Simple Summary
Essential oils (EOs) from plants are promising products for pest management. This paper describes the chemical composition and repellent action of four EOs against Ulomoides dermestoides, a common pest on several stored products. Most abundant chemical components found in the EOs were sabinene, trans-β-caryophyllene, and α-humulene for phellandrene-rich Lippia origanoides; limonene and carvone for carvone-rich Lippia alba; geranial, geraniol, and neral for citral-rich Lippia alba; and α-guaiene, α-bulnesene, and patchoulol for Pogostemon cablin. The repellent bioactivity, carried out utilizing the area preference method, showed that all EOs displayed great repellency with low mortality rates, suggesting these natural mixtures can be used in formulations of repellents against stored grain pests.
Abstract
The essential oils (EOs) from bioactive species can provide an alternative tool for the management of stored grain insects that is less environmentally damaging than synthetic chemicals. The aim of this study was to assess the repellent action and toxicity of EOs obtained from phellandrene-rich Lippia origanoides, carvone-rich Lippia alba, citral-rich L. alba, and Pogostemon cablin aerial parts on adults of Ulomoides dermestoides. These EOs were isolated by hydrodistillation and characterized by gas chromatography coupled to mass spectrometry (GC-MS). The repellency assay was carried out using the area preference method, and the toxicity evaluated utilizing a filter paper contact test. The major components (>10%) of the studied EOs were sabinene (16.9%), trans-β-caryophyllene (18.6%) and α-humulene (10.1%) for phellandrene-rich L. origanoides EO; limonene (40.1%) and carvone (37.7%) for carvone-rich L. alba EO; geranial (24.5%), geraniol (19.0%), and neral (11.9%) for citral-rich L. alba EO; and α-guaiene (13.3%), α-bulnesene (15.7%), and patchoulol (35.3%) for P. cablin EO. All EOs displayed 100% repellency at a concentration of 16 μL/mL, with lower toxicity than that elicited by the commercial repellent DEET. EO concentrations up to 8 µL/mL did not induce any mortality on the beetle. These findings show that the EOs provide active and safe molecules for natural repellent formulations to prevent and control insect infestations of stored products.
1. Introduction
About 30% of stored food products are highly vulnerable to post-harvest loss, both in terms of quality and quantity [1]. Insect pest infestations represent a major threat to these products, which in turn generate suitable conditions for the growth of bacteria and fungi [2].
The uncontrolled and excessive use of synthetic pesticides has caused serious environmental and health problems [3,4,5]. In order to avoid multiple post-harvest losses and guarantee food security, various strategies for pest control are being developed. The search for natural products is a good alternative to replace synthetic pesticides, since they are a rich source of secondary metabolites with biological activity, and beneficial properties with low toxicity in exposed organisms. Essential oils (EOs) are mixtures of small molecules that can be used as active ingredients, and are widely studied for the discovery of new bioactive compounds against pest insects. In fact, essential oil compounds such as 1,8-cineole and limonene have been used as active ingredients in mosquito repellents, flea shampoos, and insecticide-related formulations [6].
The peanut beetle, Ulomoides dermestoides Fairmaire (Coleoptera: Tenebridae), is considered a worldwide pest of stored grains (corn, oats, peanuts, rice, among others), which has been controlled with chemical insecticides. However, the use of these products has generated environmental contamination and insect resistance to these synthetic substances. Essential oils have proven to be friendly to the environment, due to their biodegradability, insect selectivity, and low toxicity to vertebrates [2,7,8], playing an important role in insect-plant interactions, and being part of defense strategies against herbivorous insects. Some of the components of EOs exert toxic effects on insects, either by contact, ingestion, or fumigation, as well as deterrence, inhibition of feeding, and repellency [9].
On the other hand, there is an interest in studying and evaluating the use of EOs for pest management, mainly as a result of insect resistance to the synthetic insecticides. In fact, various studies have investigated the bioactivity of EOs and their potential uses as bio-insecticides against insect pests. In this sense, EOs from Lippia origanoides (phellandrene), L. alba (carvone), and Pogostemon cablin have shown repellent and insecticidal properties against Aedes aegypti [10], Sitophilus zeamais [11], and Tribolium castaneum [11,12].
The present study aimed to determine the chemical composition of the essential oils from phellandrene-rich L. origanoides, carvone-rich L. alba, citral-rich L. alba, and P. cablin, and evaluate their repellent action and toxicity on adults of U. dermestoides.
