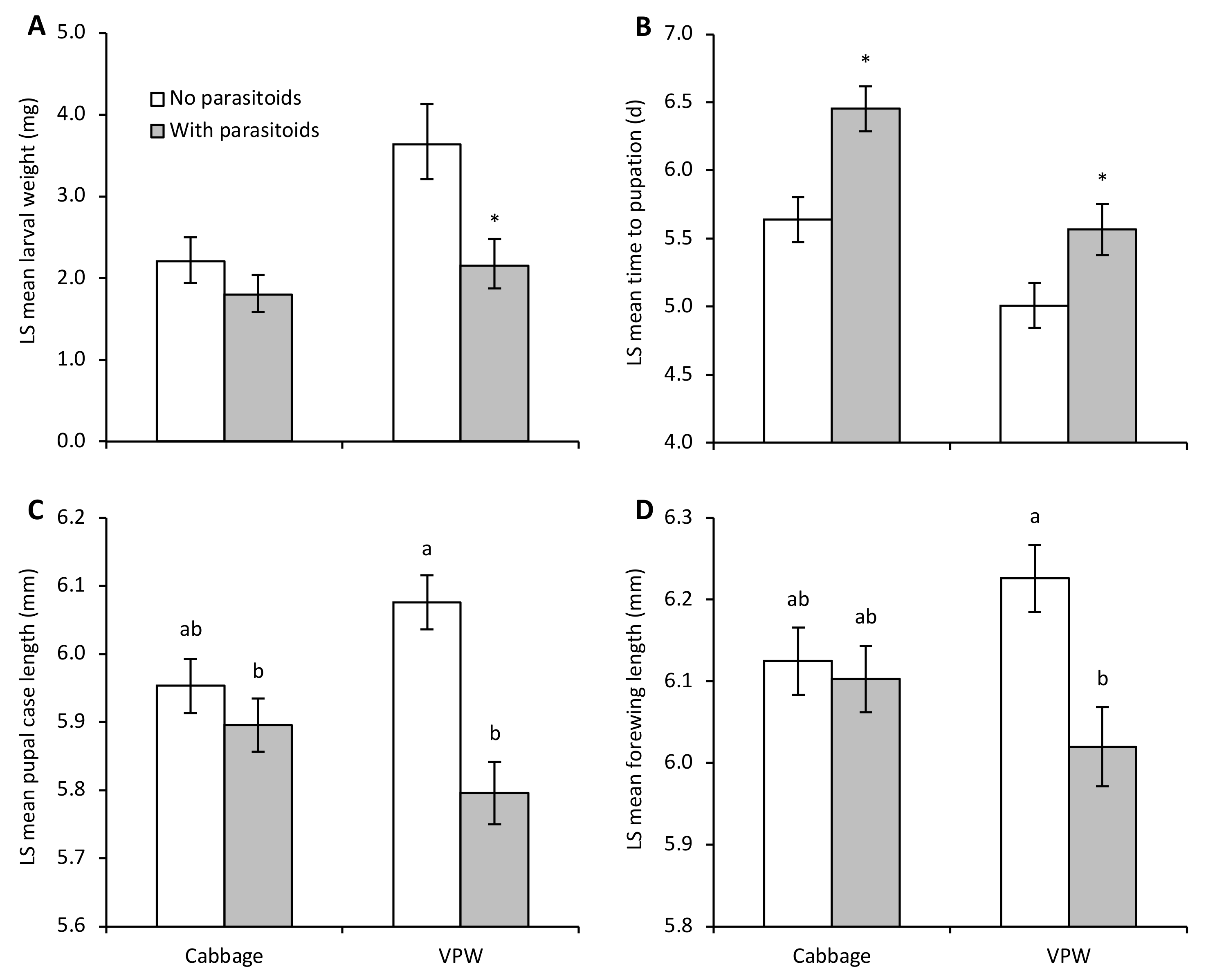
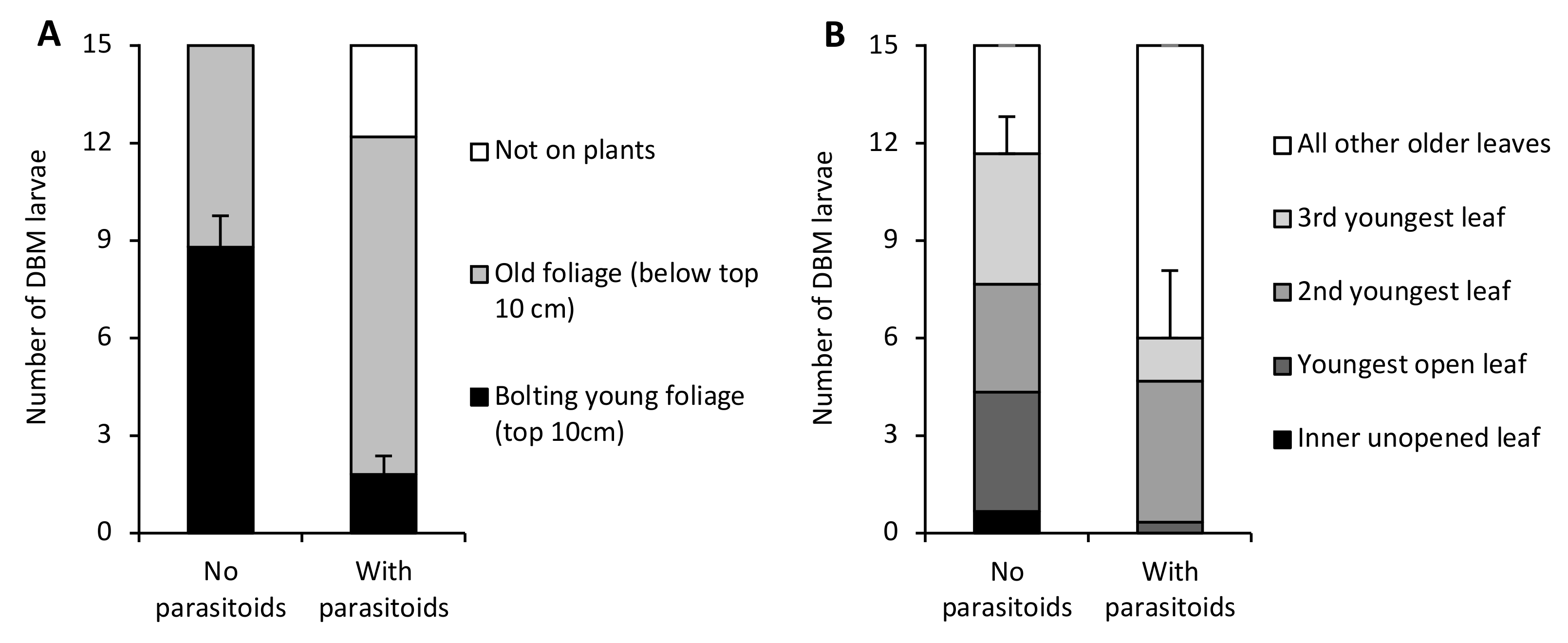
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmitz, O.J. Direct and indirect effects of predation and predation risk in old-field interaction webs. Am. Nat. 1998, 151, 327–342. [Google Scholar] [CrossRef]
- Hermann, S.L.; Landis, D.A. Scaling up our understanding of non-consumptive effects in insect systems. Curr. Opin. Insect Sci. 2017, 20, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.T.; Stahler, D.R.; Metz, M.C.; Forester, J.D.; Kauffman, M.J.; Varley, N.; White, P.J.; Smith, D.W.; MacNulty, D.R. Diel predator activity drives a dynamic landscape of fear. Ecol. Monogr. 2018, 88, 638–652. [Google Scholar] [CrossRef]
- Hoki, E.; Losey, J.; Ugine, T.A. Comparing the consumptive and non-consumptive effects of a native and introduced lady beetle on pea aphids (Acyrthosiphon pisum). Biol. Control 2014, 70, 78–84. [Google Scholar] [CrossRef]
- Culshaw-Maurer, M.; Sih, A.; Rosenheim, J.A. Bugs scaring bugs: Enemy-risk effects in biological control systems. Ecol. Lett. 2020, 23, 1693–1714. [Google Scholar] [CrossRef]
- Preisser, E.L.; Bolnick, D.I.; Benard, M.F. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 2005, 86, 501–509. [Google Scholar] [CrossRef]
- Beckerman, A.P.; Uriarte, M.; Schmitz, O.J. Experimental evidence for a behavior-mediated trophic cascade in a terrestrial food chain. Proc. Natl. Acad. Sci. USA 1997, 94, 10735–10738. [Google Scholar] [CrossRef]
- Schmitz, O.J. Top predator control of plant biodiversity and productivity in an old-field ecosystem. Ecol. Lett. 2003, 6, 156–163. [Google Scholar] [CrossRef]
- Vogelweith, F.; Moret, Y.; Thiery, D.; Moreau, J. Lobesia botrana larvae develop faster in the presence of parasitoids. PLoS ONE 2013, 8, e72568. [Google Scholar] [CrossRef]
- Minchella, D.J. Host life-history variation in response to parasitism. Parasitology 1985, 90, 205–216. [Google Scholar] [CrossRef]
- Sarfraz, M.; Keddie, A.B.; Dosdall, L.M. Biological control of the diamondback moth, Plutella xylostella: A review. Biocontrol Sci. Technol. 2005, 15, 763–789. [Google Scholar] [CrossRef]
