Modeling Thermal Developmental Trajectories and Thermal Requirements of the Ladybird Stethorus gilvifrons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mite and Coccinellid Stock Colonies
2.2. Experimental Design
2.3. The Relationship between Temperature and Developmental Rate
- The coefficient of determination (R2). A higher value R2 indicates better fit.
- The residual sum of squares (RSS). A smaller value of RSS indicated better fit. The coefficient of determination and residual sum of the square is commonly used for model evaluation. However, the R2 value is not appropriate for discrimination between models with a different number of parameters because models with more parameters will provide a better fit. Therefore, we used the Akaike information criterion (AIC) and the adjusted coefficient of determination R2adj, which are independent of the number of parameters.
- 2.
- The adjusted coefficient of determination (R2adj). A higher value of R2adj indicates better fit [38]. R2adj was calculated from the following equation:
2.4. Statistical Analysis
2.4.1. Linear Modeling
2.4.2. Non-Linear Modeling
3. Results
3.1. Developmental Time (Data from [23])
3.2. Model Evaluation
3.2.1. Linear Models
3.2.2. Non-Linear Models
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Helle, W.; Sabelis, M.W. Spider Mites: Their Biology, Natural Enemies, and Control; World Crop Pests; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1A. [Google Scholar]
- Khanamani, M.; Fathipour, Y.; Hajiqanbar, H.; Sedaratian, A. Two-spotted spider mite reared on resistant eggplant affects consumption rate and life table parameters of its predator, Typhlodromus bagdasarjani (Acari: Phytoseiidae). Exp. Appl. Acarol. 2014, 63, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.; Grissa-Lebdi, K.; Lognay, G.; Bitume, E.; Hance, T.; Mailleux, A.C. A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J. Pest Sci. 2013, 86, 361–386. [Google Scholar] [CrossRef]
- Van Leeuwen, T.; Vontas, J.; Tsagkarakou, A.; Dermauw, W.; Tirry, L. Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: A review. Insect Biochem. Mol. Biol. 2010, 40, 563–572. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Kring, T.J. Predaceous coccinellidae in biological control. Annu. Rev. Èntomol. 1998, 43, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Brodeur, J.; Cloutier, C. Effect of temperature on intrinsic rates of natural increase (rm) of a coccinellid and its spider mite prey. Biocontrol 2003, 48, 57–72. [Google Scholar] [CrossRef]
- Madadi, H.; Enkegaard, A.; Brødsgaard, H.F.; Kharrazi-Pakdel, A.; Ashouri, A.; Mohaghegh-Neishabouri, J. Interactions between Orius albidipennis (Heteroptera: Anthocoridae) and Neoseiulus cucumeris (Acari: Phytoseiidae): Effects of host plants under microcosm condition. Biol. Control 2009, 50, 137–142. [Google Scholar] [CrossRef]
- Weintraub, P.G.; Pivonia, S.; Steinberg, S. How many Orius laevigatus are needed for effective western flower thrips, Frankliniella occidentalis, management in sweet pepper? Crop. Prot. 2011, 30, 1443–1448. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Croft, B.A. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Èntomol. 1997, 42, 291–321. [Google Scholar] [CrossRef]
- Hoy, M.A. Agricultural Acarology: Introduction to Integrated Mite Management; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2011. [Google Scholar]
- Farazmand, A.; Fathipour, Y.; Kamali, K. Control of the spider mite Tetranychus urticae using phytoseiid and thrips predators under microcosm conditions: Single-predator versus combined-predators release. Syst. Appl. Acarol. 2015, 20, 162–170. [Google Scholar] [CrossRef]
- Pakyari, H. Development rate of Scolothrips longicornis (Thysanoptera: Thripidae) at various temperatures. Acad. J. Entomol. 2011, 4, 1–6. [Google Scholar]
- Osman, M.; Bayoumy, M. Effect of prey stages of the two-spotted mite Tetranychus urticae on functional response of the coccinellid predator Stethorus gilvifrons. Acta Phytopathol. et Èntomol. Hung. 2011, 46, 277–288. [Google Scholar] [CrossRef]