2. Materials and Methods
2.1. Insects
Adult insects of the beetle Ulomoides dermestoides (Coleoptera: Tenebrionidae) were housed in glass jars covered with a nylon mesh held with rubber bands, under standard laboratory conditions of 28 °C (±1 °C), a 10:14 h light:dark photoperiod, and relative humidity of 70–85%. Insect maintenance, feeding, and breeding followed the established laboratory protocols in the bioassay laboratory of the University of Cartagena (Colombia) [13]. Adult, healthy U. dermestoides, 4-8 days old, were randomly chosen for bioassays.
2.2. Plant Material and Extraction of EOs
EOs of Lippia origanoides, phellandrene-rich chemotype, Lippia alba, carvone-rich chemotype, Lippia alba, citral-rich chemotype, and Pogostemon cablin were obtained from aerial parts using steam distillation in a 0.4 m3 stainless steel column and separated by decantation. Subsequently, they were dried with Na2SO4 and stored at 4 °C in amber flasks.
2.3. Characterization of EOs
Each EO (50 mg) was dissolved in 1 mL of CH2Cl2. The dilution (2 µL) was injected into a gas chromatograph coupled to a mass selective detector. The analysis was performed in a gas chromatograph, GC 6890 Plus (Agilent Technologies, Palo Alto, CA, USA), equipped with a selective mass detector, MS 5973 Network (AT, Palo Alto, CA, USA), using electron ionization (EI, 70 eV). Helium (99.995%, AP gas, Messer, Bogotá, Colombia) was used as carrier gas, with initial inlet pressure at the head of the column of 113.5 kPa; the volumetric flow rate of carrier gas during the chromatographic run was kept constant (1 mL/min). The injection mode was split (30:1) and the injector temperature was kept at 250 °C.
Chromatographic separation of EO components was carried out in two capillary columns, one with poly(ethylene glycol), PEG, as a stationary phase (DB-WAX, J & W Scientific, Folsom, CA, USA), 60 m × 0.25 mm (i.d) × 0.25 μm (df), and the other containing 5%-phenyl-poly(methylsiloxane) (DB-5MS, J&W Scientific, Folsom, CA, USA), displaying the same dimensions as the polar one. When using the polar column (DB-WAX), the oven temperature was programmed from 50 °C (5 min) to 150 °C (7 min), at 4 °C/min, and then up to 230 °C (50 min), at 4 °C/min. When the apolar column (DB-5MS) was used, temperature was programmed from 45 °C (5 min) to 150 °C (2 min), at 4 °C/min, then up to 300 °C (10 min), at 5 °C/min. The GC/MS transfer line temperature was set at 230 °C when the polar column was used and at 300 °C for the apolar column. The temperatures of the ionization chamber and the quadrupole were 250 °C and 150 °C, respectively. The mass range for the acquisition of ionic currents was m/z 45–450 u, with an acquisition speed of 3.58 scan/s. The data was processed with the MSD Chem Station software (AT, Palo Alto, CA, USA). The integration parameters were the following: threshold = 18, with a rejection area of the peak above the baseline less than 1%. The identification of compounds was carried out based on their linear retention indices (LRI), calculated from the retention times of the compound of interest, and the C6–C25 and C8–C40 n-alkanes (Sigma-Aldrich, San Luis, MO, USA), according to the used temperature (Equation (1)):
where LRI is the linear retention index of the compound of interest (x), n is the carbon number of the n-alkane that elute first, and N the carbon number of the n-alkane that elutes after the x; tRx is the retention time of the compound of interest (min), and tRn and tRN are the retention times of n-alkanes that elute before (n) or after (N) the compound of interest (x) (min), respectively.
LRI = (100 × n) + 100 × [(tRx − tRn)/(tRN − tRn)]
For tentative identification, experimental mass spectra of each compound was compared to that from Adams [14], NIST [15], and Wiley spectral databases. Confirmatory identification of some detected compounds was performed by comparing their LRI and mass spectra with those of available standard substances.
