Aphelinus nigritus Howard (Hymenoptera: Aphelinidae) Preference for Sorghum Aphid, Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae), Honeydew Is Stronger in Johnson Grass, Sorghum halepense, Than in Grain Sorghum, Sorghum bicolor
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishing Main Aphid Colonies
2.2. Establishing Clonal Aphid Colonies
2.3. Rearing Parasitoids
2.4. Honeydew Collection
2.5. Analysis of Honeydew Composition Using HPLC
2.6. Statistical Analyses of Honeydew Composition
2.7. Measuring Parasitoid Preference
2.8. Statistical Analyses of Parasitoid Preference
3. Results
3.1. SAs Fed on Grain Sorghum and Johnson Grass Excrete Varied Concentrations of Honeydew
3.2. Sugar, Amino Acid, and Organic Acid Profiles Are Similar between Host Plants
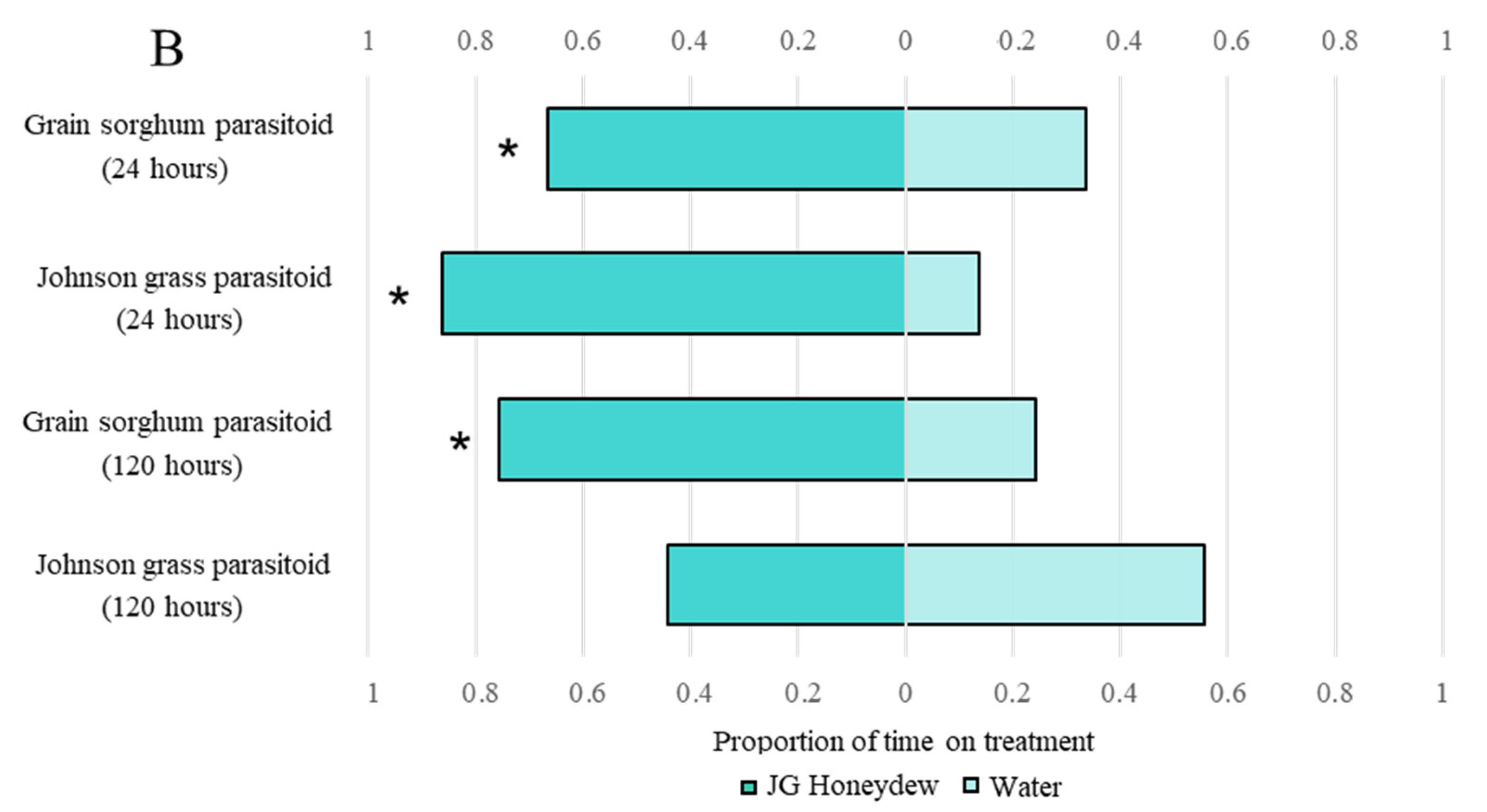
3.3. Aphelinus Nigritus Retention Behavior Observed on Honeydew Excreted by Aphids Feeding on Both Grain Sorghum and Johnson Grass
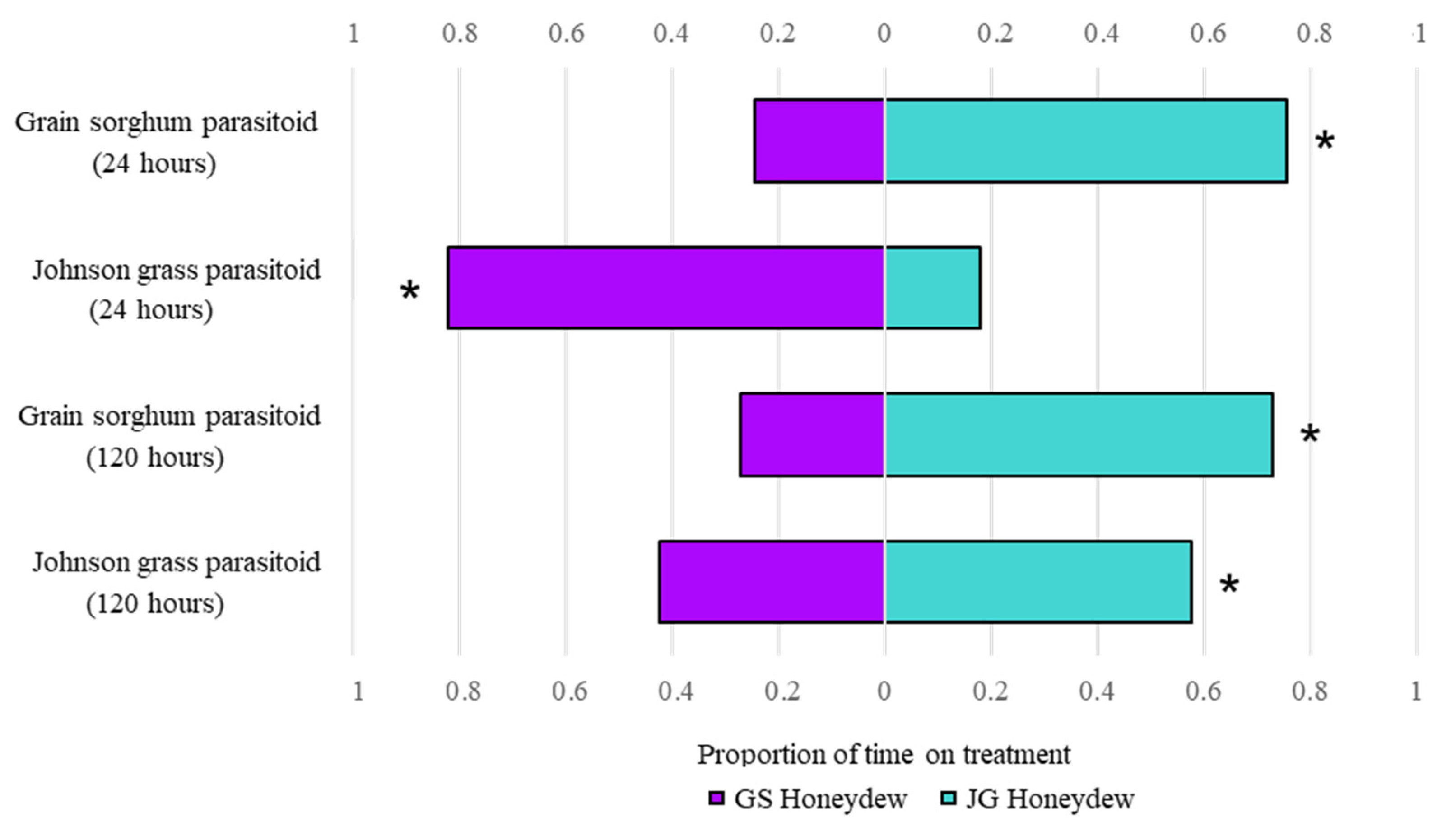
3.4. Aphelinus Nigritus Prefers Johnson Grass over Grain Sorghum Honeydew
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takabayashi, J.; Sato, Y.; Horikoshi, M.; Yamaoka, R.; Yano, S.; Ohsaki, N.; Dicke, M. Plant effects on parasitoid foraging: Differences between two tritrophic systems. Biol. Control 1998, 11, 97–103. [Google Scholar] [CrossRef]
- Feng, Y.; Wratten, S.; Sandhu, H.; Keller, M. Host plants affect the foraging success of two parasitoids that attack light brown apple moth Epiphyas postvittana (Walker) (Lepidoptera: Tortricidae). PLoS ONE 2015, 10, e0124773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wäschke, N.; Meiners, T.; Rostás, M. Foraging strategies of parasitoids in complex chemical environments. In Chemical Ecology of Insect Parasitoids; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 37–63. [Google Scholar]
- Lovinger, A.; Liewehr, D.; Lamp, W.O. Glandular trichomes on alfalfa impede searching behavior of the potato leafhopper parasitoid. Biol. Control 2000, 18, 187–192. [Google Scholar] [CrossRef]
- Ye, M.; Veyrat, N.; Xu, H.; Hu, L.; Turlings, T.C.; Erb, M. An herbivore-induced plant volatile reduces parasitoid attraction by changing the smell of caterpillars. Sci. Adv. 2018, 4, eaar4767. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.-S.; Jiang, L.-H. Differential parasitism of Plutella xylostella (Lepidoptera: Plutellidae) larvae by the parasitoid Cotesia plutellae (Hymenoptera: Braconidae) on two host plant species. Bull. Entomol. Res. 2003, 93, 65. [Google Scholar] [CrossRef]
- Lill, J.; Marquis, R.; Ricklefs, R. Host plants influence parasitism of forest caterpillars. Nature 2002, 417, 170–173. [Google Scholar] [CrossRef]
