Multi-Omics Reveals the Effect of Population Density on the Phenotype, Transcriptome and Metabolome of Mythimna separata
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Materials and Treatments
2.2. Growth Record and Sampling
2.3. Transcriptomic Analysis
2.4. Metabolomic Analysis
3. Results
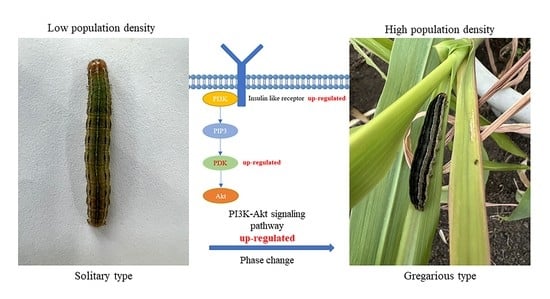
3.1. Effect of High Density on Phase Change of M. separata
3.2. Effect of Density on Transcriptome of M. separata
3.3. Effect of Density on Metabolome of M. separata
3.4. Association Analysis of Transcriptomic and Metabolomic Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Simpson, S.J.; Sword, G.A.; Lo, N. Polyphenism in insects. Curr. Biol. 2011, 21, R738–R749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kang, L. Molecular mechanisms of phase change in locusts. Annu. Rev. Entomol. 2014, 59, 225–244. [Google Scholar] [CrossRef]
- Ogawa, K.; Miura, T. Aphid polyphenisms: Trans-generational developmental regulation through viviparity. Front. Physiol. 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nakasuji, F. Effects of day length and density on development and wing form of the small brown planthopper, Laodelphax striatellus (Hemiptera: Delphacidae). Appl. Entomol. Zoolog. 1991, 26, 557–561. [Google Scholar] [CrossRef]
- Vilaplana, L.; Redman, E.M.; Wilson, K.; Cory, J.S. Density-related variation in vertical transmission of a virus in the African armyworm. Oecologia 2008, 155, 237–246. [Google Scholar] [CrossRef]
- Zhang, Y.; He, J.; Zhang, Y.; Li, Y.; Yu, Y.; Jiang, X.; Pan, W. Insect cytokine growth-blocking peptide may regulate density-dependent phase trait of cuticular melanization in the larval armyworm, Mythimna separata. J. Asia-Pac. Entomol. 2020, 23, 498–503. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, X.; Yuan, S.; Yang, P.; Li, L.; Zhao, Y.; Kang, L. Variation of TNF modulates cellular immunity of gregarious and solitary locusts against fungal pathogen Metarhizium anisopliae. Proc. Natl. Acad. Sci. USA 2022, 119, e2120835119. [Google Scholar] [CrossRef]
- Wilson, K.; Thomas, M.B.; Blanford, S.; Doggett, M.; Simpson, S.J.; Moore, S.L. Coping with crowds: Density-dependent disease resistance in desert locusts. Proc. Natl. Acad. Sci. USA 2002, 99, 5471–5475. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishide, Y. Do desert locust hoppers develop gregarious characteristics by watching a video? J. Insect Physiol. 2012, 58, 1060–1071. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Q.; Chen, D.; Wei, J.; Yang, P.; Yu, J.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Despland, E.; Hägele, B.F.; Dodgson, T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc. Natl. Acad. Sci. USA 2001, 98, 3895–3897. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y.; Liu, Q.; Liu, Z.; Jiang, F.; Wang, H.; Kang, L. A β-carotene-binding protein carrying a red pigment regulates body-color transition between green and black in locusts. Elife 2019, 8, e41362. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wu, Z.; Wang, X.; Yang, P.; Yu, D.; Zhao, C.; Kang, L. Metabolomic analysis reveals that carnitines are key regulatory metabolites in phase transition of the locusts. Proc. Natl. Acad. Sci. USA 2012, 109, 3259–3263. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.J.; Xue, J.; Lu, B.; Zhang, X.C.; Zhuo, J.C.; He, S.F.; Zhang, C.X. Two insulin receptors determine alternative wing morphs in planthoppers. Nature 2015, 519, 464–467. [Google Scholar] [CrossRef]
- Tanaka, S. Hormonal control of body-color polymorphism in Locusta migratoria: Interaction between [His7]-corazonin and juvenile hormone. J. Insect Physiol. 2000, 46, 1535–1544. [Google Scholar] [CrossRef]