- Barbar, Z.; Kerhili, S.; Aslan, L.H. Daily consumption and predation rate of different Stethorus gilvifrons (Mulsant) (Coleoptera: Coccinellidae) stages on Panonychus citri (McGregor) (Acari: Tetranychidae). Egypt J. Biol. Pest Control. 2016, 26, 413. [Google Scholar]
- Biddinger, D.J.; Weber, D.C.; Hull, L.A. Coccinellidae as predators of mites: Stethorini in biological control. Biol. Control 2009, 51, 268–283. [Google Scholar] [CrossRef]
- Farahbakhsh, G. A checklist of economically important insects and other enemies of plants and agricultural products in Iran; Ministry of Agriculture Department and Plant Protection Publication no. 1; Plan Organization Press: Tehran, Iran, 1961; pp. 1–153. [Google Scholar]
- Kaylani, S. Biology and life-history of Stethorus gilvifrons (Mulsant) in Lebanon. Magon Publ. Ser. Sci. Beirut 1967, 11, 1–24. [Google Scholar]
- Trivedi, N.P.; Patel, P.B.; Patel, J.P.; Aniyaliya, M.D. Stethorus spp.: A predator of mites. J. Pharm. Innov. 2021, 10, 389–394. [Google Scholar]
- Haji-Zadeh, J.; Kamali, G.K.; Assadi, H.B. Investigations on the functional response and populations fluctuations of Stethorus gilvifrons on red spider mite, Panonychus ulmi (Koch) in Karaj vicinity [Iran]. J. Appl. Entomol. 1993, 61, 32–34. (In Persian) [Google Scholar]
- Taghizadeh, R.; Fathipour, Y.; Kamali, K. Influence of temperature on life-table parameters of Stethorus gilvifrons (Mulsant) (Coleoptera: Coccinellidae) fed on Tetranychus urticae Koch. J. Appl. Èntomol. 2008, 132, 638–645. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Fathipour, Y.; Kamali, K. Temperature-dependent development of acarophagous ladybird, Stethorus gilvifrons (Mulsant) (Coleoptera: Coccinellidae). J. Asia-Pacific Èntomol. 2008, 11, 145–148. [Google Scholar] [CrossRef]
- Jafari, M.; Goldasteh, S.; Ranjbar Aghdam, H.; Zamani, A.A.; Soleyman-Nejadian, E. Effect of solitary and group rearing on demographic parameters of Stethorus gilvifrons (Col.: Coccinelidae) feeding on Tetranychus urticae. J. Entomol. Res. 2019, 11, 197–217. [Google Scholar]
- Jafari, M.; Goldasteh, S.; Ranjbar Aghdam, H.; Zamani, A.A.; Soleyman-Nejadian, E. Effect of temperature on two-sex life table parameters of Stethorus gilvifrons (Coleoptera: Coccinellidae) feeding on Tetranychus urticae (Acari: Tetranychidae). J. Entomol. Soc. Iran. 2020, 40, 65–82. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Insect Predator-Prey Dynamics: Ladybird Beetles and Biological Control; Cambridge University Press: Cambridge, UK, 2000; pp. 1–252. [Google Scholar]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Jerbi-Elayed, M.; Tougeron, K.; Grissa-Lebdi, K.; Hance, T. Effect of developmental temperatures on Aphidius colemani host-foraging behavior at high temperature. J. Therm. Biol. 2022, 103, 103140. [Google Scholar] [CrossRef] [PubMed]
- Gevrey, M.; Worner, S.P. Prediction of global distribution of insect pest species in relation to climate by using an ecological informatics method. J. Econ. Èntomol. 2006, 99, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Brodeur, J.; Cloutier, C. Relationship between temperature and developmental rate of Stethorus punctillum (Coleoptera: Coccinellidae) and its prey Tetranychus mcdanieli (Acarina: Tetranychidae). Environ. Èntomol. 2002, 31, 177–187. [Google Scholar] [CrossRef]
- Huffaker, C.B.; Rabb, R.L.; Logan, J.A. Some aspects of population dynamics relative to augmentation of natural enemy action. In Biological control by augmentation of natural enemies; Ridgway, R.L., Vinson, S.B., Eds.; Plenum Press: New York, NY, USA, 1977; pp. 3–38. [Google Scholar]
- Logan, J.; Wolesensky, W.; Joern, A. Temperature-dependent phenology and predation in arthropod systems. Ecol. Model. 2006, 196, 471–482. [Google Scholar] [CrossRef]
- Estay, S.A.; Lima, M.; Labra, F.A. Predicting insect pest status under climate change scenarios: Combining experimental data and population dynamics modelling. J. Appl. Èntomol. 2009, 133, 491–499. [Google Scholar] [CrossRef]
- Obrycki, J.J.; Tauber, M.J. Thermal requirements for development of Coleomegilla maculata (Coleoptera: Coccinellidae) and its parasite Perilitus coccinellae (Hymenoptera: Braconidae). Can. Èntomol. 1978, 110, 407–412. [Google Scholar] [CrossRef]