- Heard, S.B.; Stireman, J.O.; Nason, J.D.; Cox, G.H.; Kolacz, C.R.; Brown, J.M. On the elusiveness of enemy-free space: Spatial, temporal, and host-plant-related variation in parasitoid attack rates on three gallmakers of goldenrods. Oecologia 2006, 150, 421. [Google Scholar] [CrossRef]
- Elkhouly, A.; Al Hireereeq, E.A.; ALfaqi, A.B.; Shafsha, H.A. Natural Abundance and Host Plant Preference of the Larval Pupal Endoparasitoid Opius pallipes Wesmail (Hymenoptera: Braconidae) on the Serpentine Leafminer Liriomyza trifolii (Burgess) on Some Summer Host Plants. Asian J. Agric. Hortic. Res. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Vosteen, I.; Gershenzon, J.; Kunert, G. Enemy-free space promotes maintenance of host races in an aphid species. Oecologia 2016, 181, 659–672. [Google Scholar] [CrossRef]
- Holt, R.D.; Lawton, J.H. Apparent competition and enemy-free space in insect host-parasitoid communities. Am. Nat. 1993, 142, 623–645. [Google Scholar] [CrossRef]
- Gross, P. Insect behavioral and morphological defenses against parasitoids. Annu. Rev. Entomol. 1993, 38, 251–273. [Google Scholar] [CrossRef]
- Budenberg, W. Honeydew as a contact kairomone for aphid parasitoids. Entomol. Exp. Et Appl. 1990, 55, 139–148. [Google Scholar] [CrossRef]
- Lou, Y.G.; Cheng, J.A. Host-recognition kairomone from Sogatella furcifera for the parasitoid Anagrus nilaparvatae. Entomol. Exp. Et Appl. 2001, 101, 59–67. [Google Scholar] [CrossRef]
- Romeis, J.; Zebitz, C. Searching behaviour of Encarsia formosa as mediated by colour and honeydew. Entomol. Exp. Et Appl. 1997, 82, 299–309. [Google Scholar] [CrossRef]
- Lee, J.C.; Heimpel, G.E.; Leibee, G.L. Comparing floral nectar and aphid honeydew diets on the longevity and nutrient levels of a parasitoid wasp. Entomol. Exp. Et Appl. 2004, 111, 189–199. [Google Scholar] [CrossRef]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging success of parasitoid wasps on flowers: Interplay of insect morphology, floral architecture and searching behavior. Entomol. Exp. Et Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Lee, J.C.; Andow, D.A.; Heimpel, G.E. Influence of floral resources on sugar feeding and nutrient dynamics of a parasitoid in the field. Ecol. Entomol. 2006, 31, 470–480. [Google Scholar] [CrossRef]
- Woodring, J.; Wiedemann, R.; Fischer, M.; Hoffmann, K.; Völkl, W. Honeydew amino acids in relation to sugars and their role in the establishment of ant-attendance hierarchy in eight species of aphids feeding on tansy (Tanacetum vulgare). Physiol. Entomol. 2004, 29, 311–319. [Google Scholar] [CrossRef]
- Byrne, D.N.; Miller, W.B. Carbohydrate and amino acid composition of phloem sap and honeydew produced by Bemisia tabaci. J. Insect Physiol. 1990, 36, 433–439. [Google Scholar] [CrossRef]