- Ishikawa, A.; Gotoh, H.; Abe, T.; Miura, T. Juvenile hormone titer and wing-morph differentiation in the vetch aphid Megoura crassicauda. J. Insect Physiol. 2013, 59, 444–449. [Google Scholar] [CrossRef]
- Jiang, X.F.; Luo, L.Z.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of Migration in Mythimna separata (Walker) in China: A Review Integrating Environmental, Physiological, Hormonal, Genetic, and Molecular Factors. Environ. Entomol. 2011, 40, 516–533. [Google Scholar] [CrossRef]
- Iwao, S. Differences in light reactions of larvae of the armyworm, Leucania separata Walker, in relation to their phase status. Nature 1967, 213, 941–942. [Google Scholar] [CrossRef]
- Kong, H.; Dong, C.; Jing, W.; Zheng, M.; Tian, Z.; Hou, Q.; Zhu, S. Transcriptomic insight into antimicrobial peptide factors involved in the prophylactic immunity of crowded Mythimna separata larvae. Dev. Comp. Immunol. 2019, 98, 34–41. [Google Scholar] [CrossRef]
- Kong, H.; Dong, C.; Tian, Z.; Mao, N.; Wang, C.; Cheng, Y.; Luo, L. Altered immunity in crowded Mythimna separata is mediated by octopamine and dopamine. Sci. Rep. 2018, 8, 3215. [Google Scholar] [CrossRef]
- Zheng, L.; Lytle, C.; Njauw, C.; Altstein, M.; Martins-Green, M. Cloning and characterization of the pheromone biosynthesis activating neuropeptide receptor gene in Spodoptera littoralis larvae. Gene 2007, 393, 20–30. [Google Scholar] [CrossRef]
- Jurenka, R. Regulation of pheromone biosynthesis in moths. Curr. Opin. Insect Sci. 2017, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, Y.; Kurakake, M.; Oda, Y.; Tsuzuki, S.; Hayakawa, Y. Insect cytokine growth-blocking peptide signaling cascades regulate two separate groups of target genes. Febs J. 2008, 275, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, S. How do food quality and larval crowding affect performance of the autumnal moth, Epirrita autumnata? Entomol. Exp. Appl. 2008, 129, 286–294. [Google Scholar] [CrossRef]
- Yan, H.; Opachaloemphan, C.; Carmona-Aldana, F.; Mancini, G.; Mlejnek, J.; Descostes, N.; Reinberg, D. Insulin signaling in the long-lived reproductive caste of ants. Science 2022, 377, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, S.V.; Hartfelder, K. The insulin signaling pathway in honey bee (Apis mellifera) caste development—Differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. J. Insect Physiol. 2008, 54, 1064–1071. [Google Scholar] [CrossRef]
- Wu, Q.; Brown, M.R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 2006, 51, 1–24. [Google Scholar] [CrossRef]
- Eijkelenboom, A.; Burgering, B.M. FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013, 14, 83–97. [Google Scholar] [CrossRef]
- Friggi-Grelin, F.; Iché, M.; Birman, S. Tissue-specific developmental requirements of Drosophila tyrosine hydroxylase isoforms. Genesis 2003, 35, 175–184. [Google Scholar] [CrossRef]
- Sugumaran, M.; Barek, H. Critical analysis of the melanogenic pathway in insects and higher animals. Int. J. Mol. Sci. 2016, 17, 1753. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, N.H.; Koo, B.S.; Lee, H.J.; Lee, A.Y. Bee venom stimulates human melanocyte proliferation, melanogenesis, dendricity and migration. Exp. Mol. Med. 2007, 39, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Shakhmantsir, I.; Massad, N.L.; Kennell, J.A. Regulation of cuticle pigmentation in drosophila by the nutrient sensing insulin and TOR signaling pathways. Dev. Dyn. 2014, 243, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and immune function, supplementation and clinical translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131, 2515S–2522S. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Ding, D.; Ma, C.; Guo, W.; Kang, L. Locust density shapes energy metabolism and oxidative stress resulting in divergence of flight traits. Proc. Natl. Acad. Sci. USA 2022, 119, e2115753118. [Google Scholar] [CrossRef] [PubMed]