- Buckley, L.B. Temperature-sensitive development shapes insect phenological responses to climate change. Curr. Opin. Insect Sci. 2022, 52, 100897. [Google Scholar] [CrossRef]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Briere, J.-F.; Pracros, P. Comparison of temperature-dependent growth models with the development of Lobesia botrana (Lepidoptera: Tortricidae). Environ. Èntomol. 1998, 27, 94–101. [Google Scholar] [CrossRef]
- Kontodimas, D.C.; Eliopoulos, P.A.; Stathas, G.J.; Economou, L.P. Comparative temperature-dependent development of Nephus includens (Kirsch) and Nephus bisignatus (Boheman) (Coleoptera: Coccinellidae) preying on Planococcus citri (Risso) (Homoptera: Pseudococcidae): Evaluation of a linear and various nonlinear models using specific criteria. Environ. Èntomol. 2004, 33, 1–11. [Google Scholar] [CrossRef]
- Aghdam, H.R.; Fathipour, Y.; Radjabi, G.; Rezapanah, M. Temperature-dependent development and temperature thresholds of codling moth (Lepidoptera: Tortricidae) in Iran. Environ. Èntomol. 2009, 38, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, T.; Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ. Èntomol. 2000, 29, 671–682. [Google Scholar] [CrossRef]
- Analytis, S. Relationship between temperature and development times in phytopathogenic fungus and in plant pests: A mathematical model. J. Agric. Res. 1981, 5, 133–159. [Google Scholar]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in Arthropods. Environ. Èntomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Lactin, D.J.; Holliday, N.J.; Johnson, D.L.; Craigen, R. Improved rate model of temperature-dependent development by Arthropods. Environ. Èntomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- Briere, J.-F.; Pracros, P.; Le Roux, A.-Y.; Pierre, J.-S. A novel rate model of temperature-dependent development for Arthropods. Environ. Èntomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Analytis, S. Über die Relation zwischen biologischer Entwicklung und Temperatur bei phytopathogenen Pilzen. J. Phytopathol. 1977, 90, 64–76. [Google Scholar] [CrossRef]
- Analytis, S. Obtaining of sub-models for modeling the entire life cycle of a pathogen/Über die Erlangung von Sub-Modellen, die zur Beschreibung eines gesamten Lebenszyklus eines Krankheitserregers dienen. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz/. J. Plant Dis. Prot. 1980, 87, 371–382. [Google Scholar]
- Harcourt, D.G.; Yee, J.M. Polynomial algorithm for predicting the duration of insect life stages. Environ. Èntomol. 1982, 11, 581–584. [Google Scholar] [CrossRef]
- Janisch, E. The influence of temperature on the life-history of insects. Trans. R. Entomol. Soc. Lond. 1932, 80, 137–168. [Google Scholar] [CrossRef]
- Taylor, F. Sensitivity of physiological time in Arthropods to variation of its parameters. Environ. Èntomol. 1982, 11, 573–577. [Google Scholar] [CrossRef]
- Stinner, R.E.; Gutierrez, A.P.; Butler, G.D. An algorithm for temperature-dependent growth rate simulation. Can. Èntomol. 1974, 106, 519–524. [Google Scholar] [CrossRef]
- Holling, C.S. The functional response of invertebrate predators to prey density. Memoirs Èntomol. Soc. Can. 1966, 98, 5–86. [Google Scholar] [CrossRef]
- Hilbert, D.W.; Logan, J.A. Empirical model of nymphal development for the migratory grasshopper, Melanoplus sanguinipes (Orthoptera: Acrididae). Environ. Èntomol. 1983, 12, 1–5. [Google Scholar] [CrossRef]
- Lamb, R.J. Developmental rate of Acyrthosiphon pisum (Homoptera: Aphididae) at low temperatures: Implications for estimating rate parameters for insects. Environ. Èntomol. 1992, 21, 10–19. [Google Scholar] [CrossRef]
- Enkegaard, A. The poinsettia strain of the cotton white fly, Bemisia tabaci (Homoptera: Aleyrodidae), biological and demographic parameters on poinsettia (Euphorbia pulcherrima) in relation to temperature. Bull. Èntomol. Res. 1993, 83, 535–546. [Google Scholar] [CrossRef]