- Yao, I.; Akimoto, S.I. Flexibility in the composition and concentration of amino acids in honeydew of the drepanosiphid aphid Tuberculatus quercicola. Ecol. Entomol. 2002, 27, 745–752. [Google Scholar] [CrossRef]
- Blüthgen, N.; Gottsberger, G.; Fiedler, K. Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest. Austral Ecol. 2004, 29, 418–429. [Google Scholar] [CrossRef]
- Leroy, P.D.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.J.; Francis, F.; Brostaux, Y.; Felton, G.W.; Haubruge, E. Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat. Commun. 2011, 2, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, C.A.; Wäckers, F.L.; Turlings, T.C. The nutritional value of aphid honeydew for non-aphid parasitoids. Basic Appl. Ecol. 2008, 9, 286–297. [Google Scholar] [CrossRef] [Green Version]
- Tena, A.; Wackers, F.L.; Heimpel, G.E.; Urbaneja, A.; Pekas, A. Parasitoid nutritional ecology in a community context: The importance of honeydew and implications for biological control. Curr Opin Insect Sci 2016, 14, 100–104. [Google Scholar] [CrossRef]
- Hogervorst, P.A.; Wäckers, F.L.; Romeis, J. Effects of honeydew sugar composition on the longevity of Aphidius ervi. Entomol. Exp. Et Appl. 2007, 122, 223–232. [Google Scholar] [CrossRef]
- Wäckers, F.L.; Van Rijn, P.C.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Casas, J.; Driessen, G.; Mandon, N.; Wielaard, S.; Desouhant, E.; Van Alphen, J.; Lapchin, L.; Rivero, A.; Christides, J.P.; Bernstein, C. Energy dynamics in a parasitoid foraging in the wild. J. Anim. Ecol. 2003, 72, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Shik, J.Z.; Kay, A.D.; Silverman, J. Aphid honeydew provides a nutritionally balanced resource for incipient Argentine ant mutualists. Anim. Behav. 2014, 95, 33–39. [Google Scholar] [CrossRef]
- Vollhardt, I.M.; Bianchi, F.J.; Wäckers, F.L.; Thies, C.; Tscharntke, T. Spatial distribution of flower vs. honeydew resources in cereal fields may affect aphid parasitism. Biol. Control 2010, 53, 204–213. [Google Scholar] [CrossRef]
- Strand, M.R.; Vinson, S.B. Behavioral response of the parasitoid Cardiochiles nigriceps to a kairomone. Entomol. Exp. Et Appl. 1982, 31, 308–315. [Google Scholar] [CrossRef]
- Battaglia, D.; Pennacchio, F.; Marincola, G.; Tranfaglia, A. Cornicle secretion of Acyrthosiphon pisum (Homoptera: Aphididae) as a contact kairomone for the parasitoid Aphidius ervi (Hymenoptera: Braconidae). Eur. J. Entomol. 1993, 90, 423–428. [Google Scholar]
- Afsheen, S.; Wang, X.; Li, R.; Zhu, C.S.; Lou, Y.G. Differential attraction of parasitoids in relation to specificity of kairomones from herbivores and their by-products. Insect Sci. 2008, 15, 381–397. [Google Scholar] [CrossRef]
