Regulation of Aquaporin Prip Expression and Its Physiological Function in Rhyzopertha dominica (Coleoptera: Bostrichidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects R. dominica
2.2. cDNA Cloning of RdPrip by RT-PCR and RACE, and Sequence Analysis
2.3. Analysis of the Developmental and Tissue Expression Profiles of RdPrip by Real-Time Quantitative RT-PCR
2.4. Functional Studies of RdPrip through RNA Interference
2.5. Association of RdPrip Functions with Spinosad Resistance in R. dominica
3. Results
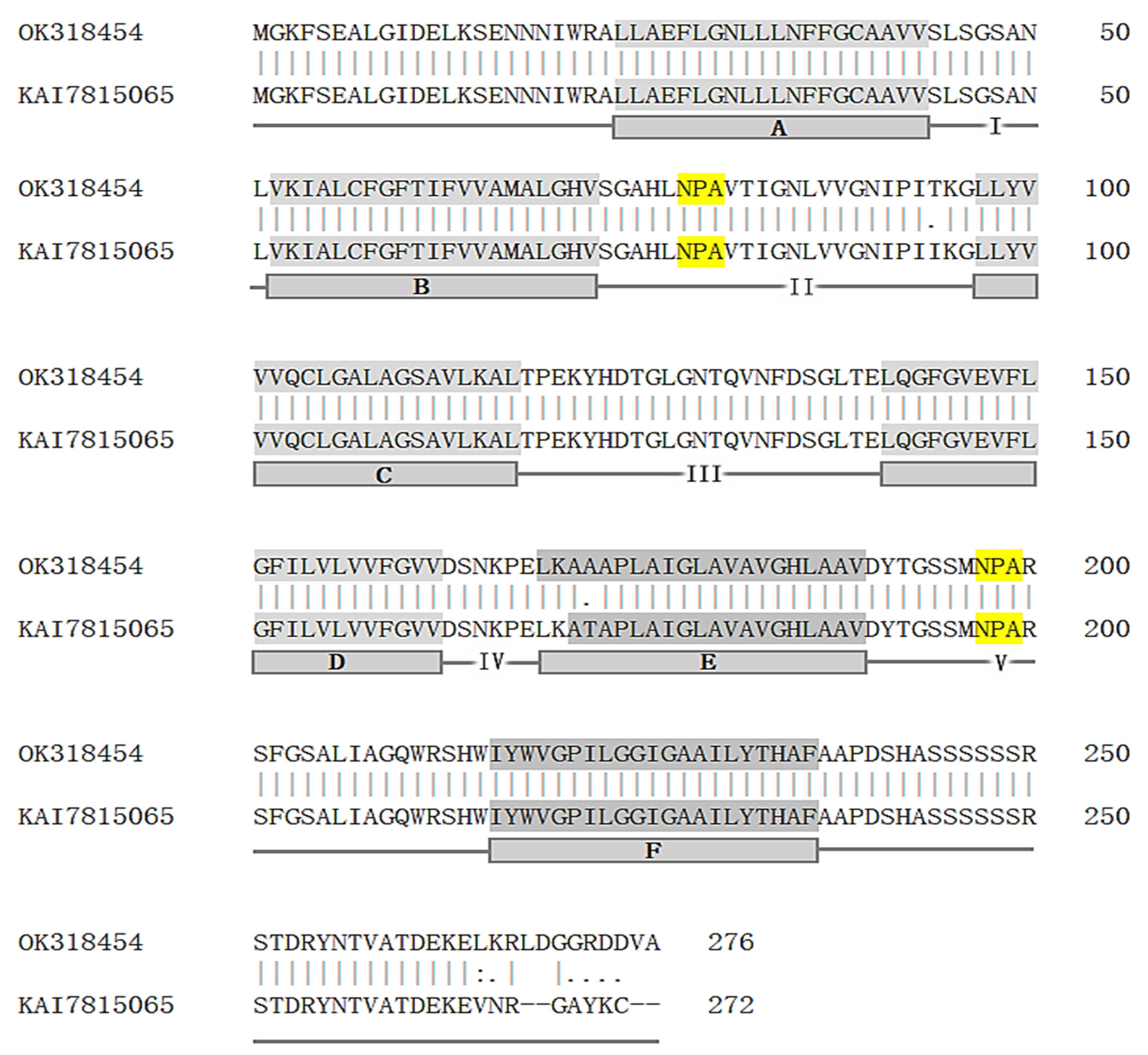
3.1. cDNA Cloning and Sequence Analyses
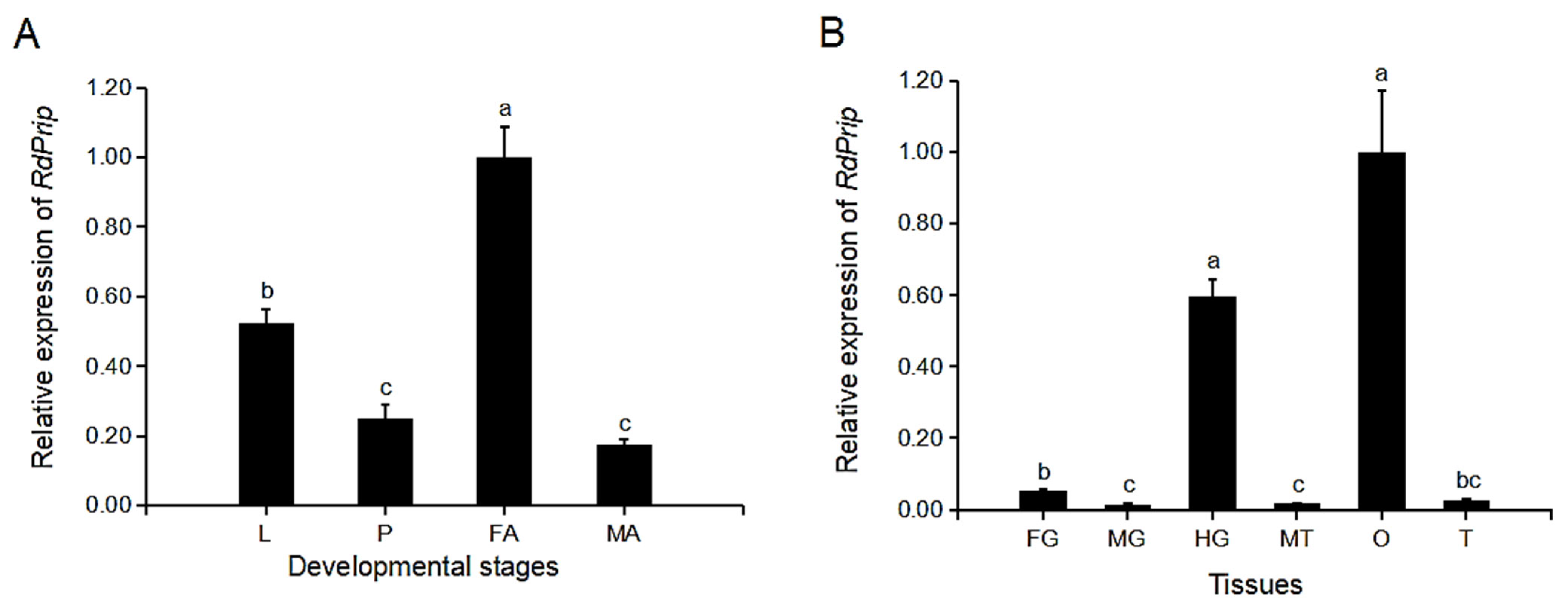
3.2. Expression Profiles of RdPrip
3.3. Functional Studies of RdPrip
3.4. Association of RdPrip Functions with Spinosad Resistance in R. dominica
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, B.L.; Agre, P. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to chnnel proteins. J. Biol. Chem. 1991, 266, 6407–6415. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.N.; Chauvigné, F.; Stavang, J.A.; Belles, X.; Cerdà, J. Insect glycerol transporters evolved by functional co-option and gene replacement. Nat. Commun. 2015, 6, 7814. [Google Scholar] [CrossRef]
- Kaufmann, N.; Mathai, J.C.; Hill, W.G.; Dow, J.A.T.; Zeidel, M.L.; Brodsky, J.L. Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. Am. J. Physiol.-Cell Physiol. 2005, 289, C397–C407. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tsujimoto, H.; Cha, S.-J.; Agre, P.; Rasgon, J.L. Aquaporin water channel AgAQP1 in the malaria vector mosquito Anopheles gambiae during blood feeding and humidity adaptation. Proc. Natl. Acad. Sci. USA 2011, 108, 6062–6066. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.L.; Rodriguez, S.D.; Hansen, I.A. Functional characterization of aquaporins and aquaglyceroporins of the yellow fever mosquiot, Aedes aegypti. Sci. Rep. 2015, 5, 7795. [Google Scholar] [CrossRef] [PubMed]
- Shakesby, A.J.; Wallace, I.S.; Isaacs, H.V.; Pritchard, J.; Roberts, D.M.; Douglas, A.E. A water-specific aquaporin involved in aphid osmoregulation. Insect Biochem. Mol. Biol. 2009, 39, 1–10. [Google Scholar] [CrossRef]