- Hardie, D.G. AMP-activated protein kinase: Maintaining energy homeostasis at the cellular and whole body levels. Annu. Rev. Nutr. 2014, 34, 31. [Google Scholar] [CrossRef]
- Stanton, R.C. Glucose-6-phosphate dehydrogenase, NADPH, and cell survival. IUBMB Life 2012, 64, 362–369. [Google Scholar] [CrossRef]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Ralser, M. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef] [PubMed]
- Rui, L. Energy metabolism in the liver. Compr. Physiol. 2014, 4, 177. [Google Scholar]
- Sarov-Blat, L.; So, W.V.; Liu, L.; Rosbash, M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell 2000, 101, 647–656. [Google Scholar] [CrossRef]
- Meunier, N.; Belgacem, Y.H.; Martin, J.R. Regulation of feeding behaviour and locomotor activity by takeout in Drosophila. J. Exp. Biol. 2007, 210, 1424–1434. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, S.; Ma, L.; Tian, L.; Wang, S.; Sheng, Z.; Li, S. Transcriptional regulation of the insulin signaling pathway genes by starvation and 20-hydroxyecdysone in the Bombyx fat body. J. Insect Physiol. 2010, 56, 1436–1444. [Google Scholar] [CrossRef]
- Li, H.; Dai, C.; Zhu, Y.; Hu, Y. Larvae crowding increases development rate, improves disease resistance, and induces expression of antioxidant enzymes and heat shock proteins in Mythimna separata (Lepidoptera: Noetuidae). J. Econ. Entomol. 2021, 114, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.H.; Yu, Q.; Deng, Z.L.; Yang, K.; Ye, Y.; Ge, M.X.; Kong, Q.P. ETS1 acts as a regulator of human healthy aging via decreasing ribosomal activity. Sci. Adv. 2022, 8, eabf2017. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, J.; Peric-Mataruga, V.; Vlahovic, M.; Mrdakovic, M.; Cvetanovic, D. Effects of rearing density on larval growth and activity of digestive enzymes in Lymantria dispar L. (Lepidoptera: Lymantriidae). Folia Biol.-Krakow 2004, 52, 105–112. [Google Scholar]
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Rahfeld, J.; Schierborn, M.; Hartrodt, B.; Neubert, K.; Heins, J. Are diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) inhibitors or substrates of dipeptidyl peptidase IV? Biochim Biophys Acta. 1991, 1076, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yang, H.; Hu, Y.; Zhang, C.; Fan, D. Multi-Omics Reveals the Effect of Population Density on the Phenotype, Transcriptome and Metabolome of Mythimna separata. Insects 2023, 14, 68. https://doi.org/10.3390/insects14010068
Wang S, Yang H, Hu Y, Zhang C, Fan D. Multi-Omics Reveals the Effect of Population Density on the Phenotype, Transcriptome and Metabolome of Mythimna separata. Insects. 2023; 14(1):68. https://doi.org/10.3390/insects14010068
Chicago/Turabian StyleWang, Sibo, Hongjia Yang, Yushuo Hu, Chunyu Zhang, and Dong Fan. 2023. "Multi-Omics Reveals the Effect of Population Density on the Phenotype, Transcriptome and Metabolome of Mythimna separata" Insects 14, no. 1: 68. https://doi.org/10.3390/insects14010068
APA StyleWang, S., Yang, H., Hu, Y., Zhang, C., & Fan, D. (2023). Multi-Omics Reveals the Effect of Population Density on the Phenotype, Transcriptome and Metabolome of Mythimna separata. Insects, 14(1), 68. https://doi.org/10.3390/insects14010068