- Brown, R.L.; El-Sayed, A.M.; Unelius, C.R.; Beggs, J.R.; Suckling, D.M. Invasive Vespula wasps utilize kairomones to exploit honeydew produced by sooty scale insects, Ultracoelostoma. J. Chem. Ecol. 2015, 41, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Mehrnejad, M.R.; Copland, M.J. Behavioral responses of the parasitoid Psyllaephagus pistaciae (Hymenoptera: Encyrtidae) to host plant volatiles and honeydew. Entomol. Sci. 2006, 9, 31–37. [Google Scholar] [CrossRef]
- Nibouche, S.; Costet, L.; Medina, R.F.; Holt, J.R.; Sadeyen, J.; Zoogones, A.-S.; Brown, P.; Blackman, R.L. Morphometric and molecular discrimination of the sugarcane aphid, Melanaphis sacchari, (Zehntner, 1897) and the sorghum aphid Melanaphis sorghi (Theobald, 1904). PLoS ONE 2021, 16, e0241881. [Google Scholar] [CrossRef] [PubMed]
- Uyi, O.; Lahiri, S.; Ni, X.; Buntin, D.; Jacobson, A.; Reay-Jones, F.P.; Punnuri, S.; Huseth, A.S.; Toews, M.D. Host plant resistance, foliar insecticide application and natural enemies play a role in the management of Melanaphis sorghi (Hemiptera: Aphididae) in grain sorghum. Front. Plant Sci. 2022, 13, 1006225. [Google Scholar] [CrossRef]
- Lahiri, S.; Ni, X.; Buntin, G.D.; Punnuri, S.; Jacobson, A.; Reay-Jones, F.P.; Toews, M.D. Combining host plant resistance and foliar insecticide application to manage Melanaphis sacchari (Hemiptera: Aphididae) in grain sorghum. Int. J. Pest Manag. 2021, 67, 10–19. [Google Scholar] [CrossRef]
- Seiter, N.J.; Miskelley, A.D.; Lorenz, G.M.; Joshi, N.K.; Studebaker, G.E.; Kelley, J.P. Impact of planting date on Melanaphis sacchari (Hemiptera: Aphididae) population dynamics and grain sorghum yield. J. Econ. Entomol. 2019, 112, 2731–2736. [Google Scholar] [CrossRef]
- Völkl, W.; Woodring, J.; Fischer, M.; Lorenz, M.W.; Hoffmann, K.H. Ant-aphid mutualisms: The impact of honeydew production and honeydew sugar composition on ant preferences. Oecologia 1999, 118, 483–491. [Google Scholar] [CrossRef]
- Detrain, C.; Verheggen, F.J.; Diez, L.; Wathelet, B.; Haubruge, E. Aphid–ant mutualism: How honeydew sugars influence the behaviour of ant scouts. Physiol. Entomol. 2010, 35, 168–174. [Google Scholar] [CrossRef]
- Zhou, A.; Wu, D.; Liang, G.; Lu, Y.; Xu, Y. Effects of tending by Solenopsis invicta (Hymenoptera: Formicidae) on the sugar composition and concentration in the honeydew of an invasive mealybug, Phenacoccus solenopsis (Hemiptera: Pseudococcidae). Ethology 2015, 121, 492–500. [Google Scholar] [CrossRef]
- Hendrix, D.L.; Wei, Y.-a.; Leggett, J.E. Homopteran honeydew sugar composition is determined by both the insect and plant species. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 101, 23–27. [Google Scholar] [CrossRef]
- Akbar, W.; Showler, A.; Reagan, T.; Davis, J.; Beuzelin, J. Feeding by sugarcane aphid, M elanaphis sacchari, on sugarcane cultivars with differential susceptibility and potential mechanism of resistance. Entomol. Exp. Et Appl. 2014, 150, 32–44. [Google Scholar] [CrossRef]