- Drake, L.L.; Boudko, D.Y.; Marinotti, O.; Carpenter, V.K.; Dawe, A.L.; Hansen, I.A. The aquaporin gene family of the yellow fever mosquito, Aedes aegypti. PLoS ONE 2010, 5, e15578. [Google Scholar] [CrossRef]
- Mathew, L.G.; Campbell, E.M.; Yool, A.J.; Fabrick, J.A. Identification and characterization of functional aquaporin water channel protein from alimentary tract of whitefly, Bemisia tabaci. Insect Biochem. Mol. Biol. 2011, 41, 178–190. [Google Scholar] [CrossRef]
- Esquivel, C.J.; Cassone, B.J.; Piermarini, P.M. A de novo transcriptome of the Malpighian tubules in non-blood-fed and blood-fed Asian tiger mosquitoes Aedes albopictus: Insights into diuresis, detoxification, and blood meal processing. PeerJ 2016, 4, e1784. [Google Scholar] [CrossRef]
- Mitchell, S.N.; Stevenson, B.J.; Müller, P.; Wilding, C.S.; Egyir-Yawson, A.; Field, S.G.; Hemingway, J.; Paine, M.J.I.; Ranson, H.; Donnelly, M.J. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. USA 2012, 109, 6147–6152. [Google Scholar] [CrossRef]
- Karaagaç, S.U. Insecticide Resistance. In Insecticides—Advances in Integrated Pest Management; Perveen, F., Ed.; Intech: Rijeka, Croatia, 2012; pp. 469–478. [Google Scholar]
- Herraiz, A.; Chauvigné, F.; Cerdà, J.; Bellés, X.; Piulachs, M.-D. Identification and functional characterization of an ovarian aquaporin from the cockroach Blattella germanica L. (Dictyoptera, Blattellidae). J. Exp. Biol. 2011, 214, 3630–3638. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Hansen, I.A.; Attardo, G.M.; Michalková, V.; Mireji, P.O.; Bargul, J.L.; Drake, L.L.; Masiga, D.K.; Aksoy, S. Aquaporins are critical for provision of water during lactation and intrauterine progeny hydration to maintain tsetse fly reproductive success. PLoS Negl. Trop. Dis. 2014, 8, e2517. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Kambara, K.; Naka, H.; Azuma, M. Insect water-specific aquaporins in developing ovarian follicles of the silk moth Bmobyx mori: Role in hydration during egg maturation. Biol. Bull. 2015, 229, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Santana, L.; Dohanik, V.T.; Serrão, J.E. Differential expression of aquaporin genes during ovary activation in the honeybee Apis mellifera (Hymenoptera: Apidae) queens. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 253, 110551. [Google Scholar] [CrossRef] [PubMed]
