Detection of Arthropod-Borne Bacteria and Assessment of MALDI-TOF MS for the Identification of Field-Collected Immature Bed Bugs from Mauritania
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
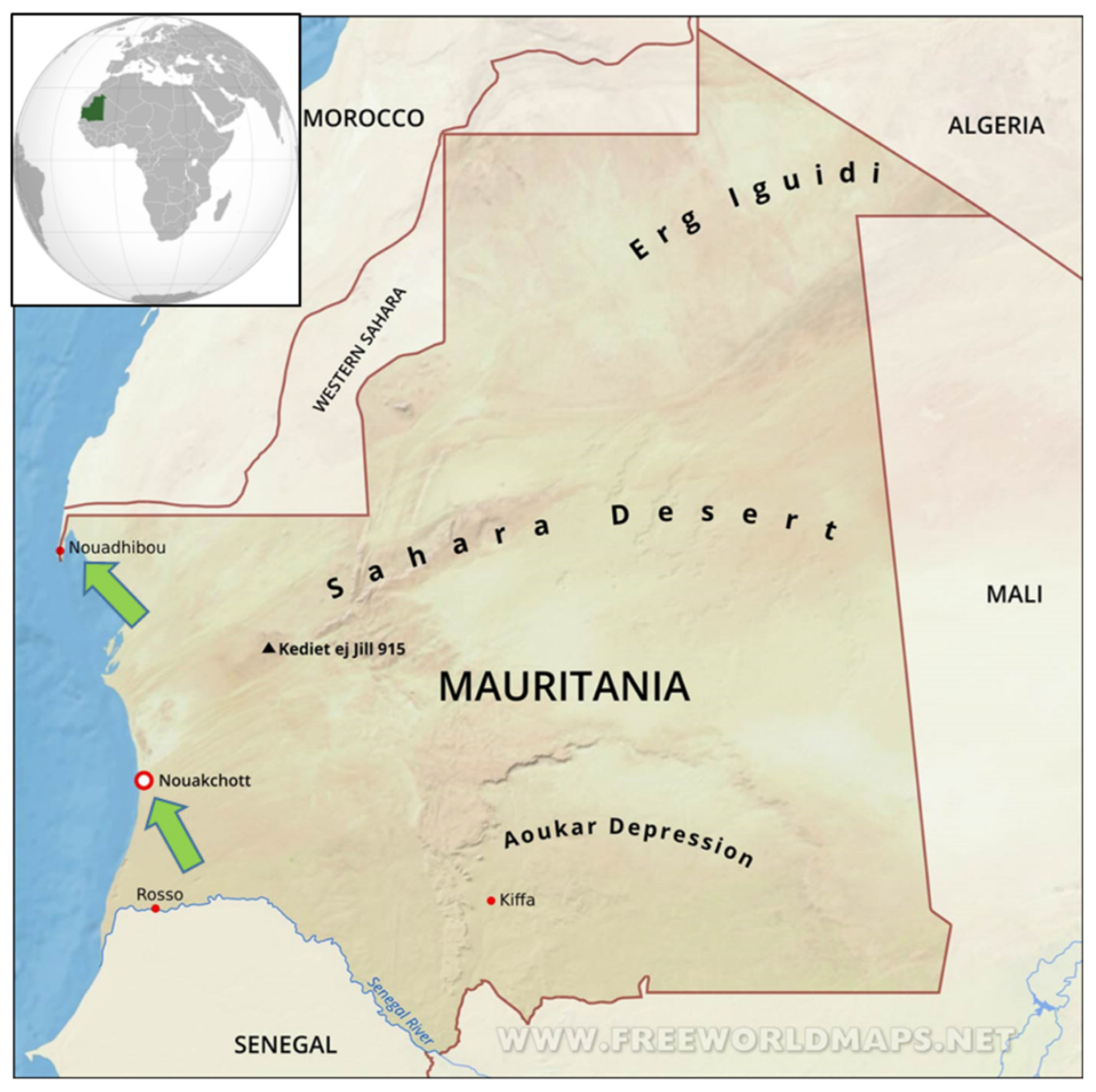
2.1. Sampling Sites
2.2. Bed Bugs: Collection, Morphological Identification and Dissection
2.3. MALDI-TOF MS Identification
2.4. DNA Extraction and Molecular Identification of Cimex sp.
2.5. Detection and Identification of Bacteria in Cimex sp.
3. Results
3.1. Specimen Collection and Morphological Identification
3.2. MALDI-TOF MS Identification
3.3. Molecular Identification of Cimex Specimens
3.4. Detection and Identification of Bacteria in Cimex Specimens
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panagiotakopulu, E.; Buckland, P.C. Cimex lectularius L., the common bed bug from Pharaonic Egypt. Antiquity 1999, 73, 908–911. [Google Scholar] [CrossRef]
- Parola, P.; Izri, A. Bedbugs. N. Engl. J. Med. 2020, 382, 2230–2237. [Google Scholar] [CrossRef]
- Kweka, E.J.; Mwang’Onde, B.J.; Kimaro, E.; Msangi, S.; Tenu, F.; Mahande, A.M. Insecticides susceptibility status of the bedbugs (Cimex lectularius) in a rural area of Magugu, Northern Tanzania. J. Glob. Infect. Dis. 2009, 1, 102–106. [Google Scholar] [CrossRef]
- Myamba, J.; Maxwell, C.A.; Asidi, A.; Curtis, C.F. Pyrethroid resistance in tropical bedbugs, Cimex hemipterus, associated with use of treated bednets: Pyrethroid resistance in tropical bedbugs. Med. Vet. Entomol. 2002, 16, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Newberry, K.; Mchunu, Z. Changes in the relative frequency of occurrence of infestations of two sympatric species of bedbug in northern Natal and KwaZulu, South Africa. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 262–264. [Google Scholar] [CrossRef]
- Emmanuel, O.I.; Cyprian, A.; Agbo, O.E. A Survey of Bedbug (Cimex lectularius) Infestation in Some Homes and Hostels in Gboko, Benue State, Nigeria. Psyche A J. Entomol. 2014, 2014, 762704. [Google Scholar] [CrossRef]
- Ndiaye, E.H.I.; Diatta, G.; Diarra, A.Z.; Berenger, J.M.; Bassene, H.; Mediannikov, O.; Bouganali, C.; Sokhna, C.; Parola, P. Morphological, Molecular and MALDI-TOF MS Identification of Bedbugs and Associated Wolbachia Species in Rural Senegal. J. Med. Èntomol. 2022, 59, 1019–1032. [Google Scholar] [CrossRef]
- Gushulak, B.D.; MacPherson, D.W. Globalization of Infectious Diseases: The Impact of Migration. Clin. Infect. Dis. 2004, 38, 1742–1748. [Google Scholar] [CrossRef]