- Bieri, M.; Baumgartner, J.; Bianchi, G.; Delucchi, V.; Arx, R.V. Development and fecundity of pea aphid (Acyrthosiphon pisum Harris) as affected by constant temperatures and by pea varieties. Mitt. Schweiz. Entomol. Ges. 1983, 56, 163–171. [Google Scholar]
- Sharpe, P.J.; DeMichele, D.W. Reaction kinetics of poikilotherm development. J. Theor. Biol. 1977, 64, 649–670. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Vucetich, J.A.; Peterson, R.O.; Schaefer, C.C. The effect of prey and predator densities on wolf predation. Ecology 2002, 83, 3003–3013. [Google Scholar] [CrossRef]
- Chi, H.S.I.N.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Èntomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A computer program for the age-atage, two-sex life table analysis. 2017. Available online: http://140.120.197.173/Ecology/ (accessed on 16 September 2017).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993; p. 320. [Google Scholar]
- Akca, I.; Ayvaz, T.; Yazici, E.; Smith, C.L.; Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): With additional comments on life table research criteria. J. Econ. Èntomol. 2015, 108, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.D. The Coccinellidae (Coleoptera) of America North of Mexico. J. N. Y. Entomol. 1985, 93, 88–99. [Google Scholar]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Aksit, T.; Cakmak, I.; Ozer, G. Effect of temperature and photoperiod on development and fecundity of an acarophagous ladybird beetle, Stethorus gilvifrons. Phytoparasitica 2007, 35, 357–366. [Google Scholar] [CrossRef]
- Shih, C.I.T.; Lin, P.J.; Chang, T.W. Biology, predation, life table and intrinsic rate of increase of Stethorus loi Sasaji. Prot. Bull. Taipei. 1991, 33, 290–300. [Google Scholar]
- Fiaboe, K.K.M.; Gondim, M.G.C.; De Moraes, G.J.; Ogol, C.K.P.O.; Knapp, M. Bionomics of the acarophagous ladybird beetle Stethorus tridens fed Tetranychus evansi. J. Appl. Èntomol. 2007, 131, 355–361. [Google Scholar] [CrossRef]
- Khan, I.; Spooner-Hart, R. Temperature-dependent development of immature stages of predatory ladybird beetle Stethorus vagans (Coleoptera: Coccinellidae) at constant and fluctuating temperatures. Acta Zoöl. Acad. Sci. Hung. 2017, 63, 83–96. [Google Scholar] [CrossRef]
- Mori, K.; Nozawa, M.; Arai, K.; Gotoh, T. Life-history traits of the acarophagous lady beetle, Stethorus japonicus at three constant temperatures. Biocontrol 2005, 50, 35–51. [Google Scholar] [CrossRef]
- Rattanatip, J.; Siri, N.; Chandrapatya, A. Comparitive biology and lifetable of Stethorus pauperculus (Weise) and S. siphonulus (Kapur) (Coleoptera: Coccinellidae) fed on Tetranychus urticae Koch (Acari: Tetranychidae) in Thailand. Thai J. Agric. Sci. 2008, 41, 117–126. [Google Scholar]
- Perumalsamy, K.; Selvasundaram, R.; Roobakkumar, A.; Rahman, V.J.; Muraleedharan, N. Life table and predatory efficiency of Stethorus gilvifrons (Coleoptera: Coccinellidae), an important predator of the red spider mite, Oligonychus coffeae (Acari: Tetranychidae), infesting tea. Exp. Appl. Acarol. 2010, 50, 141–150. [Google Scholar] [CrossRef]
- Matter, M.M.; El-Shershaby, M.M.; Farag, N.A.; Gesraha, M.A. Impact of temperature and prey density on the predacious capacity and behaviour of Stethorus punctillum Weise. Arch. Phytopathol. Plant Prot. 2011, 44, 127–134. [Google Scholar] [CrossRef]
- Handoko, H.; Affandi, A. Life-history traits of Stethorus gilvifrons (Mulsant) (Coleoptera: Coccinellidae) on phytophagous mites Eutetranychus orientalis Klein (Acari: Tetranychidae). AGRIVITA J. Agric. Sci. 2012, 34, 7–13. [Google Scholar] [CrossRef]
- Worner, S.P. Performance of phenological models under variable temperature regimes: Consequences of the Kaufmann or rate summation effect. Environ. Èntomol. 1992, 21, 689–699. [Google Scholar] [CrossRef]