- Henter, H.J.; Via, S. The Potential for coevolution in a host-parasitoid system. I. Genetic variation within an aphid population in susceptibility to a parasitic wasp. Evolution 1995, 49, 427–438. [Google Scholar]
- Hufbauer, R.A.; Via, S. Evolution of an aphid-parasitoid interaction: Variation in resistance to parasitism among aphid populations specialized on different plants. Evolution 1999, 53, 1435–1445. [Google Scholar] [PubMed]
- Barbosa, P.; Segarra, A.; Gross, P.; Caldas, A.; Ahlstrom, K.; Carlson, R.; Ferguson, D.; Grissell, E.; Hodges, R.; Marsh, P. Differential parasitism of macrolepidopteran herbivores on two deciduous tree species. Ecology 2001, 82, 698–704. [Google Scholar] [CrossRef]
- EDDMapS; Grass, J. Sorghum halepense Distribution. In Early Detection & Distribution Mapping System; The University of Georgia-Center for Invasive Species and Ecosystem Health: Athens, GA, USA, 2019. [Google Scholar]
- McWhorter, C. Introduction and spread of johnsongrass in the United States. Weed Sci. 1971, 19, 496–500. [Google Scholar] [CrossRef]
- Longley, M.; Jepson, P.C. Effects of honeydew and insecticide residues on the distribution of foraging aphid parasitoids under glasshouse and field conditions. Entomol. Exp. Et Appl. 1996, 81, 189–198. [Google Scholar] [CrossRef]
- Gale, C.C.; Lesne, P.; Wilson, C.; Helms, A.M.; Suh, C.P.; Sword, G.A. Foliar herbivory increases sucrose concentration in bracteal extrafloral nectar of cotton. PLoS ONE 2021, 16, e0258836. [Google Scholar] [CrossRef]
- Steppuhn, A.; Wäckers, F. HPLC sugar analysis reveals the nutritional state and the feeding history of parasitoids. Funct. Ecol. 2004, 18, 812–819. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Package Version 2013, 2, 1–295. [Google Scholar]
- Clavijo Mccormick, A.; Gershenzon, J.; Unsicker, S.B. Little peaks with big effects: Establishing the role of minor plant volatiles in plant–insect interactions. Plant Cell Environ. 2014, 37, 1836–1844. [Google Scholar] [CrossRef] [PubMed]
- Friard, O.P.; Gamba, M. Behavioral Observation Research Interactive Software (BORIS); University of Torino Via dell’Accademia Albertina: Torino, PIE, Italy, 2016. [Google Scholar]
- Shaaban, B.; Seeburger, V.; Schroeder, A.; Lohaus, G. Sugar, amino acid and inorganic ion profiling of the honeydew from different hemipteran species feeding on Abies alba and Picea abies. PLoS ONE 2020, 15, e0228171. [Google Scholar] [CrossRef] [PubMed]
- Brinza, L.; Viñuelas, J.; Cottret, L.; Calevro, F.; Rahbé, Y.; Febvay, G.; Duport, G.; Colella, S.; Rabatel, A.; Gautier, C. Systemic analysis of the symbiotic function of Buchnera aphidicola, the primary endosymbiont of the pea aphid Acyrthosiphon pisum. Comptes Rendus Biol. 2009, 332, 1034–1049. [Google Scholar] [CrossRef]