- Kikawada, T.; Saito, A.; Kanamori, Y.; Fujita, M.; Snigórska, K.; Watanabe, M.; Okuda, T. Dehydration-inducible changes in expression of two auaporins in the sleeping chironomid Polypedilum vanderplanki. Biochim. Biophys. Acta 2008, 1778, 514–520. [Google Scholar] [CrossRef]
- Philip, B.N.; Kiss, A.J.; Lee, R.E., Jr. The protective role of aquaporins in the freeze-tolerant insect Eurosta silidaginis: Functional characterization and tissue abundance of EsAQP1. J. Exp. Biol. 2011, 214, 848–857. [Google Scholar] [CrossRef]
- Yi, S.-X.; Benoit, J.B.; Elnitsky, M.A.; Kaufmann, N.; Brodsky, J.L.; Zeidel, M.L.; Denlinger, D.L.; Lee, R.E., Jr. Function and immune-localization of aquaporins in the Antarctic midge Belgica antarctica. J. Insect Physiol. 2011, 57, 1096–1105. [Google Scholar] [CrossRef]
- Yang, L.; Piermarini, P.M. Molecular expression of aquaporin mRNAs in the northern house mosquito, Culex pipiens. J. Insect Physiol. 2017, 96, 35–44. [Google Scholar] [CrossRef]
- Potter, C. The biology and distribution of Rhyzopertha dominica (Fab.). Trans. R. Entomol. Soc. Lond. 1935, 83, 449–482. [Google Scholar] [CrossRef]
- Edde, P.A. A review of the biology and control of Rhyzopertha dominica (F.) the lesser grain borer. J. Stored Prod. Res. 2012, 48, 1–18. [Google Scholar] [CrossRef]
- Saini, R.S. Histology and physiology of the cryptonephridial system of insects. Trans. R. Entomol. Soc. London 1964, 116, 347–392. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, M.-E. Susceptibility of field populations of the lesser grain borer, Rhyzopertha dominica (F.), to deltamethrin and spinosad on paddy rice in Taiwan. J. Stored Prod. Res. 2013, 55, 124–127. [Google Scholar] [CrossRef]
- Nagae, T.; Miyake, S.; Kosaki, S.; Azuma, M. Identification and characterization of a functional aquaporin water channel (Anomala cuprea DRIP) in a coleopteran insect. J. Exp. Biol. 2013, 216, 2564–2572. [Google Scholar] [PubMed]