2.4. Repellent Action of EOs
The experiments were carried out in Petri dishes (9 cm in diameter) employing the area preference method on filter paper [16] under standard laboratory conditions of 28 °C (±1 °C), a 10:14 h light:dark photoperiod, and relative humidity of 70–85%. Briefly, each paper filter was cut in two identical pieces, one (left) treated with 500 µL acetone (negative control), and the other (right) with 500 µL of 2–16 μL/mL of each EO dissolved in acetone. The solvent was allowed to evaporate for 10 min, and then the two halves were re-attached with adhesive tape. Ten unsexed adults of U. dermestoides were deposited in the center of the filter paper and the Petri dish was closed with its fitting cover and stored in the absence of light. After exposure (2 and 4 h), experimental units were counted in both areas. A commercial repellent containing 99.8% DEET was utilized as a positive control. The Percentage of Repellency (PR) and Preference Index (PI) were defined as PI = (percentage of insects in treated paper − percentage of insects in control paper)/(percentage of insects in treated paper + percentage of insects in control paper). PI values ranging between −1.0 and −0.1 indicate the EO has repellent properties; values from −0.1 to +0.1 suggest the EO has neither repellent nor attractant behaviors; and those between +0.1 and +1.0 make the EO an insect attractant. Each experiment was carried out twice, with four replicates each.
2.5. Contact Toxicity on Filter Paper
The contact toxicity on filter papers was conducted using Whatman grade 1 filter (Catalog number 1001090, Whatman International Ltd, Maidstone, UK), 9 cm in diameter [17]. One mL of acetone (Merck, Darmstadt, Germany) or treatment (EO in acetone, 2–16 µL/mL) was dispensed on the surface of the paper that was then placed in a glass Petri dish. DEET (99.8%) (WPC Brands, Inc, Bridgeton, MO, USA) was utilized as positive control. Once the solvent evaporated (10 min), ten unsexed adults were added on each disc, closed with a fitting cover and then stored in darkness under laboratory conditions. Mortality was assessed after 24 and 48 h. The insects were considered dead when no leg or antennal movements were observed. The experiment was repeated twice, using for replicates.
2.6. Data Analysis
The data are presented as the mean ± standard error (x ± SE). The paired t-test was utilized to compare mean number of insects on the treated and untreated area of the filter paper. Percentage repellency was preceded with a positive or negative sign, indicating repellency or attraction, respectively. Data obtained from each bioassay were subjected to Probit analysis, and 50% repellence concentrations (RC50) were determined by log-Probit regression. Normal distribution and homogeneity of variances were assessed using Shapiro–Wilk and Bartlett tests, respectively. Data from the assays were arcsine-transformed and subjected to ANOVA to determine differences between means of different treatments, employing Dunnett as a post-hoc test. Chi-square was applied to establish relationships between repellency and treatments. Calculations were carried out using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Significance was set at p < 0.05.
3. Results
3.1. Chemical Composition of EOs
The major compounds (relative amount ≥5%) found in phellandrene-rich L. origanoides, carvone-rich L. alba, citral-rich L. alba, and P. cablin EOs are listed in Table 1. In the phellandrene-rich L. origanoides EO, sabinene (16.9%), trans-β-caryophyllene (18.6%), α-humulene (10.1%), p-cimene (8.7%), and 1,8-cineol (6.5%) were the major constituents, representing 60.8% of the EO. The major components in the carvone-rich L. alba EO were limonene (40.1%), carvone (37.7%), and germacrene D (8.1%), accounting for 85.9% of the EO. In the L. alba, citral-rich chemotype EO, geranial (24.5%), geraniol (19.0%), neral (11.9%), and trans-β-caryophyllene (9.1%), were the most abundant, accounting for 64.5% of the EO. The major compounds in the P. cablin EO were patchoulol (35.3%), α-bulnesene (15.7%), α-guaiene (13.3%), seychellene (8.5%), and α-patchoulene (6.3%), adding up 79.1% of the EO. Chromatographic profiles for testes EOs, as well as the mass spectra for most identified compounds, are provided in Supplementary Material (Figure S1–S4).

Table 1.
Chemical composition of phellandrene-rich L. origanoides, carvone-rich L. alba, citral-rich L. alba, and P. cablin essential oils.