- Mohammed, N.A.; Ahmed, I.A.M.; Babiker, E.E. Nutritional evaluation of sorghum flour (Sorghum bicolor L. Moench) during processing of injera. World Acad. Sci. Eng. Technol. 2011, 51, 72–76. [Google Scholar]
- Ajakaiye, C.O. Amino acid composition of sorghum grains as influenced by grain maturity, genotype, and nitrogen fertilization. J. Agric. Food Chem. 1984, 32, 47–50. [Google Scholar] [CrossRef]
- McWhorter, C. Water-soluble carbohydrates in johnsongrass. Weed Sci. 1974, 22, 159–163. [Google Scholar] [CrossRef]
- Horowitz, M. Seasonal development of established johnsongrass. Weed Sci. 1972, 20, 392–395. [Google Scholar] [CrossRef]
- Neucere, N.J.; Sumrell, G. Chemical composition of different varieties of grain sorghum. J. Agric. Food Chem. 1980, 28, 19–21. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, J.-J.; Li, Y.; Gou, Y.; Quandahor, P.; Liu, C. Trehalose and glucose levels regulate feeding behavior of the phloem-feeding insect, the pea aphid Acyrthosiphon pisum Harris. Sci. Rep. 2021, 11, 15864. [Google Scholar] [CrossRef]
- Prosser, W.; Simpson, S.; Douglas, A. How an aphid (Acyrthosiphon pisum) symbiosis responds to variation in dietary nitrogen. J. Insect Physiol. 1992, 38, 301–307. [Google Scholar] [CrossRef]
- Simpson, S.J.; Abisgold, J.D.; Douglas, A.E. Response of the pea aphid (Acyrthosiphon pisum) to variation in dietary levels of sugar and amino acids: The significance of amino acid quality. J. Insect Physiol. 1995, 41, 71–75. [Google Scholar] [CrossRef]
- Shimron, O.; Hefetz, A.; Gerling, D. Arrestment responses of Eretmocerus species and Encarsia deserti (Hymenoptera: Aphelinidae) to Bemisia tabaci honeydew. J. Insect Behav. 1992, 5, 517–526. [Google Scholar] [CrossRef]
- Hirose, Y.; Mitsunaga, T.; Yano, E.; Goto, C. Effects of sugars on the longevity of adult females of Eretmocerus eremicus and Encarsia formosa (Hymenoptera: Aphelinidae), parasitoids of Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Alyerodidae), as related to their honeydew feeding and host feeding. Appl. Entomol. Zool. 2009, 44, 175–181. [Google Scholar]
- Wäckers, F.L. Do oligosaccharides reduce the suitability of honeydew for predators and parasitoids? A further facet to the function of insect-synthesized honeydew sugars. Oikos 2000, 90, 197–201. [Google Scholar] [CrossRef]
- Vollhardt, I.M.; Bianchi, F.J.; Wäckers, F.L.; Thies, C.; Tscharntke, T. Nectar vs. honeydew feeding by aphid parasitoids: Does it pay to have a discriminating palate? Entomol. Exp. Et Appl. 2010, 137, 1–10. [Google Scholar] [CrossRef]