- Kataoka, N.; Miyake, S.; Azuma, M. Aquaporin and aquaglyceroporin in silkworms, differently expressed in the hindgut and midgut of Bombyx mori. Insect Mol. Biol. 2009, 18, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Staniscuaski, F.; Paluzzi, J.-P.; Real-Guerra, R.; Carlini, C.R.; Orchard, I. Expression analysis and molecular characterization of aquaporins in Rhodnius prolixus. J. Insect Physiol. 2013, 59, 1140–1150. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Armenteros, J.J.A.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. Deep TMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Madeira, F.; Pearce, M.; Tivey, A.R.N.; Basutkar, P.; Lee, J.; Edbali, O.; Madhusoodanan, N.; Kolesnikov, A.; Lopez, R. Search and sequence analysis tools services from EMBL-EBI in 2022. Nucleic Acids Res. 2022, 50, W276–W279. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Preston, G.G.; Smith, B.L.; Guggino, W.B.; Agre, P. Molecular structure of the water channel through aquaporin CHIP: The hourglass model. J. Biol. Chem. 1994, 269, 14648–14654. [Google Scholar] [CrossRef]
- Gonen, T.; Walz, T. The structure of aquaporins. Q. Rev. Biophys. 2006, 39, 361–396. [Google Scholar] [CrossRef]
- Oppert, B.; Muszewska, A.; Steczkiewicz, K.; Satovic-Vuksic, E.; Plohl, M.; Fabrick, J.A.; Vinokurov, K.S.; Koloniuk, I.; Johnston, J.S.; Smith, T.P.L.; et al. The genome of Rhyzopertha dominica (Fab.) (Coleoptera: Bostrichidae): Adaptation for success. Genes 2022, 13, 446. [Google Scholar] [CrossRef]
- Yao, X.X.; Meng, Q.-W.; Li, G.-Q. RNA interference-mediated functional characterization of aquaporin genes in Tribolium castaneum. Insect Mol. Biol. 2018, 27, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-X.; He, F.-J.; Xu, J.; Liu, Y.; Wang, G.-R.; Du, Y.-Z. Identification and physiological function of CsPrip, a new aquaporin in Chilo suppressalis. Int. J. Biol. Macromol. 2021, 184, 721–730. [Google Scholar] [CrossRef] [PubMed]
- York-Andersen, A.H.; Wood, B.W.; Wilby, E.L.; Berry, A.S.; Weil, T.T. Osmolarity0regulated swelling initiates egg activation in Drosophila. Open Biol. 2021, 11, 210067. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Sakamoto, J.M.; Rasgon, J.L. Functional characterization of aquaporin-like genes in the human bed bug Cimex lectularius. Sci. Rep. 2017, 7, 3214. [Google Scholar] [CrossRef]
- Toé, K.H.; N’Falé, S.; Dabire, R.K.; Ranson, H.; Jones, C.M. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genom. 2015, 16, 146. [Google Scholar] [CrossRef]