3.2. Repellent Action on U. dermestoides Adults
The repellent effects of the EOs and the commercial repellent DEET on adult U. dermestoides are displayed in Table 2. The EOs from L. alba, carvone and citral chemotypes, as well as the EO extracted from P. cablin were strongly repellent to U. dermestoides. These EOs exhibited PR greater than 80% at the lowest concentration (2 μL/mL) after 2 h exposure. In the four evaluated EOs, no attractive action was found, in contrast with that observed for DEET at the lowest concentration after exposure to 2 h (−13 ± 11) and 4 h (−25 ± 13). The commercial repellent displayed less efficacy than the four tested EOs, with PR lower than 100% at the highest tested concentration (16 μL/mL, 2 h exposure). Interestingly, all four EOs were found to be more repellent (RC50 1.0–4.3 μL/mL) than DEET (RC50 5.9–9.1 μL/mL) under the same experimental conditions. Significant differences at all tested concentrations in L. alba, carvone and citral chemotypes, and P. cablin EOs, as well as commercial repellent DEET were registered between the average number of insects in the treated and untreated areas. In addition, no statistical differences were detected between PRs when the same tested concentrations of EOs were compared at 2 and 4 h exposure.

Table 2.
Percentage repellency (PR) and mean repellent concentration value (RC50) for EOs and DEET against U. dermestoides after two exposure times.
The preference index calculated for tested EOs are shown in Table 3. All EOs displayed PI values between −1.0 and −0.1, indicating that these EOs are repellents against U. dermestoides adults. This same pattern was found in the commercial repellent DEET except for the lowest concentration (2 μL/mL) where the PI had an attractant effect.

Table 3.
Preference index (PI) for insects treated with essential oils or commercial repellent in different concentrations after two exposure times.
3.3. Contact Toxicity
The results of contact toxicity are presented in Figure 1. Compared to DEET, the four evaluated EOs exhibited lower toxicity against U. dermestoides. The toxicity decreased in the order carvone-rich L. alba > citral-rich L. alba> phellandrene-rich L. origanoides > P. cablin. In contrast, the commercial repellent caused more than 80% mortality at the lowest tested concentration (2 μL/mL) at both 24 and 48 h exposure. An interesting point displayed in Figure 1 is that the biological activity of the EOs was more variable than that elicited by DEET.
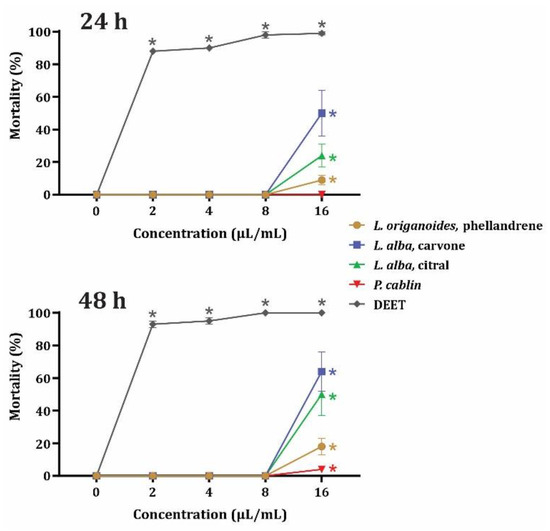
Figure 1.
Percentage mortality of U. dermestoides adults exposed to EOs from phellandrene-rich L. origanoide, carvone-rich L. alba, citral-rich L. alba, and P. cablin, after 24 and 48 h exposures. Data (n = 8) are presented as mean ± SEM. *. Significant difference compared to the negative control.
4. Discussion
Essential oils and their components possess different bioactivities that have been employed for pharmacological, medicinal, aromatic or cosmetic purposes. In addition, they are considered to be an alternative for the control of insect pests of stored products [21]. In this study, several EOs were chemically characterized by GC-MS and tested their repellent and toxicity properties against U. dermestoides under laboratory conditions.
EOs from L. alba (carvone and citral chemotypes) exhibited a high repellency action against U. dermestoides adults. These results were similar to those reported for others insects, such as Sitophilus zeamais [11], Tribolium castaneum [11], and Rhipicephalus microplus [22]. Species of the genus Lippia (Verbenaceae) are characterized by their wide distribution and medicinal importance. In Colombia, L. origanoides species have been reported to have properties such as antioxidant [23], repellent [24], antifungal [25], and fumigant [13]. Moreover, L. alba species have shown antifungal, antigenotoxic [26], antibacterial [27], and repellent properties [28], among others.