- Lewis, W.; Stapel, J.O.; Cortesero, A.M.; Takasu, K. Understanding how parasitoids balance food and host needs: Importance to biological control. Biol. Control 1998, 11, 175–183. [Google Scholar] [CrossRef]
- Křivan, V.; Sirot, E. Searching for food or hosts: The influence of parasitoids behavior on host–parasitoid dynamics. Theor. Popul. Biol. 1997, 51, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Heimpel, G.; Lee, J.; Wu, Z.; Weiser, L.; Wäckers, F.; Jervis, M. Gut sugar analysis in field-caught parasitoids: Adapting methods originally developed for biting flies. Int. J. Pest Manag. 2004, 50, 193–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, N.; Wang, J.; Wan, F. Effect of six carbohydrate sources on the longevity of a whitefly parasitoid Eretmocerus hayati (Hymenoptera: Aphelinidae). J. Asia-Pac. Entomol. 2014, 17, 723–728. [Google Scholar] [CrossRef]
- Tena, A.; Senft, M.; Desneux, N.; Dregni, J.; Heimpel, G.E. The influence of aphid-produced honeydew on parasitoid fitness and nutritional state: A comparative study. Basic Appl. Ecol. 2018, 29, 55–68. [Google Scholar] [CrossRef]
- McWHORTER, C.G.; Paul, R.N.; Ouzts, J.C. Bicellular trichomes of johnsongrass (Sorghum halepense) leaves: Morphology, histochemistry, and function. Weed Sci. 1995, 43, 201–208. [Google Scholar] [CrossRef]
- Mulatu, B.; Applebaum, S.W.; Coll, M. Effect of tomato leaf traits on the potato tuber moth and its predominant larval parasitoid: A mechanism for enemy-free space. Biol. Control 2006, 37, 231–236. [Google Scholar] [CrossRef]
- Keller, M.A. Influence of leaf surfaces on movements by the hymenopterous parasitoid Trichogramma exiguum. Entomol. Exp. Et Appl. 1987, 43, 55–59. [Google Scholar] [CrossRef]
- Giunti, G.; Canale, A.; Messing, R.; Donati, E.; Stefanini, C.; Michaud, J.; Benelli, G. Parasitoid learning: Current knowledge and implications for biological control. Biol. Control 2015, 90, 208–219. [Google Scholar] [CrossRef]
- Fischer, C.Y.; Lognay, G.C.; Detrain, C.; Heil, M.; Grigorescu, A.; Sabri, A.; Thonart, P.; Haubruge, E.; Verheggen, F.J. Bacteria may enhance species association in an ant–aphid mutualistic relationship. Chemoecology 2015, 25, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Peach, D.A.; Gries, R.; Young, N.; Lakes, R.; Galloway, E.; Alamsetti, S.K.; Ko, E.; Ly, A.; Gries, G. Attraction of female Aedes aegypti (L.) to aphid honeydew. Insects 2019, 10, 43. [Google Scholar] [CrossRef] [Green Version]
- Sanchéz, E.R.; Ramírez, A.G.; Ramírez, A.R.; Angulo, M.G.; Suárez, J.M.T.; Alejo, J.C. Biological activity of Bacillus thuringiensis culture supernatant on Bemisia tabaci and its parasitoid Eretmocerus eremicus. Trop. Subtrop. Agroecosyst. 2019, 22. Available online: https://www.revista.ccba.uady.mx/ojs/index.php/TSA/article/view/2717 (accessed on 20 December 2022).