- Gao, Y.; Kim, M.J.; Kim, J.H.; Jeong, I.H.; Clark, J.M.; Lee, S.H. Transcriptomic identification and characterization of genes responding to sublethal doses of three different insecticides in the western flower thrips, Frankliniella occidentalis. Pestic. Biochem. Physiol. 2020, 167, 104596. [Google Scholar] [CrossRef]
- Liu, K.; Tsujimoto, H.; Huang, Y.; Rasgon, J.L.; Agre, P. Aquaglyceroporin function in the malaria mosquito Anopheles gambiae. Biol. Cell 2016, 108, 294–305. [Google Scholar] [CrossRef]



| Primer Name | Primer Sequence |
|---|---|
| RdDripdf | GGAKGHCAYRTHAAYCCSGCKGTRAC |
| RdDripdr | ABBGGWCCMRCCCARTAMAYCCAWTG |
| RdDrip450-1 | ATCAAGGGACTGCTATACGTCGTCG |
| RdDrip450-2 | CATTGTCCTGCGATCAAAGCAGACC |
| RdDrip450-3 | TACAATGTCTGGGAGCATTAGCTGG |
| RdDrip450-4 | TGTAGTCTACAGCCGCCAAATGTCC |
| RdPrip1008-1 | GCGATTCCTACAGTACAGTTCGGTTACG |
| RdPrip1008-2 | ATGTAGGCATTAGATGAGTGTTGTGCGC |
| RdPrip qPCR-1 | GGCAATCTAGTTGTAGGTAATATAC |
| RdPrip qPCR-2 | CTCAGTCAAACCAGAATCG |
| Rd-18s qPCR-01 | CGAGACTCTGGCCTGCTAAC |
| Rd-18s qPCR-02 | CCGCCTGTCCCTCTAAGAA |
| RdPripdsRNA-03 | TAATACGACTCACTATAGGGAGATTCTGGTTTGACTGAGCTGCAAG |
| RdPripdsRNA-04 | TAATACGACTCACTATAGGGAGAGCATTAGATGAGTGTTGTGCGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, L.-P.; Chen, M.-E. Regulation of Aquaporin Prip Expression and Its Physiological Function in Rhyzopertha dominica (Coleoptera: Bostrichidae). Insects 2023, 14, 70. https://doi.org/10.3390/insects14010070
Tan L-P, Chen M-E. Regulation of Aquaporin Prip Expression and Its Physiological Function in Rhyzopertha dominica (Coleoptera: Bostrichidae). Insects. 2023; 14(1):70. https://doi.org/10.3390/insects14010070
Chicago/Turabian StyleTan, Lan-Pin, and Mei-Er Chen. 2023. "Regulation of Aquaporin Prip Expression and Its Physiological Function in Rhyzopertha dominica (Coleoptera: Bostrichidae)" Insects 14, no. 1: 70. https://doi.org/10.3390/insects14010070
APA StyleTan, L.-P., & Chen, M.-E. (2023). Regulation of Aquaporin Prip Expression and Its Physiological Function in Rhyzopertha dominica (Coleoptera: Bostrichidae). Insects, 14(1), 70. https://doi.org/10.3390/insects14010070