- Wang, X.G.; Keller, M.A. A comparison of the host-searching efficiency of two larval parasitoids of Plutella xylostella. Ecol. Entomol. 2002, 27, 105–114. [Google Scholar] [CrossRef]
- Greeney, H.F.; Dyer, L.A.; Smilanich, A.M. Feeding by lepidopteran larvae is dangerous: A review of caterpillars’ chemical, physiological, morphological, and behavioral defenses against natural enemies. Invertebr. Surviv. J. 2012, 9, 7–34. [Google Scholar]
- Kim, Y.; Park, C.; No, S.; Yoo, S.; Suh, S.S.; Kang, C. Hanging by a thread: Post-attack defense of caterpillars. J. Asia-Pac. Entomol. 2022, 25, 101893. [Google Scholar] [CrossRef]
- Pugh, M.; Kihata, N.; Uyeda, J.; Wang, K.H.; Shikano, I. The effects of a naturalized weed, Lepidium virginicum, on the development and behaviors of the diamondback moth and its natural enemies in Hawaii. Biol. Control 2022, 173, 104994. [Google Scholar] [CrossRef]
- Begum, S.; Tsukuda, R.; Fujisaki, K.; Nakasuji, F. The effects of wild cruciferous host plants on morphology, reproductive performance and flight activity in the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Pop. Ecol. 1996, 38, 257–263. [Google Scholar] [CrossRef]
- Moreira, L.F.; Teixeira, N.C.; Santos, N.A.; Valim, J.O.S.; Maurício, R.M.; Guedes, R.N.C.; Oliveira, M.G.A.; Campos, W.G. Diamondback moth performance and preference for leaves of Brassica oleracea of different ages and strata. J. Appl. Entomol. 2016, 140, 627–635. [Google Scholar] [CrossRef]
- Kirschman, L.J.; Quade, A.H.; Zera, A.J.; Warne, R.W. Immune function trade-offs in response to parasite threats. J. Insect Physiol. 2017, 98, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Cotter, S.C.; Reeson, A.F.; Pell, J.K. Melanism and disease resistance in insects. Ecol. Lett. 2001, 4, 637–649. [Google Scholar] [CrossRef]
- Wilson, K.; Thomas, M.B.; Blanford, S.; Doggett, M.; Simpson, S.J.; Moore, S.L. Coping with crowds: Density-dependent disease resistance in desert locusts. Proc. Natl. Acad. Sci. USA 2002, 99, 5471–5475. [Google Scholar] [CrossRef]