- Zamani, A.A.; Talebi, A.; Fathipour, Y.; Baniameri, V. Effect of temperature on life history of Aphidius colemani and Aphidius matricariae (Hymenoptera: Braconidae), two parasitoids of Aphis gossypii and Myzus persicae (Homoptera: Aphididae). Environ. Èntomol. 2007, 36, 263–271. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; x Ratkowsky, D.A.; Hoddle, M.S. The influence of temperature variation on life history parameters and thermal performance curves of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the Asian citrus psyllid (Hemiptera: Liviidae). J. Econ. Èntomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Èntomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Tarusikirwa, V.; Nyamukondiwa, C.; Chidawanyika, F. Fluctuating environments impact thermal tolerance in an invasive insect species Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Èntomol. 2020, 144, 885–896. [Google Scholar] [CrossRef]
- Gutierrez, J. Etude Biologique et Ecologique de Tetranychus neocaledonicus Andre (Acari: Tetranychidae); Cahiers Orstom, n57; ORSTOM: Paris, France, 1976. [Google Scholar]
- Bounfour, M.; Tanigoshi, L.K. Effect of temperature on development and demographic parameters of Tetranychus urticae and Eotetranychus carpini borealis (Acari: Tetranychidae). Ann. Èntomol. Soc. Am. 2001, 94, 400–404. [Google Scholar] [CrossRef]
- Praslička, J.; Huszár, J. Influence of temperature and host plants on the development and fecundity of the spider mite Tetranychus urticae (Acarina: Tetranychidae). Plant Prot. Sci. 2004, 40, 141–144. [Google Scholar] [CrossRef]
- El-Wahed, N.A.; El-Halawany, A.S. Effect of temperature degrees on the biology and life table parameters of Tetranychus urticae Koch on two pear varieties. Egypt. Acad. J. Biol. Sci. B. Zoöl. 2012, 4, 103–109. [Google Scholar] [CrossRef]
- Bayu, M.S.Y.I.; Ullah, M.S.; Takano, Y.; Gotoh, T. Impact of constant versus fluctuating temperatures on the development and life history parameters of Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 2017, 72, 205–227. [Google Scholar] [CrossRef]
- Afshari, M.; Mossadegh, S.; Soleyman-Nejadian, E.; Kamali, K. Geographical distribution and hostplants of Stethorus gilvifrons (Mulsant) (Col.: Coccinellidae) and its biology under laboratory conditions in Khuzestan province. J. Agri. Sci. Nat. Res. 2007, 14, 201–210. [Google Scholar]
- Perdikis, D.C.; Lykouressis, D.P. Thermal requirements for development of the polyphagous predator Macrolophus pygmaeus (Hemiptera: Miridae). Environ. Èntomol. 2002, 31, 661–667. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ahn, J.J. Temperature effects on development, fecundity, and life table parameters of Amblyseius womersleyi (Acari: Phytoseiidae). Environ. Èntomol. 2000, 29, 265–271. [Google Scholar] [CrossRef]
- Imani, Z.; Shishehbor, P.; Sohrabi, F. The effect of Tetranychus turkestani and Eutetranychus orientalis (Acari: Tetranychidae) on the development and reproduction of Stethorus gilvifrons (Coleoptera: Coccinellidae). J. Asia-Pacific Èntomol. 2009, 12, 213–216. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Shih, C.-I.T. Development of Neoseiulus womersleyi (Schicha) and Euseius ovalis (Evans) feeding on four tetranychid mites (Acari: Phytoseiidae, Tetranychidae) and pollen. J. Asia-Pacific Èntomol. 2010, 13, 289–296. [Google Scholar] [CrossRef]
- Ullah, M.; Gotoh, T. Life-table attributes of Neoseiulus womersleyi (Schicha) feeding on five tetranychid mites (Acari: Phytoseiidae, Tetranychidae). Int. J. Acarol. 2014, 40, 337–348. [Google Scholar] [CrossRef]
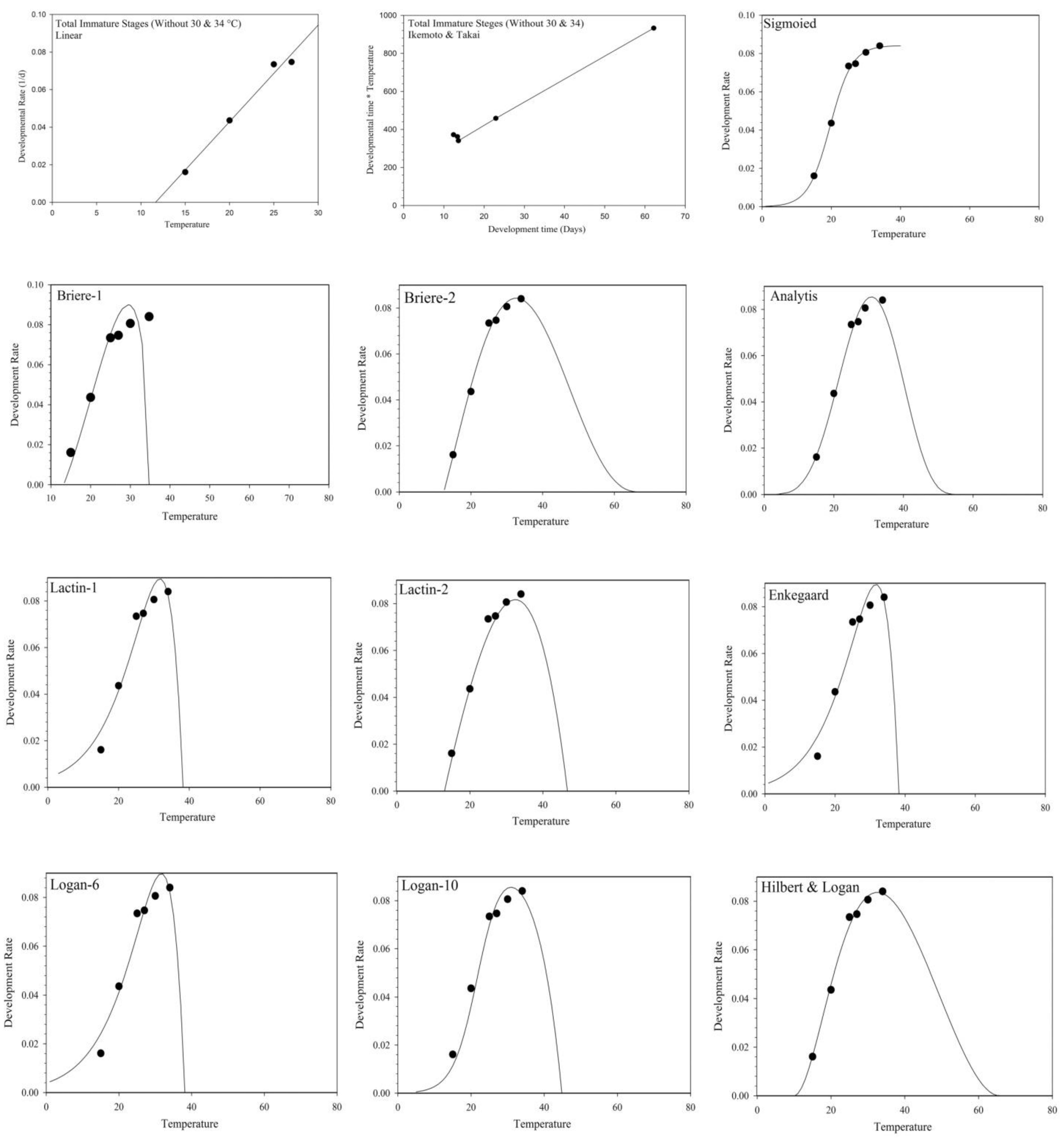
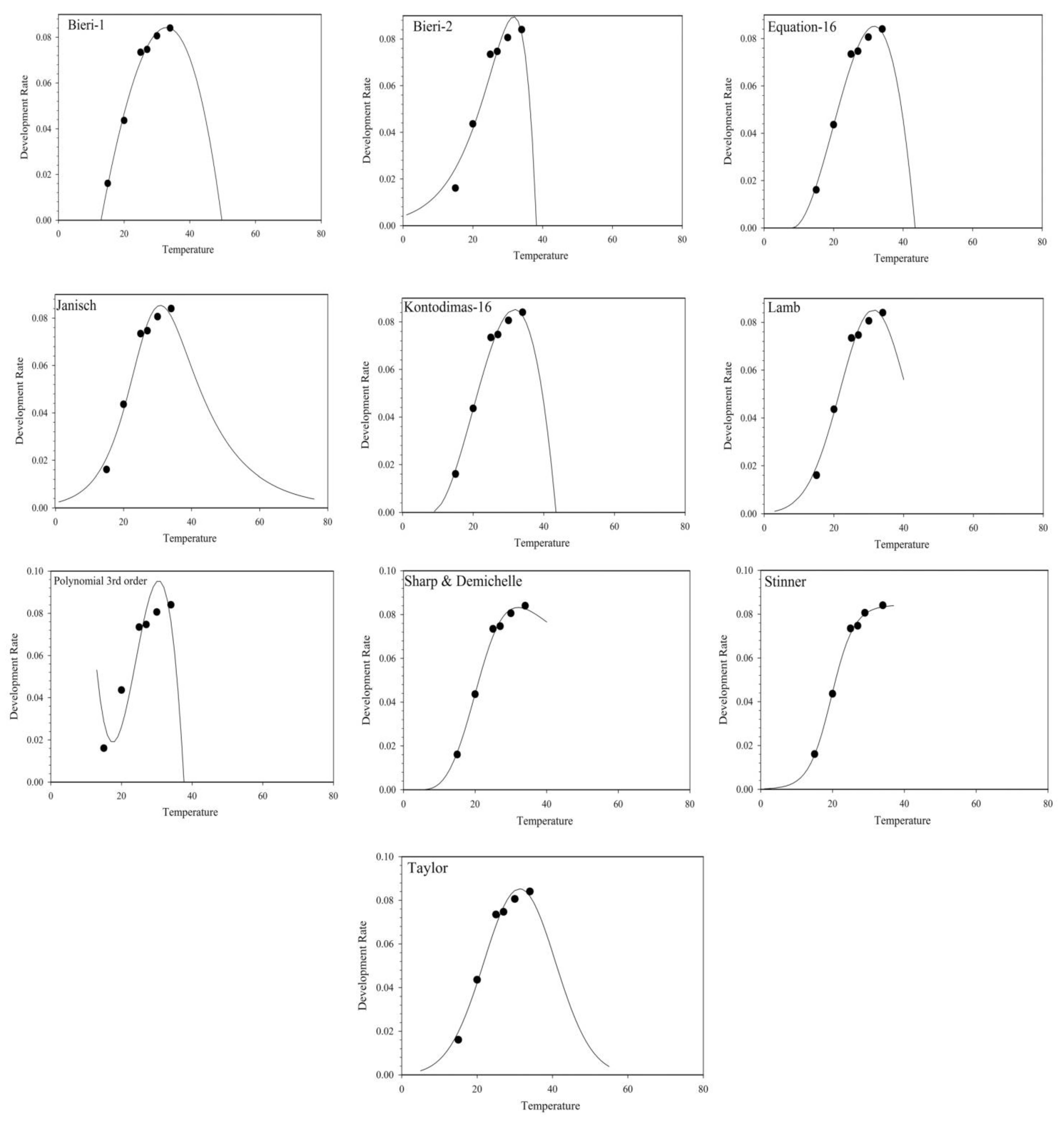
| Equation | Model | References |
|---|---|---|
| Ordinary linear regression | [35] | |
| Ikemoto & Takai | [39] | |
| Sigmoid | [40] | |
| Logan-6 | [41] | |
| Logan-10 | [41] | |
| Lactin-1 | [42] | |
| Lactin-2 | [42] | |
| Briere-1 | [43] | |
| Briere-2 | [43] | |
| Analytis | [44,45] | |
| Polynomial 3rd order | [46] | |
| Kontodimas-16 | [37] | |
| Janisch | [47] | |
| Taylor | [48] | |
| Stinner | [49] | |
| Hilbert & Logan, or Holling III | [50,51] | |
| Lamb | [52] | |
| Equation-16 | [37] | |
| Enkegaard | [53] | |
| Bieri-1 | [54] | |
| Bieri-2 | [54] | |
| Sharpe & DeMichele | [55] |
| Sex | Stage | Temperatures (°C) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 | n | 20 | n | 25 | n | 27 | n | 30 | n | 34 | n | ||
| Female | Egg | 15.7 ± 0.5 a | 139 | 8.7 ± 0.2 b | 125 | 3.3 ± 0.0 d | 115 | 3.2 ± 0.1 de | 118 | 3.0 ± 0.0 e | 118 | 3.7 ± 0.0 c | 92 |