The repellent action of the different L. alba chemotypes is a function of their composition. In this study, most abundant compounds present in the L. alba EOs, such as limonene, carvone, geranial, geraniol, and neral have been evaluated as repellents against different insect pest of stored products [28,29,30]. In the case of carvone and geraniol, main compounds present in L. alba, displayed repellency percentages greater than 90% against Tribolium castaneum [28]. In addition, Zhang et al. [31] reported that geraniol was a strong repellent on the booklouse, Liposcelis bostrychophila, and limonene showed great repellent activity against T. castaneum and Lasioderma serricorne.
Pogostemon cablin, also known as “patchouli”, is an aromatic plant member of the Lamiaceae family, widely cultivated in many tropical and subtropical regions. The main compounds found in P. cablin oil were similar to those reported by other authors, with relatively small differences in concentrations, reaching a maximum of 2.1% in the case of α-guaiene [32,33]; 3.8% for α-bulnesene [32]; 2,7% for α-patchoulene [32]; 4.8% for seychellene [33]; and 12.7–15.8% for patchoulol [32,34]. This EO has also been tested as a repellent in Tribolium castaneum, Lasioderma serricorne, and Liposcelis bostrychophila [34] with moderate results, suggesting a broader-spectrum use, in particular combined with other potent EOs, such as those from Lippia species.
According to Peixoto et al. [11] carvone chemotypes were more toxic than the citral chemotypes against Sitophilus zeamais and Tribolium castaneum, a behavior similar to that presented here. Interestingly, compared to DEET, EOs evaluated in this research showed lower toxicity against adult U. dermestoides. This has a two-fold consequence. First, the EOs from species reported here are more environmentally friendly from a contact toxicity perspective; and second, their combined use guarantees the presence of multiple molecules that can alleviate possible resistance-related problems linked to the use of individual compounds when used as repellents.
5. Conclusions
The EOs from L. alba carvone and citral chemotypes, as well as that from P. cablin have great potential to act as repellents on U. dermestoides, with lower toxicity compared to DEET. The combination of these EOs in formulations of environmentally friendly repellents is highly encouraged.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14010041/s1, Figure S1: Chromatographic profiles obtained by GC/MS (full scan) of L. origanoides, phellandrene chemotype EO, DB-5MS and DB-WAX columns (60 m), split 1:30, MSD (EI, 70 eV). Compounds: α-Pinene (1), Camphene (2), Sabinene (3), β-Pinene (4), β-Myrcene (5), α-Phellandrene (6), p-Cymene (7), Limonene (8), β-Phellandrene (9), 1,8-Cineole (10), γ-Terpinene (11), Linalool (12), Borneol (13), Terpinen-4-ol (14), α-Cubebene (15), α-Copaene (16), β-Elemene (17), trans-β-Caryophyllene (18), β-Copaene (19), α-Humulene (20), γ-Muurolene (21), Amorpha-4,7(11)-diene (22), Germacrene D (23), β-Selinene (24), α-Muurolene (25), α-Selinene (26), δ-Cadinene (27), cis-Calamenene (28), N.I. M+• m/z 204 (Figure S1a) (29), Caryophyllene oxide (30), Guaiol (31), Humulene epoxide II (32), γ-Eudesmol (33), Coelution N.I. M+• m/z 220 + N.I. M+• m/z 204 (34), Caryophylla-4(12),8(13)-dien-5β-ol (35), α-Cadinol (36), α-Eudesmol (37), and N.I. M+• m/z 222 (Figure S1b) (38); Figure S1a: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 29 (N.I. M+• m/z 204); Figure S1b: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 38 (N.I. M+• m/z 222); Figure S2: Chromatographic profiles obtained by GC/MS (full scan) of L. alba, carvone chemotype EO, DB-5MS and DB-WAX columns (60 m), split 1:30, MSD (EI, 70 eV). Compounds: cis-Hex-3-en-1-ol (1), α-Pinene (2), Camphene (3), Oct-1-en-3-ol (4), β-Mircene (5), Octan-3-ol (6), Limonene (7), trans-β-Ocimene (8), Linalool (9), trans-p-Mentha-2,8-dien-1-ol (10), cis-Limonene oxide (11), Borneol (12), cis-Dihydrocarvone (13), trans-Dihydrocarvone (14), cis-Carveol (15), neo-iso-Dihydrocarveol (16), Carvone (17), Piperitone (18), Geranial (19), trans-Carvyl acetate (20), Piperitenone (21), α-Copaene (22), β-Bourbonene (23), β-Elemene (24), β-Copaene (25), trans-β-Caryophyllene (26), β-Gurjunene (27), trans-β-Farnesene (28), trans-9-epi-Caryophyllene (29), Germacrene D (30), Bicyclogermacrene (31), and α-Cadinene (32); Figure S3: Chromatographic profiles obtained by GC/MS (full scan) of L. alba, citral chemotype EO, DB-5MS column (60 m), split 1:30, MSD (EI, 70 eV). Compounds: cis-Hex-3-en-1-ol (1), α-Pinene (2), Oct-1-en-3-ol (3), 6-Methyl-hept-5-en-2-ona (4), α-Phellandrene (5), Limonene (6), trans-β-Ocimene (7), Linalool (8), Citronellal (9), Isocitral (10), Isogeranial (11), Nerol (12), Neral (13), Geraniol (14), Geranial (15), Neryl acetate (16), β-Elemene (17), Geranyl acetate (18), trans-β-Caryophyllene (19), trans-β-Caryophyllene (20), trans-β-Farnesene (21), α-Humulene (22), Germacrene D (23), Geranyl isobutanoate (24), α-Bulnesene (25), trans-α-Bisabolene (26), Caryophyllene oxide (27), N.I. M+• m/z 286 (Figure S3a) (28);Figure S3a: mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 286 (N.I. M+• m/z 286); Figure S4: Chromatographic profiles obtained by GC/MS (full scan) of L. alba, citral chemotype EO, DB-5MS and DB-WAX columns (60 m), split 1:30, MSD (EI, 70 eV). Compounds: α-Pinene (1), β-Pinene (2), β-Patchoulene (3), Cycloseychellene (4), α-Patchoulene (5), trans-β-Caryophyllene (6), α-Patchoulene (7), Seychellene (8), γ-Patchoulene (9), α-Humulene (10), N.I. M+• m/z 204 (Figure S4a) (11), δ-Selinene (12), Aciphyllene (13), γ-Gurjunene (14), α-Bulnesene (15), α-Selinene (16), 7-epi-α-Selinene (17), Nootkatene (18), N.I. M+• m/z 220 (Figure S4b) (19), N.I. M+• m/z 220 (Figure S4c) (20), Caryophyllene oxide (21), Humulene epoxide II (22), Norpatchoulenol (23), N.I. M+• m/z 222 (Figure S4d) (24), N.I. M+• m/z 222 (Figure S4e) (25), Patchoulol (26), N.I. M+• m/z 206 (Figure S4f) (27), Pogostol (28), Coelution N.I. M+• m/z 222 + N.I. M+• m/z 220 (29), Rotundone (30), N.I. M+• m/z 220 (Figure S4g) (31), N.I. M+• m/z 222 (Figure S4h) (32), N.I. M+• m/z 218 (Figure S4i) (33), Dehydrofukinone (34), N.I. M+• m/z 218 (Figure S4j) (35), and Pogostone (36); Figure S4a: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 204 (N.I. M+• m/z 204); Figure S4b: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 220 (N.I. M+• m/z 220); Figure S4c: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 220 (N.I. M+• m/z 220); Figure S4d: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 222 (N.I. M+• m/z 222); Figure S4e: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 222 (N.I. M+• m/z 222); Figure S4f: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 206 (N.I. M+• m/z 206); Figure S4g: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 220 (N.I. M+• m/z 220); Figure S4h: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 222 (N.I. M+• m/z 222); Figure S4i: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 218 (N.I. M+• m/z 218); Figure S4j: Mass spectra (EI, 70 eV) of the unidentified compound, Peak No. 218 (N.I. M+• m/z 218).
Author Contributions
Conceptualization, J.O.-V. and K.C.-G.; methodology, J.O.-V., K.C.-G., K.F.-L. and E.E.S.; software, E.E.S., J.O.-V. and K.C.-G.; validation, E.E.S. and J.O.-V.; formal analysis, J.O.-V. and K.C.-G.; investigation, E.E.S., J.O.-V., K.F.-L. and K.C.-G.; resources, J.O.-V.; data curation, K.C.-G. and J.O.-V.; writing—original draft preparation, K.C.-G. and J.O.-V.; writing—review and editing, K.C.-G. and J.O.-V.; visualization, J.O.-V.; supervision, J.O.-V.; project administration, K.C.-G. and J.O.-V.; funding acquisition, E.E.S. and J.O.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research and the APC were funded by the Ministry of Science, Technology and Innovation (Minciencias), the Ministry of Education, the Ministry of Industry, Commerce and Tourism, and ICETEX, Program Ecosistema Científico–Colombia Científica, from the Francisco José de Caldas Fund (Grant RC-FP44842-212-2018). The Ministry of Environment and Sustainable Development of Colombia supported the Universidad Industrial de Santander through access permits to genetic resources and derivatives for bioprospecting (Contract No. 270-2019). The funders had no role in the design of the study, the collection, analyses, or interpretation of data, the writing of the manuscript or the decision to publish the results.