- Shikano, I. Evolutionary ecology of multitrophic interactions between plants, insect herbivores and entomopathogens. J. Chem. Ecol. 2017, 43, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Karlsson Green, K. The effects of host plant species and larval density on immune function in the polyphagous moth Spodoptera littoralis. Ecol. Evol. 2021, 11, 10090–10097. [Google Scholar] [CrossRef]
- Thaler, J.S.; Griffin, C.A. Relative importance of consumptive and non-consumptive effects of predators on prey and plant damage: The influence of herbivore ontogeny. Entomol. Exp. Et Appl. 2008, 128, 34–40. [Google Scholar] [CrossRef]
- Jandricic, S.E.; Schmidt, D.; Bryant, G.; Frank, S.D. Non-consumptive predator effects on a primary greenhouse pest: Predatory mite harassment reduces western flower thrips abundance and plant damage. Biol. Control. 2016, 95, 5–12. [Google Scholar] [CrossRef]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar] [CrossRef]
- Shikano, I.; Oak, M.C.; Halpert-Scanderbeg, O.; Cory, J.S. Trade-offs between transgenerational transfer of nutritional stress tolerance and immune priming. Funct. Ecol. 2015, 29, 1156–1164. [Google Scholar] [CrossRef]
- Triggs, A.M.; Knell, R.J. Parental diet has strong transgenerational effects on offspring immunity. Funct. Ecol. 2012, 26, 1409–1417. [Google Scholar] [CrossRef]
- Behmer, S.T. Insect herbivore nutrient regulation. Annu. Rev. Entomol. 2009, 54, 165–187. [Google Scholar] [CrossRef]
- Ingerslew, K.S.; Finke, D.L. Mechanisms underlying the nonconsumptive effects of parasitoid wasps on aphids. Environ. Entomol. 2017, 46, 75–83. [Google Scholar] [CrossRef]
- Höller, C.; Micha, S.G.; Schulz, S.; Francke, W.; Pickett, J.A. Enemy-induced dispersal in a parasitic wasp. Experientia 1994, 50, 182–185. [Google Scholar] [CrossRef]
- Kaplan, I.; McArt, S.H.; Thaler, J.S. Plant defenses and predation risk differentially shape patterns of consumption, growth, and digestive efficiency in a guild of leaf-chewing insects. PLoS ONE 2014, 9, e93714. [Google Scholar] [CrossRef] [Green Version]
