A 12-Year Experimental Design to Test the Recovery of Butterfly Biodiversity in an Urban Ecosystem: Lessons from the Parc Urbain des Papillons
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
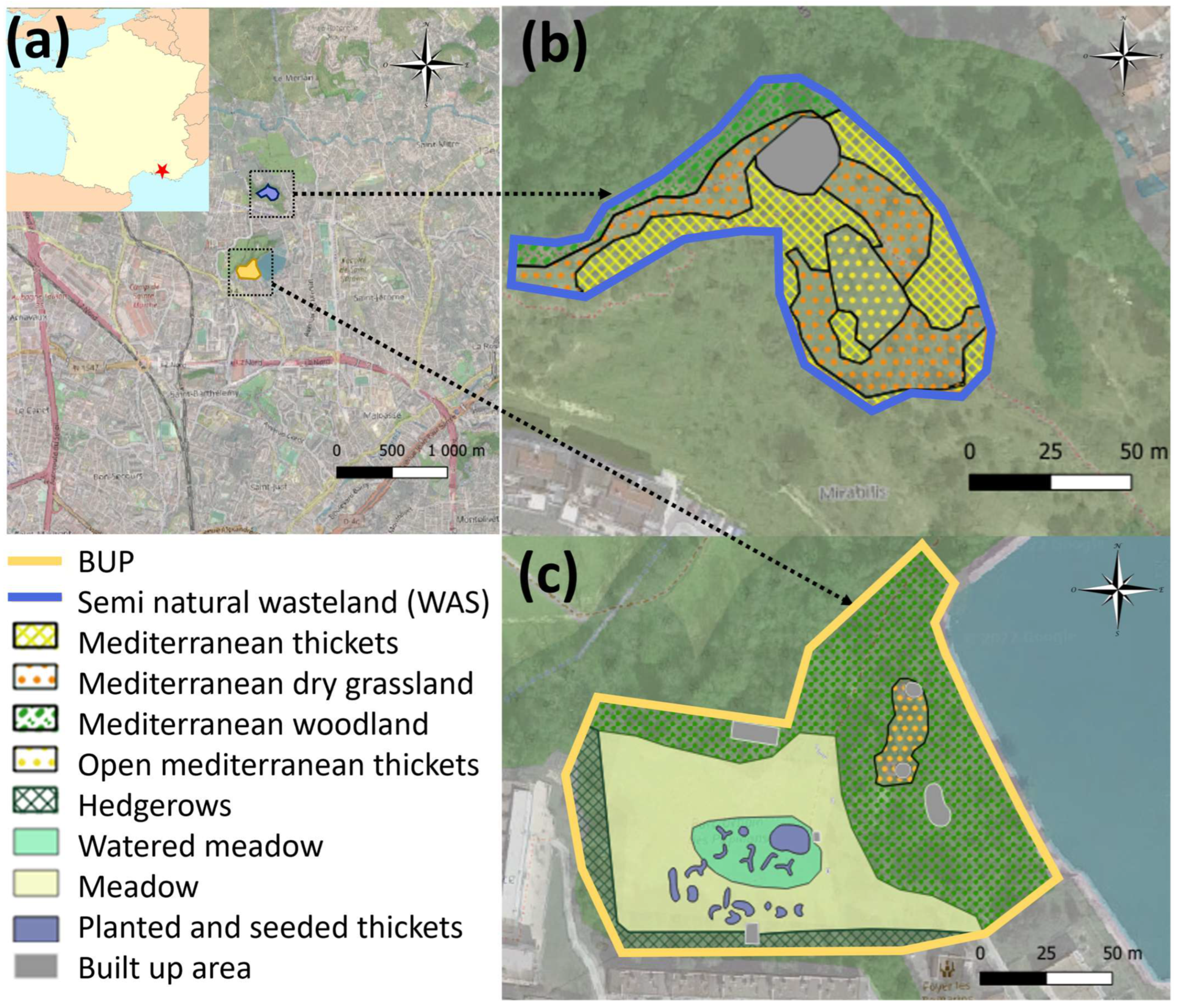
2.1. Study Sites
2.2. Butterfly Field Inventory
2.3. Data Analysis
3. Results
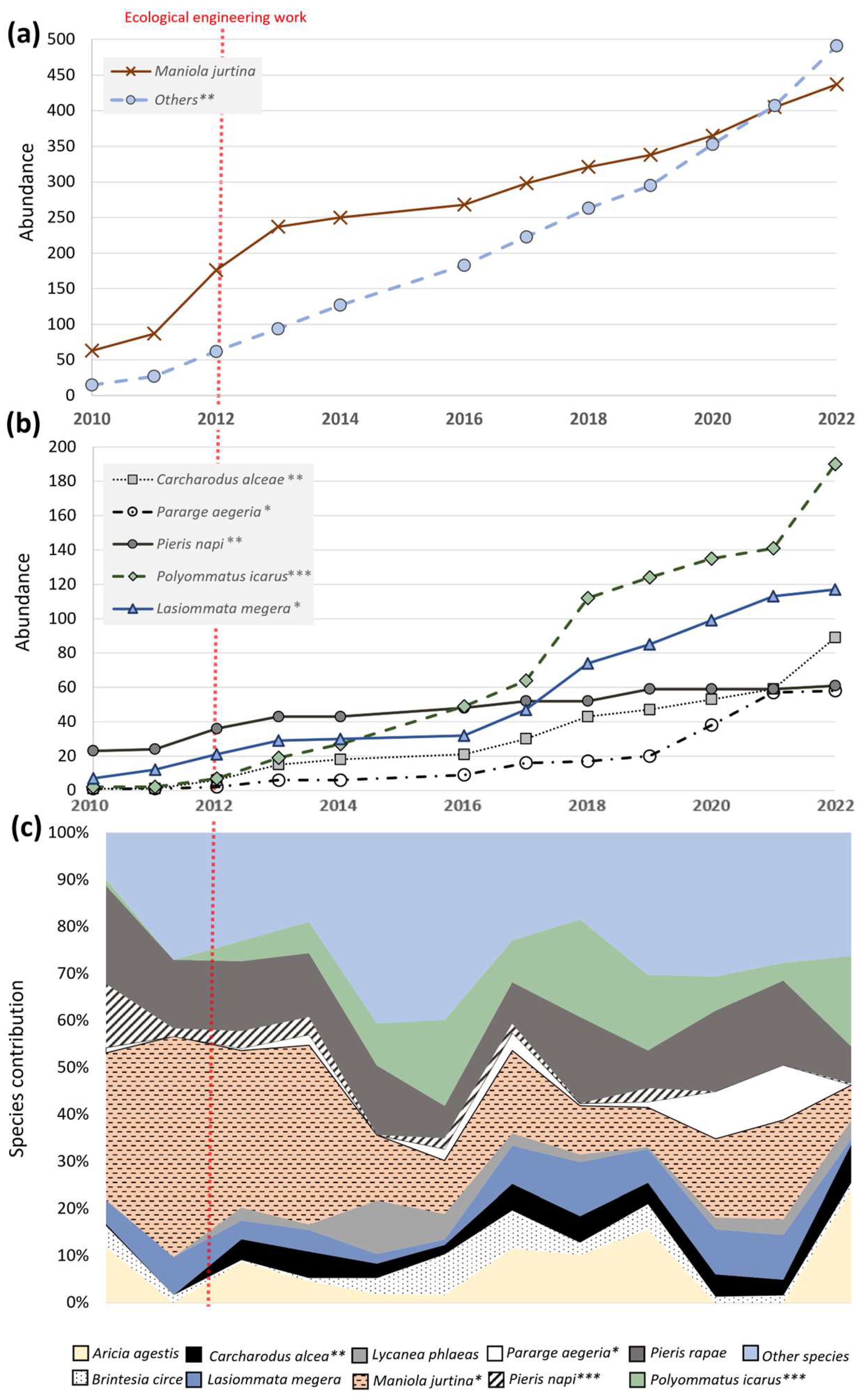
3.1. General Characteristics of Butterfly Communities
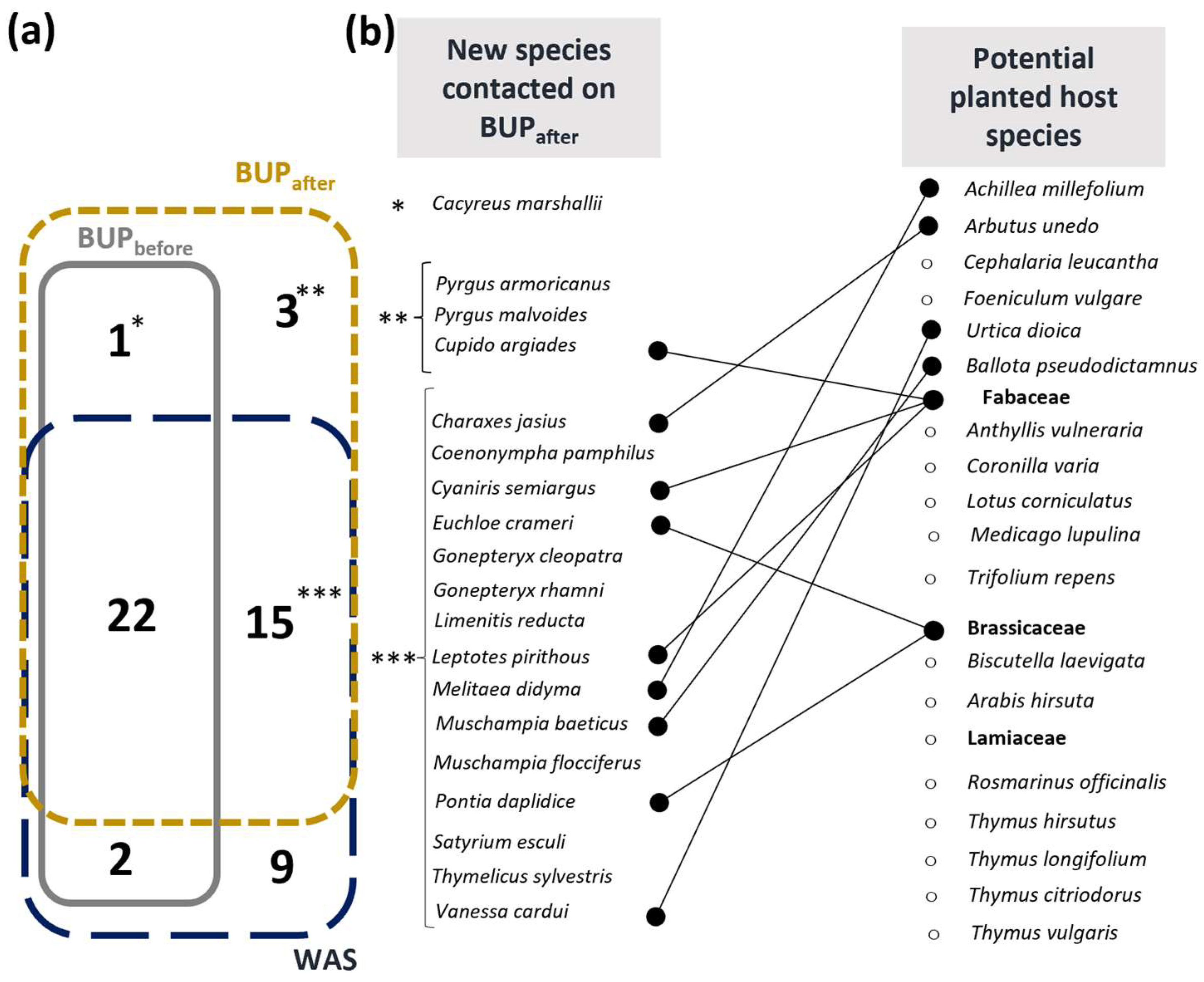
3.2. Impact of the Planted Bed on Targeted Butterfly Species