Data Availability Statement
All datasets used in this study can be provided by the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boxall, R.A. Damage and loss caused by the larger grain borer prostephanus truncates. Integr. Pest Manag. Rev. 2002, 7, 105–121. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Sabarwal, A.; Kumar, K.; Singh, R.P. Hazardous effects of chemical pesticides on human health–Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018, 63, 103–114. [Google Scholar] [CrossRef]
- Saroj, A.; Oriyomi, O.V.; Nayak, A.K.; Haider, S.Z. Phytochemicals of plant-derived essential oils: A novel green approach against pests. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–79. [Google Scholar]
- Sánchez-Gómez, S.; Pagán, R.; Pavela, R.; Mazzara, E.; Spinozzi, E.; Marinelli, O.; Zeppa, L.; Morshedloo, M.R.; Maggi, F.; Canale, A. Lethal and sublethal effects of essential oil-loaded zein nanocapsules on a zoonotic disease vector mosquito, and their non-target impact. Ind. Crops Prod. 2022, 176, 114413. [Google Scholar] [CrossRef]
- Oguh, C.; Okpaka, C.; Ubani, C.; Okekeaji, U.; Joseph, P.; Amadi, E. Natural pesticides (biopesticides) and uses in pest management-a critical review. Asian J. Biochem. Genet. Eng. 2019, 2, 1–18. [Google Scholar]
- Plata-Rueda, A.; Martínez, L.C.; da Silva Rolim, G.; Coelho, R.P.; Santos, M.H.; de Souza Tavares, W.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and repellent activities of Cymbopogon citratus (Poaceae) essential oil and its terpenoids (citral and geranyl acetate) against Ulomoides dermestoides. Crop Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Ríos, N.; Stashenko, E.E.; Duque, J.E. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae). Rev. Bras. Entomol. 2017, 61, 307–311. [Google Scholar] [CrossRef]
- Peixoto, M.G.; Bacci, L.; Blank, A.F.; Araújo, A.P.A.; Alves, P.B.; Silva, J.H.S.; Santos, A.A.; Oliveira, A.P.; da Costa, A.S.; de Fátima Arrigoni-Blank, M. Toxicity and repellency of essential oils of Lippia alba chemotypes and their major monoterpenes against stored grain insects. Ind. Crops Prod. 2015, 71, 31–36. [Google Scholar] [CrossRef]
- Bagade, R.P.; Jadhav, A.D.; Chavan, R.V. Toxicity and repellency of four plant essential oils against Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). Int. J. Trop. Insect Sci. 2021, 41, 1505–1512. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Stashenko, E.E.; Olivero-Verbel, J. Repellent and fumigant actions of the essential oils from Elettaria cardamomum (L.) Maton, Salvia officinalis (L.) Linnaeus, and Lippia origanoides (V.) Kunth against Tribolium castaneum and Ulomoides dermestoides. J. Essent. Oil-Bear. Plants 2019, 22, 18–30. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Flow, IL, USA, 2007; ISBN 1932633219. [Google Scholar]
- NIST. NIST Database. Versión 2.3; NIST: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Zandi-Sohani, N.; Hojjati, M.; Carbonell-Barrachina, Á.A. Bioactivity of Lantana camara L. essential oil against Callosobruchus maculatus (Fabricius). Chil. J. Agric. Res. 2012, 72, 502. [Google Scholar] [CrossRef]
- Tapondjou, A.; Adler, C.; Fonte, D.; Bouda, H.; Reichmuth, C. Bioactivities of cymol and essential oils of Cupressus sempervirens and Eucalyptus saligna against Sitophilus zeamais Motschulsky and Tribolium confusum du Val. J. Stored Prod. Res. 2005, 41, 91–102. [Google Scholar] [CrossRef]
- Babushok, V.; Linstrom, P.; Zenkevich, I. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011, 40, 1–47. [Google Scholar] [CrossRef]