- Thaler, J.S.; Contreras, H.; Davidowitz, G. Effects of predation risk and plant resistance on Manduca sexta caterpillar feeding behaviour and physiology. Ecol. Entomol. 2014, 39, 210–216. [Google Scholar] [CrossRef]
- Rabus, M.; Laforsch, C. Growing large and bulky in the presence of the enemy: Daphnia magna gradually switches the mode of inducible morphological defences. Funct. Ecol. 2011, 25, 1137–1143. [Google Scholar] [CrossRef]
- Venkanna, Y.; Suroshe, S.S.; Dahuja, A. Non-consumptive effects of the zigzag ladybird beetle, Cheilomenes sexmaculata (Fab.) on its prey, the cotton aphid, Aphis gossypii Glover. Biocontrol Sci. Technol. 2021, 31, 1204–1219. [Google Scholar] [CrossRef]
- Sloggett, J.J.; Weisser, W.W. Parasitoids induce production of the dispersal morph of the pea aphid, Acyrthosiphon pisum. Oikos 2002, 98, 323–333. [Google Scholar] [CrossRef]
- Muhamad, O.; Tsukuda, R.; Oki, Y.; Fujisaki, K.; Nakasuji, F. Influences of wild crucifers on life history traits and flight ability of the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Res. Popul. Ecol. 1994, 36, 53–62. [Google Scholar] [CrossRef]
- Roff, D.A.; Fairbairn, D.J. Wing dimorphisms and the evolution of migratory polymorphisms among the Insecta. Am. Zool. 1991, 31, 243–251. [Google Scholar] [CrossRef]
- Gingras, D.; Dutilleul, P.; Boivin, G. Modeling the impact of plant structure on host-finding behavior of parasitoids. Oecologia 2002, 130, 396–402. [Google Scholar] [CrossRef]
- Andow, D.A.; Prokrym, D.R. Plant structural complexity and host-finding by a parasitoid. Oecologia 1990, 82, 162–165. [Google Scholar] [CrossRef]
- Bukovinszky, T.; Gols, R.; Posthumus, M.A.; Vet, L.E.M.; Van Lenteren, J.C. Variation in plant volatiles and attraction of the parasitoid Diadegma semiclausum (Hellén). J. Chem. Ecol. 2005, 31, 461–480. [Google Scholar] [CrossRef]
- Fox, L.R.; Eisenbach, J. Contrary choices: Possible exploitation of enemy-free space by herbivorous insects in cultivated vs. wild crucifers. Oecologia 1992, 89, 574–579. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Hu, G.; Johanowicz, D. Management of diamondback moth (Lepidoptera: Plutellidae) in cabbage using collard as a trap crop. HortScience 2000, 35, 875–879. [Google Scholar] [CrossRef]