3.3. Patterns of Change in BUP Butterfly Community Composition
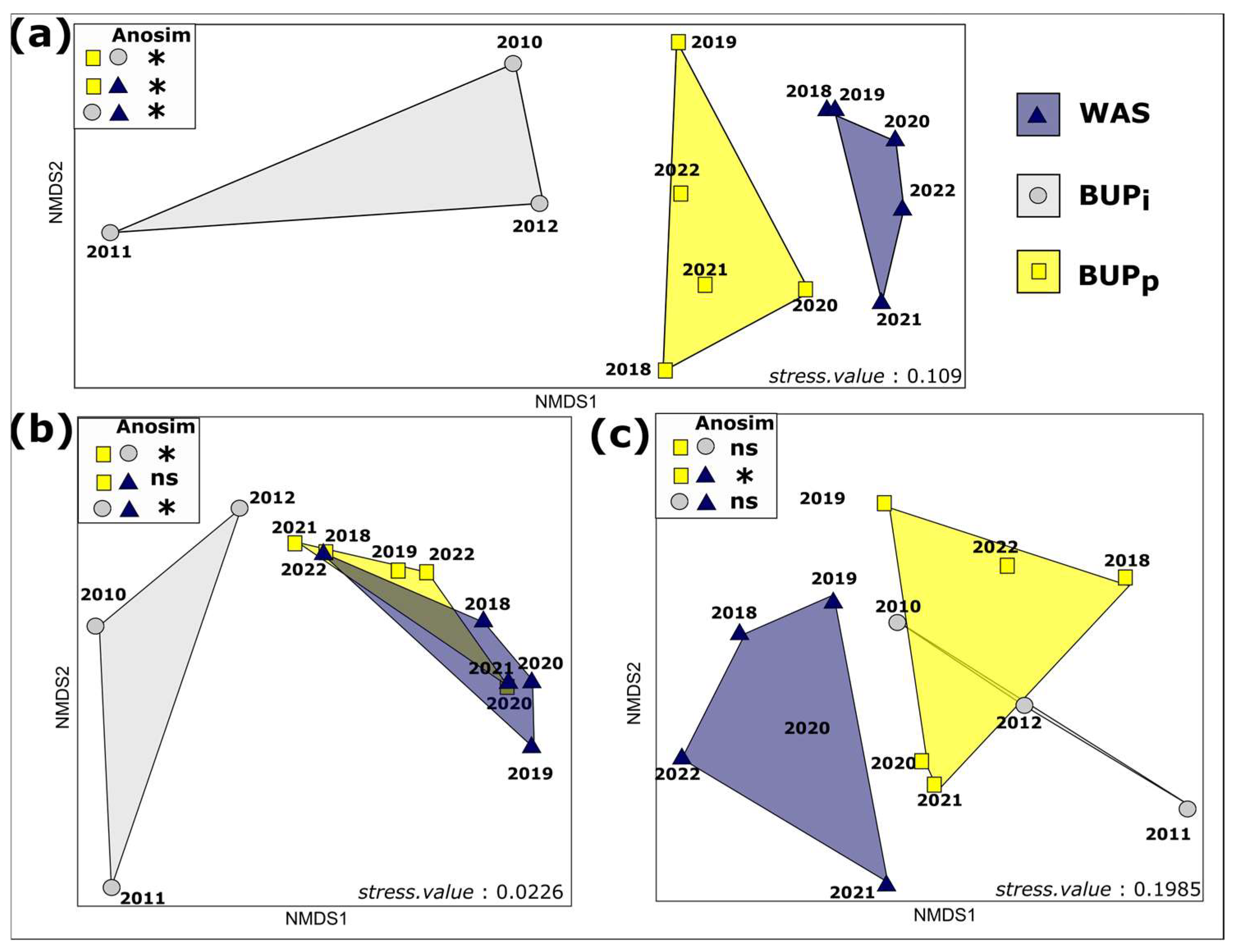
3.3.1. Changes in the Community Composition over Time
3.3.2. Trajectory of the Community Composition
4. Discussion
4.1. Impact of Species Phenology on Results
4.2. Ecological Engineering: Effects of Host and Nectar Source Diversification on Butterfly Community
4.3. Ecological Engineering: Microhabitat Alterations to the Butterfly Community