- Fand, B.B.; Amala, U.; Yadav, D.; Rathi, G.; Mhaske, S.; Upadhyay, A.; Shabeer, T.A.; Kumbhar, D. Bacterial volatiles from mealybug honeydew exhibit kairomonal activity toward solitary endoparasitoid Anagyrus dactylopii. J. Pest Sci. 2020, 93, 195–206. [Google Scholar] [CrossRef]



| Variables | F Value | df | p Value |
|---|---|---|---|
| Collection Timepoint | 11.2749 | 2.104 | <0.0001 |
| Host Plant | 6.0142 | 2.104 | 0.0159 |
| Collection Timepoint × Host Plant | 0.4947 | 2.104 | 0.6112 |
| 24 h | 72 h | 120 h | ||||
|---|---|---|---|---|---|---|
| Sugar/ Organic Acid | Grain Sorghum | Johnson Grass | Grain Sorghum | Johnson Grass | Grain Sorghum | Johnson Grass |
| Fructose | 0.66 ± 0.71 | 1.18 ± 0.73 | 2.47 ± 0.71 | 4.67 ± 0.68 | 3.32 ± 0.73 | 4.87 ± 0.70 |
| Glucose | 0.76 ± 0.66 | 1.3 ± 0.68 | 2.39 ± 0.66 | 4.32 ± 0.63 | 2.91 ± 0.68 | 4.55 ± 0.64 |
| Melezitose | 0.1 ± 0.10 | 0.11 ± 0.10 | 0.39 ± 0.10 | 0.43 ± 0.10 | 0.35 ± 0.12 | 0.52 ± 0.10 |
| Stachyose | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| Sucrose | 1.36 ± 0.99 | 1.34 ± 0.96 | 3.47 ± 0.96 | 4.58 ± 0.89 | 3.15 ± 0.96 | 4.17 ± 0.91 |
| Fumaric Acid | 1.16 ± 0.35 | 1.82 ± 0.37 | 2.39 ± 0.36 | 3.24 ± 0.34 | 2.34 ± 0.37 | 3.76 ± 0.35 |
| ANCOVA | |||
|---|---|---|---|
| Variables | F Value | df | p Value |
| Collection Timepoint | 1.096 | 1.71 | 0.2986 |
| Original Parasitoid Aphid host | 0.6485 | 1.71 | 0.4223 |
| Collection Timepoint × Original Parasitoid Aphid host | 0.4947 | 2.104 | 0.6112 |
| Choice Treatment | 0.0729 | 1.71 | 0.7879 |
| Honeydew Concentration (covariate) | 4.9370 | 1.71 | 0.0295 |
| ANCOVA | |||
|---|---|---|---|
| Variables | F Value | df | p Value |
| Collection Timepoint | 1.107 | 1.35 | 0.300 |
| Original Parasitoid Aphid host | 3.616 | 1.35 | 0.066 |
| Collection Timepoint × Original Parasitoid Aphid host | 1.331 | 1.35 | 0.256 |
| Honeydew Concentration (covariate) | 0.136 | 1.35 | 0.714 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, C.; Helms, A.M.; Bernal, J.S.; Grunseich, J.M.; Medina, R.F. Aphelinus nigritus Howard (Hymenoptera: Aphelinidae) Preference for Sorghum Aphid, Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae), Honeydew Is Stronger in Johnson Grass, Sorghum halepense, Than in Grain Sorghum, Sorghum bicolor. Insects 2023, 14, 10. https://doi.org/10.3390/insects14010010
Wright C, Helms AM, Bernal JS, Grunseich JM, Medina RF. Aphelinus nigritus Howard (Hymenoptera: Aphelinidae) Preference for Sorghum Aphid, Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae), Honeydew Is Stronger in Johnson Grass, Sorghum halepense, Than in Grain Sorghum, Sorghum bicolor. Insects. 2023; 14(1):10. https://doi.org/10.3390/insects14010010
Chicago/Turabian StyleWright, Crys, Anjel M. Helms, Julio S. Bernal, John M. Grunseich, and Raul F. Medina. 2023. "Aphelinus nigritus Howard (Hymenoptera: Aphelinidae) Preference for Sorghum Aphid, Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae), Honeydew Is Stronger in Johnson Grass, Sorghum halepense, Than in Grain Sorghum, Sorghum bicolor" Insects 14, no. 1: 10. https://doi.org/10.3390/insects14010010
APA StyleWright, C., Helms, A. M., Bernal, J. S., Grunseich, J. M., & Medina, R. F. (2023). Aphelinus nigritus Howard (Hymenoptera: Aphelinidae) Preference for Sorghum Aphid, Melanaphis sorghi (Theobald, 1904) (Hemiptera: Aphididae), Honeydew Is Stronger in Johnson Grass, Sorghum halepense, Than in Grain Sorghum, Sorghum bicolor. Insects, 14(1), 10. https://doi.org/10.3390/insects14010010