- Vinson, S.B. The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 1998, 11, 79–96. [Google Scholar] [CrossRef]
- Broadway, R.M.; Colvin, A.A. Influence of cabbage proteinase inhibitors in situ on the growth of larval Trichoplusia ni and Pieris rapae. J. Chem. Ecol. 1992, 18, 1009–1024. [Google Scholar] [CrossRef]
- Agrawal, A.A. Benefits and costs of induced plant defense for Lepidium virginicum (Brassicaceae). Ecology 2000, 81, 1804–1813. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Giunti, G.; Canale, A.; Messing, R.H.; Donati, E.; Stefanini, C.; Michaud, J.P.; Benelli, G. Parasitoid learning: Current knowledge and implications for biological control. Biol. Control 2015, 90, 208–219. [Google Scholar] [CrossRef]
- Wang, X.G.; Duff, J.; Keller, M.A.; Zalucki, M.P.; Liu, S.S.; Bailey, P. Role of Diadegma semiclausum (Hymenoptera: Ichneumonidae) in controlling Plutella xylostella (Lepidoptera: Plutellidae): Cage exclusion experiments and direct observation. Biocontrol Sci. Technol. 2004, 14, 571–586. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kihata, N.; Shikano, I. Enemy-Risk Effects in Parasitoid-Exposed Diamondback Moth Larvae: Potential Mediation of the Interaction by Host Plants. Insects 2022, 13, 818. https://doi.org/10.3390/insects13090818
Kihata N, Shikano I. Enemy-Risk Effects in Parasitoid-Exposed Diamondback Moth Larvae: Potential Mediation of the Interaction by Host Plants. Insects. 2022; 13(9):818. https://doi.org/10.3390/insects13090818
Chicago/Turabian StyleKihata, Naoki, and Ikkei Shikano. 2022. "Enemy-Risk Effects in Parasitoid-Exposed Diamondback Moth Larvae: Potential Mediation of the Interaction by Host Plants" Insects 13, no. 9: 818. https://doi.org/10.3390/insects13090818
APA StyleKihata, N., & Shikano, I. (2022). Enemy-Risk Effects in Parasitoid-Exposed Diamondback Moth Larvae: Potential Mediation of the Interaction by Host Plants. Insects, 13(9), 818. https://doi.org/10.3390/insects13090818