- Stoek, F.; Barry, Y.; Ba, A.; Schulz, A.; Rissmann, M.; Wylezich, C.; Sadeghi, B.; Beyit, A.D.; Eisenbarth, A.; N’Diaye, F.B.; et al. Mosquito survey in Mauritania: Detection of Rift Valley fever virus and dengue virus and the determination of feeding patterns. PLoS Negl. Trop. Dis. 2022, 16, e0010203. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Usinger, R.L. Monograph of Cimicidae (Hemiptera-Heteroptera). J. Parasitol. 1967, 53, 222. [Google Scholar] [CrossRef]
- Sevestre, J.; Diarra, A.Z.; Laroche, M.; Almeras, L.; Parola, P. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: An emerging tool for studying the vectors of human infectious diseases. Future Microbiol. 2021, 16, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Yssouf, A.; Almeras, L.; Raoult, D.; Parola, P. Emerging tools for identification of arthropod vectors. Future Microbiol. 2016, 11, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Benkacimi, L.; Gazelle, G.; El Hamzaoui, B.; Bérenger, J.-M.; Parola, P.; Laroche, M. MALDI-TOF MS identification of Cimex lectularius and Cimex hemipterus bedbugs. Infect. Genet. Evol. 2020, 85, 104536. [Google Scholar] [CrossRef]
- Hamlili, F.Z.; Bérenger, J.-M.; Diarra, A.Z.; Parola, P. Molecular and MALDI-TOF MS identification of swallow bugs Cimex hirundinis (Heteroptera: Cimicidae) and endosymbionts in France. Parasites Vectors 2021, 14, 587. [Google Scholar] [CrossRef] [PubMed]
- Diarra, A.Z.; Almeras, L.; Laroche, M.; Berenger, J.-M.; Koné, A.K.; Bocoum, Z.; Dabo, A.; Doumbo, O.; Raoult, D.; Parola, P. Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Negl. Trop. Dis. 2017, 11, e0005762. [Google Scholar] [CrossRef]
- Boucheikhchoukh, M.; Laroche, M.; Aouadi, A.; Dib, L.; Benakhla, A.; Raoult, D.; Parola, P. MALDI-TOF MS identification of ticks of domestic and wild animals in Algeria and molecular detection of associated microorganisms. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 39–49. [Google Scholar] [CrossRef]
- Weirauch, C.; Munro, J.B. Molecular phylogeny of the assassin bugs (Hemiptera: Reduviidae), based on mitochondrial and nuclear ribosomal genes. Mol. Phylogenet. Evol. 2009, 53, 287–299. [Google Scholar] [CrossRef]
- Diarra, A.Z.; Kone, A.K.; Niare, S.D.; Laroche, M.; Diatta, G.; Atteynine, S.A.; Coulibaly, M.; Sangare, A.K.; Kouriba, B.; Djimde, A.; et al. Molecular Detection of Microorganisms Associated with Small Mammals and Their Ectoparasites in Mali. Am. J. Trop. Med. Hyg. 2020, 103, 2542–2551. [Google Scholar] [CrossRef]