- Davies, N. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Joulain, D. The essential oil of patchouli, Pogostemon cablin: A review. Flavour Fragr. J. 2018, 33, 6–51. [Google Scholar] [CrossRef]
- Mota Filho, T.M.M.; da Silva Camargo, R.; de Menezes, C.W.G.; Zanuncio, J.C.; Osorio, A.M.B.; Ferraz, V.P.; Gomes Brito, E.S.; Araújo, C.R.R. Chemical composition of Cymbopogon flexuosus (Poaceae) essential oil, its insecticidal and repellency activity against Sitophilus zeamais (Coleoptera: Curculionidae). Int. J. Trop. Insect Sci. 2022, 42, 2701–2708. [Google Scholar] [CrossRef]
- Da Silva Lima, A.; De Carvalho, J.F.; Peixoto, M.G.; Blank, A.F.; Borges, L.M.F.; Costa Junior, L.M. Assessment of the repellent effect of Lippia alba essential oil and major monoterpenes on the cattle tick Rhipicephalus microplus. Med. Vet. Entomol. 2016, 30, 73–77. [Google Scholar] [CrossRef]
- Stashenko, E.; Ruiz, C.; Muñoz, A.; Castañeda, M.; Martínez, J. Composition and antioxidant activity of essential oils of Lippia origanoides HBK grown in Colombia. Nat. Prod. Commun. 2008, 3, 1934578X0800300417. [Google Scholar]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. J. Stored Prod. Res. 2012, 50, 62–65. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.; Zapata, B.; Baena, A.; Bueno, J.; Ruíz-Nova, C.A.; Stashenko, E.; Mesa-Arango, A.C. Antifungal, cytotoxic and chemical analyses of essential oils of Lippia origanoides HBK grown in Colombia. Rev. Univ. Ind. Santander 2011, 43, 141–148. [Google Scholar]
- López, M.A.; Stashenko, E.E.; Fuentes, J.L. Chemical composition and antigenotoxic properties of Lippia alba essential oils. Genet. Mol. Biol. 2011, 34, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Barreto-Maya, A.; Bertel-Sevilla, A.; Stashenko, E.E. Composition, anti-quorum sensing and antimicrobial activity of essential oils from Lippia alba. Braz. J. Microbiol. 2014, 45, 759–767. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J. Agric. Food Chem. 2011, 59, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Zhang, W.; Liang, J.; You, C.; Geng, Z.; Wang, C.; Du, S. Contact and repellent activities of the essential oil from Juniperus formosana against two stored product insects. Molecules 2016, 21, 504. [Google Scholar] [CrossRef]
- Reis, S.L.; Mantello, A.G.; Macedo, J.M.; Gelfuso, E.A.; Da Silva, C.P.; Fachin, A.L.; Cardoso, A.M.; Beleboni, R.O. Typical monoterpenes as insecticides and repellents against stored grain pests. Molecules 2016, 21, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Zhao, N.N.; Liu, Q.Z.; Liu, Z.L.; Du, S.S.; Zhou, L.; Deng, Z.W. Repellent constituents of essential oil of Cymbopogon distans aerial parts against two stored-product insects. J. Agric. Food Chem. 2011, 59, 9910–9915. [Google Scholar] [CrossRef]
- Gokulakrishnan, J.; Kuppusamy, E.; Shanmugam, D.; Appavu, A.; Kaliyamoorthi, K. Pupicidal and repellent activities of Pogostemon cablin essential oil chemical compounds against medically important human vector mosquitoes. Asian Pac. J. Trop. Dis. 2013, 3, 26–31. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R. Chemical composition of leaves, inflorescence, whole aerial-parts and root essential oils of patchouli {Pogostemon cablin (Blanco) Benth.}. J. Essent. Oil Res. 2019, 31, 319–325. [Google Scholar] [CrossRef]
- Feng, Y.-X.; Wang, Y.; You, C.-X.; Guo, S.-S.; Du, Y.-S.; Du, S.-S. Bioactivities of patchoulol and phloroacetophenone from Pogostemon cablin essential oil against three insects. Int. J. Food Prop. 2019, 22, 1365–1374. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).