| Larva | 33.9 ± 0.7 a | 17 | 15.1 ± 0.4 b | 49 | 7.5 ± 0.3 c | 70 | 7.2 ± 0.1 c | 79 | 6.4 ± 0.2 d | 70 | 5.3 ± 0.1 e | 59 | |
| Pupa | 11.8 ± 0.2 a | 17 | 7.7 ± 0.4 b | 39 | 3.5 ± 0.1 c | 64 | 2.8 ± 0.0 d | 62 | 2.9 ± 0.1 e | 62 | 2.6 ± 0.0 de | 58 | |
| Male | Egg | 15.7 ± 0.5 a | 139 | 7.3 ± 0.1 b | 125 | 3.8 ± 0.1 c | 115 | 3.5 ± 0.1 c | 118 | 3.0 ± 0.0 d | 118 | 3.5 ± 0.1 c | 92 |
| Larva | 35.4 ± 1.0 a | 17 | 14.4 ± 0.3 b | 49 | 6.6 ± 0.3 cd | 70 | 7.0 ± 0.2 c | 79 | 6.3 ± 0.1 d | 70 | 5.8 ± 0.1 e | 59 | |
| Pupa | 12.1 ± 0.2 a | 17 | 6.6 ± 0.3 b | 39 | 2.8 ± 0.1 c | 64 | 2.9 ± 0.0 c | 62 | 2.9 ± 0.0 c | 62 | 2.9 ± 0.0 c | 58 | |
| Linear Model | Stage | Thermal Range | Linear Equation | Thermal Constant (DD) ± SE (°C) | Tmin ± SE (°C) | R2 | R2adj | p |
|---|---|---|---|---|---|---|---|---|
| Common | Egg | 15–27 | DR = 0.0208T − 0.261 | 48.02 ± 12.76 | 12.55 ± 0.73 | 0.9610 | 0.9415 | 0.019 |
| Larva | 15–27 | DR = 0.0103T − 0.129 | 96.95 ± 14.51 | 12.51 ± 0.27 | 0.9509 | 0.9263 | 0.025 | |
| Pupa | 15–27 | DR = 0.0240T − 0.295 | 41.70 ± 22.10 | 12.33 ± 0.70 | 0.9608 | 0.9413 | 0.019 | |
| Egg-Pupa | 15–27 | DR = 0.0051T − 0.059 | 194.50 ± 36.04 | 11.64 ± 0.29 | 0.9816 | 0.9724 | 0.009 | |
| Ikemoto & Takai | Egg | 15–27 | DT = 11.79D + 53.25 | 53.25 ± 0.008 | 11.79 ± 0.46 | 0.9807 | 0.9711 | 0.009 |
| Larva | 15–27 | DT = 12.16D + 102.34 | 102.34 ± 0.004 | 12.16 ± 0.61 | 0.9941 | 0.9911 | 0.002 | |
| Pupa | 15–27 | DT = 11.58D + 46.01 | 46.01 ± 0.009 | 11.58 ± 0.40 | 0.9777 | 0.9661 | 0.011 | |
| Egg-Pupa | 15–27 | DT = 11.96D + 187.87 | 187.87 ± 0.002 | 11.96 ± 0.51 | 0.9951 | 0.9934 | 0.0006 |
| Model | Parameters | Egg | Larva | Pupa | Total Development |
|---|---|---|---|---|---|
| Briere-1 | a | 30.51 × 10−5 | 17.50 × 10−5 | 35.61 × 10−5 | 7.86 × 10−5 |
| tmin | 13.48 | 14.38 | 13.64 | 13.00 | |
| topt | 29.75 | 29.75 | 29.75 | 29.75 | |
| tmax | 35.00 | 35.00 | 35.00 | 35.00 | |
| Briere-2 | a | 3.55 × 10−4 | 1.67 × 10−6 | 1.82 × 10−4 | 6.30 × 10−9 |
| m | 3.09 | 0.64 | 1.68 | 0.358 | |
| tmin | 11.17 | 12.20 | 11.24 | 11.96 | |
| topt | 31.00 | 35.75 | 31.50 | 32.00 | |
| tmax | 34.93 | 57.80 | 38.80 | 67.48 | |
| Logan-6 | −0.047 | 1.066 | −0.045 | 0.570 | |
| 0.185 | 0.166 | 0.167 | 0.151 | ||
| 6.83 | 5.59 | 6.09 | 6.41 | ||
| topt | 30.75 | 32.50 | 31.75 | 31.75 | |
| tmax | 36.22 | 38.68 | 37.55 | 38.21 | |
| Logan-10 | 0.466 | 0.517 | 0.419 | 0.105 | |
| 0.236 | 0.176 | 0.315 | 0.291 | ||
| 3.71 | 7.60 | 4.67 | 6.49 | ||
| k | 249.56 | 138.09 | 847.63 | 586.62 | |
| TL | 38.66 | 44.05 | 44.05 | 44.04 | |
| topt | 33.75 | 34.25 | 31.00 | 32.75 | |
| tmax | 38.43 | 44.03 | 36.97 | 44.03 | |
| Lactin-1 | 5.4453 | 6.1696 | 6.0314 | 6.5082 | |
| 0.1834 | 0.1619 | 0.1654 | 0.1535 | ||
| topt | 31.00 | 33.00 | 31.09 | 32.00 | |
| tmax | 36.3378 | 38.7646 | 37.5502 | 38.2170 | |
| Lactin-2 | 2.70 | 9.87 | 5.01 | 26.62 | |
| 0.01 | 0.011 | 0.018 | 0.017 | ||
| e | 1.21 | 1.13 | 1.24 | 0.86 | |
| TL | 39.87 | 59.56 | 44.67 | 70.78 | |
| tmin | 12.50 | 12.48 | 12.09 | 13.04 | |
| topt | 30.75 | 34.00 | 31.50 | 32.25 | |
| tmax | 36.50 | 47.27 | 36.62 | 46.73 | |
| Sigmoid | a | 7.6721 | 5.8402 | 6.5996 | 6.2261 |
| b | −0.3775 | −0.2714 | −0.3224 | −0.3167 | |
| c | 0.3105 | 0.1824 | 0.3772 | 0.0842 | |
| Hilbert & Logan, or Holling III | 0.8069 | 1.1954 | 2.6544 | 1.4284 | |
| T0 | 5.1643 | −0.0286 | 2.2864 | −5.4646 | |
| d | 2.6455 | 34.8905 | 10.2500 | 62.5963 | |
| TL | 33.6489 | 71.6097 | 46.7278 | 75.7471 | |
| 17.1084 | 20.7993 | 20.0060 | 19.6420 | ||
| tmin | 6.18 | 7.3623 | 11.2824 | 12.4085 | |
| topt | 31.00 | 36.11 | 31.18 | 33.00 | |
| tmax | 38.21 | 67.99 | 45.91 | 66.11 | |
| Stinner (T > Topt) | a | −2.9618 | −1.7579 | −2.4292 | −2.6428 |
| b | 0.1898 | 0.1355 | 0.1612 | 0.1583 | |
| c | 0.3102 | 0.1824 | 0.3772 | 0.0842 | |
| topt | - | - | - | - | |
| Polynomial 3rd order | a | 1.5108 | 0.0316 | 0.9548 | 0.8113 |
| b | −0.2282 | −0.0137 | −0.1512 | −0.1118 | |
| c | 0.0112 | 0.0011 | 0.0079 | 5.02 × 10−3 | |
| d | −1.65 × 10−4 | −1.90 × 10−5 | −1.18 × 10−4 | −6.97 × 10−5 | |
| topt | 30.00 | 34.25 | 31.42 | 32.50 | |