- Sevestre, J.; Diarra, A.Z.; Oumarou, H.A.; Durant, J.; Delaunay, P.; Parola, P. Detection of emerging tick-borne disease agents in the Alpes-Maritimes region, southeastern France. Ticks Tick-Borne Dis. 2021, 12, 101800. [Google Scholar] [CrossRef]
- Doggett, S.L.; Orton, C.J.; Lilly, D.G.; Russell, R.C. Bed Bugs: The Australian Response. Insects 2011, 2, 96–111. [Google Scholar] [CrossRef]
- Doggett, S.L.; Miller, D.M.; Lee, C.-Y. Advances in the Biology and Management of Modern Bed Bugs; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-1-119-17152-2. [Google Scholar]
- Deku, G.; Combey, R.; Doggett, S.L.; Mensah, B.A. Assessment of Tropical Bed Bug (Hemiptera: Cimicidae), Infestations in Cape Coast, Ghana: Household Control Practices and Efficacy of Commercial Insecticides and Long-Lasting Insecticidal Nets Against Field Bed Bugs. J. Med. Èntomol. 2021, 58, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Sereno, D.; Durand, R.; Mirzaei, A.; Bruel, C.; Delaunay, P.; Marty, P.; Izri, A. Bed Bugs (Hemiptera, Cimicidae): Overview of Classification, Evolution and Dispersion. Int. J. Environ. Res. Public Health 2020, 17, 4576. [Google Scholar] [CrossRef]
- Deku, G.; Combey, R.; Doggett, S.L. Morphometrics of the Tropical Bed Bug (Hemiptera: Cimicidae) From Cape Coast, Ghana. J. Med. Èntomol. 2022, 59, 1534–1547. [Google Scholar] [CrossRef] [PubMed]
- Nabet, C.; Kone, A.K.; Dia, A.K.; Sylla, M.; Gautier, M.; Yattara, M.; Thera, M.A.; Faye, O.; Braack, L.; Manguin, S.; et al. New assessment of Anopheles vector species identification using MALDI-TOF MS. Malar. J. 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Raharimalala, F.N.; Andrianinarivomanana, T.M.; Rakotondrasoa, A.; Collard, J.M.; Boyer, S. Usefulness and accuracy of MALDI-TOF mass spectrometry as a supplementary tool to identify mosquito vector species and to invest in development of international database. Med. Veter-Entomol. 2017, 31, 289–298. [Google Scholar] [CrossRef]
- Fisher, M.L.; Watson, D.W.; Osborne, J.A.; Mochizuki, H.; Breen, M.; Schal, C. Growth kinetics of endosymbiont Wolbachia in the common bed bug, Cimex lectularius. Sci. Rep. 2018, 8, 11444. [Google Scholar] [CrossRef] [PubMed]
- Landmann, F. The Wolbachia Endosymbionts. Microbiol. Spectr. 2019, 7, 7.2.25. [Google Scholar] [CrossRef]
- El Hamzaoui, B.; Laroche, M.; Bechah, Y.; Bérenger, J.M.; Parola, P. Testing the Competence of Cimex lectularius Bed Bugs for the Transmission of Borrelia recurrentis, the Agent of Relapsing Fever. Am. J. Trop. Med. Hyg. 2019, 100, 1407–1412. [Google Scholar] [CrossRef]