| tmax | 38.00 | 47.47 | 40.57 | 37.69 | |
| Equation-16 | a | 8.9669 × 10−5 | 2.2398 × 10−5 | 6.9155 × 10−5 | 1.2974 × 10−5 |
| tmin | 10.7196 | 8.7399 | 9.2019 | 8.0642 | |
| topt | 30.00 | 34.00 | 31.40 | 32.00 | |
| tmax | 39.6390 | 46.5227 | 42.4350 | 43.4616 | |
| Kontodimas-16 | a | 8.9669 × 10−5 | 2.2398 × 10−5 | 6.9155 × 10−5 | 1.2973 × 10−5 |
| tmin | 10.7196 | 8.7399 | 9.2019 | 8.0643 | |
| topt | 30.00 | 34.01 | 31.19 | 31.90 | |
| tmax | 39.6390 | 46.5227 | 42.3350 | 43.4615 | |
| Lamb (T > Topt) | Rm | 0.3245 | 0.1780 | 0.3782 | 0.0852 |
| tm (=topt) | 29.6197 | 33.1083 | 30.8630 | 31.2873 | |
| T0 | 7.4244 | 9.7908 | 8.6507 | 9.5089 | |
| Analytis | a | 1.3038 × 10−15 | 1.1464 × 10−15 | 1.3474 × 10−14 | 2.2536 × 10−17 |
| n | 7.6175 | 6.1802 | 5.3244 | 5.8251 | |
| m | 2.1428 | 2.5713 | 3.9925 | 4.7378 | |
| tmin | −9.4633 | −17.2948 | −0.3747 | −1.9163 | |
| topt | 30.10 | 37.40 | 31.02 | 31.00 | |
| tmax | 41.1822 | 60.0903 | 54.5616 | 57.4548 | |
| Enkegaard | a | 0.0248 | 0.0178 | 0.0407 | 0.0122 |
| b | −68.3956 × 10−5 | −46.1022 × 10−5 | −10.8593 × 10−4 | −31.9438 × 10−5 | |
| c | 1.1245 | 1.1075 | 1.1075 | 1.1075 | |
| d | −0.1835 | −0.1620 | −0.1656 | −0.1535 | |
| topt | 31.11 | 33.00 | 31.90 | 32.01 | |
| tmax | 36.66 | 38.76 | 37.55 | 38.21 | |
| Taylor | Rm | 0.3245 | 0.1780 | 0.3782 | 0.0852 |
| tm (=topt) | 29.6197 | 33.1083 | 30.8630 | 31.2874 | |
| 7.4244 | 9.7909 | 8.6509 | 9.5093 | ||
| Janisch | Dmin | 3.0985 | 5.6899 | 3.6422 | 12.3639 |
| k | −0.1834 | −0.1414 | −0.2354 | −0.1547 | |
| 0.1221 | −0.0975 | −0.0364 | −0.0785 | ||
| topt | 28.0868 | 30.7786 | 23.6969 | 27.90 | |
| Sharpe & DeMichele | a | 5.1991 | −10.5143 | −5.2836 | −8.4871 |
| b | −322.3149 | −1502.5915 | −259.0272 | −1360.8376 | |
| c | 7.9817 | −6.6145 | −2.8555 | −4.5433 | |
| d | −365.7124 | −1536.7211 | −304.9700 | −1403.3453 | |
| f | 17.7782 | 0.1576 | 3.076 | 0.1380 | |
| g | −38.2689 | −1276.7436 | −108.8371 | −1252.6541 | |
| topt | 30.00 | 32.23 | 31.00 | 32.05 | |
| Bieri-1 | a | 0.0209 | 0.103 | 0.0249 | 0.0177 |
| b | 1.5436 | 1.3103 | 1.274 | 1.0288 | |
| xmax | 12.5366 | 12.4235 | 12.2484 | −6.9928 | |
| xmin | 37.99 | 45.5230 | 40.99 | 49.43 | |
| tmin | 12.69 | 12.52 | 12.32 | 12.96 | |
| topt | 31.01 | 33.50 | 32.00 | 33.04 | |
| tmax | 36.33 | 41.03 | 39.38 | 49.76 | |
| Bieri-2 | a | −0.1749 | −0.0813 | −0.1801 | −0.0373 |
| b | 0.8323 | 0.8504 | 0.8473 | 0.8575 | |
| xmin | 36.33 | 38.76 | 37.55 | 38.21 | |
| topt | 31.00 | 33.00 | 31.53 | 32.00 | |
| tmax | 36.33 | 38.76 | 37.55 | 38.21 |
| Model | Parameters | Egg | Larva | Pupa | Total Development |
|---|---|---|---|---|---|
| Briere-1 | R2 | 0.9177 | 0.6358 | 0.8053 | 0.7871 |
| R2adj | 0.8628 | 0.3930 | 0.6755 | 0.6451 | |
| AIC | −35.69 | −35.49 | −28.75 | −46.31 | |
| RSS (10−4) | 57.63 | 83.13 | 183.13 | 72.60 | |
| Briere-2 | R2 | 0.9773 | 0.9716 | 0.9627 | 0.9923 |
| R2adj | 0.9432 | 0.9290 | 0.9069 | 0.9807 | |
| AIC | −42.72 | −47.75 | −38.04 | −65.33 | |
| RSS (10−4) | 12.79 | 5.53 | 27.90 | 0.29 | |
| Logan-6 | R2 | 0.9634 | 0.9444 | 0.9447 | 0.9493 |
| R2adj | 0.9085 | 0.8661 | 0.8617 | 0.8732 | |
| AIC | −39.55 | −44.16 | −35.46 | −54.10 | |
| RSS (10−4) | 21.70 | 10.05 | 42.90 | 1.92 | |
| Logan-10 | R2 | 0.9903 | 0.9757 | 0.9785 | 0.9956 |
| R2adj | 0.9515 | 0.8785 | 0.8925 | 0.9780 | |
| AIC | −47.67 | −49.33 | −41.12 | −69.08 | |
| RSS (10−4) | 5.60 | 4.25 | 16.69 | 1.34 | |
| Lactin-1 | R2 | 0.9735 | 0.9696 | 0.9586 | 0.9919 |
| R2adj | 0.9559 | 0.9494 | 0.9311 | 0.9154 | |
| AIC | −43.52 | −46.19 | −37.45 | −56.10 | |
| RSS (10−4) | 15.61 | 9.99 | 42.90 | 1.91 | |
| Lactin-2 | R2 | 0.9576 | 0.9648 | 0.9587 | 0.9776 |
| R2adj | 0.8940 | 0.9120 | 0.8967 | 0.9440 | |
| AIC | −31.02 | −47.04 | −37.41 | −57.99 | |
| RSS (10−4) | 89.97 | 6.22 | 30.98 | 0.61 | |
| Sigmoid | R2 | 0.9362 | 0.9777 | 0.9731 | 0.9969 |
| R2adj | 0.8936 | 0.9629 | 0.9552 | 0.9948 | |
| AIC | −38.03 | −51.85 | −41.50 | −73.20 | |
| RSS (10−4) | 38.96 | 3.89 | 21.85 | 0.11 | |
| Hilbert & Logan, or Holling III | R2 | 0.9792 | 0.9737 | 0.9697 | 0.9937 |
| R2adj | 0.8963 | 0.8685 | 0.8485 | 0.9686 | |
| AIC | −41.32 | −46.84 | −37.22 | −64.80 | |
| RSS (10−4) | 11.57 | 4.61 | 22.91 | 0.23 | |
| Stinner (T > Topt) | R2 | 0.9362 | 0.9777 | 0.9731 | 0.9969 |
| R2adj | 0.8406 | 0.9440 | 0.9329 | 0.9949 | |
| AIC | −36.03 | −49.85 | −39.50 | −71.20 | |
| RSS (10−4) | 38.96 | 3.89 | 21.85 | 0.11 | |
| Polynomial 3rd order | R2 | 0.9840 | 0.9714 | 0.9728 | 0.9924 |
| R2adj | 0.9600 | 0.9285 | 0.9321 | 0.9810 | |
| AIC | −43.44 | −48.35 | −39.94 | −65.87 | |
| RSS (10−4) | 11.34 | 5.01 | 20.34 | 0.27 | |
| Equation-16 | R2 | 0.9696 | 0.9712 | 0.9661 | 0.9880 |
| R2adj | 0.9493 | 0.9521 | 0.9436 | 0.9801 | |
| AIC | −43.24 | −50.31 | −40.54 | −65.08 | |
| RSS (10−4) | 0.16 | 5.03 | 25.63 | 0.42 | |
| Kontodimas-16 | R2 | 0.9723 | 0.9712 | 0.9659 | 0.9880 |
| R2adj | 0.9539 | 0.9521 | 0.9433 | 0.9801 | |
| AIC | −43.24 | −50.13 | −40.54 | −65.08 | |
| RSS (10−4) | 16.35 | 5.03 | 25.63 | 0.42 | |
| Lamb (T > Topt) | R2 | 0.9870 | 0.9674 | 0.9735 | 0.9825 |
| R2adj | 0.9783 | 0.9456 | 0.9559 | 0.9708 | |
| AIC | −35.68 | −49.51 | −42.08 | −62.64 | |
| RSS (10−4) | 57.64 | 5.76 | 19.85 | 0.64 | |
| Analytis | R2 | 0.9878 | 0.9517 | 0.9718 | 0.9848 |
| R2adj | 0.9493 | 0.7587 | 0.8592 | 0.9241 | |
| AIC | −44.37 | −42.41 | −37.68 | −58.50 | |
| RSS (10−4) | 6.95 | 9.62 | 21.23 | 0.55 | |
| Enkegaard | R2 | 0.9735 | 0.9450 | 0.9447 | 0.9492 |
| R2adj | 0.9339 | 0.8625 | 0.8618 | 0.8732 | |
| AIC | −41.52 | −44.19 | −35.45 | −54.10 | |
| RSS (10−4) | 15.61 | 9.90 | 42.90 | 1.91 | |
| Taylor | R2 | 0.9870 | 0.9674 | 0.9735 | 0.9825 |
| R2adj | 0.9783 | 0.9456 | 0.9559 | 0.9708 | |
| AIC | −47.52 | −49.51 | −42.08 | −62.64 | |
| RSS (10−4) | 8.02 | 5.76 | 19.85 | 0.64 | |
| Janisch | R2 | 0.9928 | 0.9595 | 0.9821 | 0.9743 |
| R2adj | 0.9821 | 0.8988 | 0.9553 | 0.9358 | |
| AIC | −49.55 | −45.92 | −42.25 | −58.34 | |
| RSS (10−4) | 4.09 | 7.49 | 13.83 | 0.95 | |
| Sharpe & DeMichele | R2 | 0.9875 | 0.9668 | 0.9759 | 0.9939 |
| R2adj | * | * | * | * | |
| AIC | −42.09 | −45.54 | −40.50 | −63.08 | |
| RSS (10−4) | 7.29 | 5.82 | 18.51 | 0.22 | |
| Bieri-1 | R2 | 0.9700 | 0.9676 | 0.9565 | 0.9915 |
| R2adj | 0.9250 | 0.9190 | 0.8913 | 0.9792 | |
| AIC | −41.04 | −47.59 | −37.11 | −65.24 | |
| RSS (10−4) | 16.90 | 5.67 | 32.65 | 0.29 | |
| Bieri-2 | R2 | 0.9735 | 0.9449 | 0.9447 | 0.9492 |
| R2adj | 0.9559 | 0.9083 | 0.9079 | 0.9154 | |
| AIC | −43.52 | −49.59 | −39.11 | −56.10 | |
| RSS (10−4) | 15.61 | 9.99 | 42.90 | 1.91 |
| Life Stage | Tmin ± SE (°C) | Model | Topt ± SE a (°C) | Model | Tmax ± SE (°C) | Model |
|---|---|---|---|---|---|---|
| Egg | 11.17 ± 3.0183 | Briere-2 | 31.00 | Briere-2 | 38.43 ± 3.40 | Logan-10 |
| Larva | 12.20 ± 3.7379 | Briere-2 | 35.75 | Briere-2 | 44.03 ± 3.74 | Logan-10 |
| Pupa | 11.24 ± 4.2108 | Briere-2 | 31.50 | Briere-2 | 36.97 ± 4.98 | Logan-10 |
| Total development | 11.96 ± 1.3177 | Briere-2 | 32.00 | Briere-2 | 44.03 ± 3.52 | Logan-10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, M.; Goldasteh, S.; Aghdam, H.R.; Zamani, A.A.; Soleyman-Nejadian, E.; Schausberger, P. Modeling Thermal Developmental Trajectories and Thermal Requirements of the Ladybird Stethorus gilvifrons. Insects 2023, 14, 11. https://doi.org/10.3390/insects14010011
Jafari M, Goldasteh S, Aghdam HR, Zamani AA, Soleyman-Nejadian E, Schausberger P. Modeling Thermal Developmental Trajectories and Thermal Requirements of the Ladybird Stethorus gilvifrons. Insects. 2023; 14(1):11. https://doi.org/10.3390/insects14010011
Chicago/Turabian StyleJafari, Maryam, Shila Goldasteh, Hossein Ranjbar Aghdam, Abbas Ali Zamani, Ebrahim Soleyman-Nejadian, and Peter Schausberger. 2023. "Modeling Thermal Developmental Trajectories and Thermal Requirements of the Ladybird Stethorus gilvifrons" Insects 14, no. 1: 11. https://doi.org/10.3390/insects14010011
APA StyleJafari, M., Goldasteh, S., Aghdam, H. R., Zamani, A. A., Soleyman-Nejadian, E., & Schausberger, P. (2023). Modeling Thermal Developmental Trajectories and Thermal Requirements of the Ladybird Stethorus gilvifrons. Insects, 14(1), 11. https://doi.org/10.3390/insects14010011