- Potts, R.; Scholl, J.L.; Baugh, L.A.; Pietri, J.E. A Comparative Study of Body Lice and Bed Bugs Reveals Factors Potentially Involved in Differential Vector Competence for the Relapsing Fever Spirochete Borrelia recurrentis. Infect. Immun. 2022, 90, e0068321. [Google Scholar] [CrossRef]
- Welker, M.; Van Belkum, A.; Girard, V.; Charrier, J.-P.; Pincus, D. An update on the routine application of MALDI-TOF MS in clinical microbiology. Expert Rev. Proteom. 2019, 16, 695–710. [Google Scholar] [CrossRef]
- Fall, B.; Lo, C.I.; Samb-Ba, B.; Perrot, N.; Diawara, S.; Gueye, M.W.; Sow, K.; Aubadie-Ladrix, M.; Mediannikov, O.; Sokhna, C.; et al. The Ongoing Revolution of MALDI-TOF Mass Spectrometry for Microbiology Reaches Tropical Africa. Am. J. Trop. Med. Hyg. 2015, 92, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.I.; Fall, B.; Sambe-Ba, B.; Diawara, S.; Gueye, M.W.; Mediannikov, O.; Sokhna, C.; Faye, N.; Diemé, Y.; Wade, B.; et al. MALDI-TOF Mass Spectrometry: A Powerful Tool for Clinical Microbiology at Hôpital Principal de Dakar, Senegal (West Africa). PLoS ONE 2015, 10, e0145889. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevestre, J.; Lemrabott, M.A.O.; Bérenger, J.-M.; Zan Diarra, A.; Ould Mohamed Salem Boukhary, A.; Parola, P. Detection of Arthropod-Borne Bacteria and Assessment of MALDI-TOF MS for the Identification of Field-Collected Immature Bed Bugs from Mauritania. Insects 2023, 14, 69. https://doi.org/10.3390/insects14010069
Sevestre J, Lemrabott MAO, Bérenger J-M, Zan Diarra A, Ould Mohamed Salem Boukhary A, Parola P. Detection of Arthropod-Borne Bacteria and Assessment of MALDI-TOF MS for the Identification of Field-Collected Immature Bed Bugs from Mauritania. Insects. 2023; 14(1):69. https://doi.org/10.3390/insects14010069
Chicago/Turabian StyleSevestre, Jacques, Mohamed Aly Ould Lemrabott, Jean-Michel Bérenger, Adama Zan Diarra, Ali Ould Mohamed Salem Boukhary, and Philippe Parola. 2023. "Detection of Arthropod-Borne Bacteria and Assessment of MALDI-TOF MS for the Identification of Field-Collected Immature Bed Bugs from Mauritania" Insects 14, no. 1: 69. https://doi.org/10.3390/insects14010069
APA StyleSevestre, J., Lemrabott, M. A. O., Bérenger, J.-M., Zan Diarra, A., Ould Mohamed Salem Boukhary, A., & Parola, P. (2023). Detection of Arthropod-Borne Bacteria and Assessment of MALDI-TOF MS for the Identification of Field-Collected Immature Bed Bugs from Mauritania. Insects, 14(1), 69. https://doi.org/10.3390/insects14010069