4.4. Role of the BUP in Mediterranean Butterfly Conservation: Appearance and Reproduction of Targeted Species
4.5. Toward a Specific Community: The Theory of the Neo-Ecosystem
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olsen, S.L.; Evju, M.; Endrestøl, A. Fragmentation in Calcareous Grasslands: Species Specialization Matters. Biodivers. Conserv. 2018, 27, 2329–2361. [Google Scholar] [CrossRef]
- Nunez, S.; Alkemade, R. Exploring Interaction Effects from Mechanisms between Climate and Land-Use Changes and the Projected Consequences on Biodiversity. Biodivers. Conserv. 2021, 30, 3685–3696. [Google Scholar] [CrossRef]
- McDonald, R.I.; Mansur, A.V.; Ascensão, F.; Colbert, M.; Crossman, K.; Elmqvist, T.; Gonzalez, A.; Güneralp, B.; Haase, D.; Hamann, M.; et al. Research Gaps in Knowledge of the Impact of Urban Growth on Biodiversity. Nat. Sustain. 2020, 3, 16–24. [Google Scholar] [CrossRef]
- Lizée, M.-H. Diversité, Organisation Spatiale et Fonctionnelle Des Communautés de Papillons (Lépidoptères, Rhopalocères) En Milieu Urbain et Périurbain: Rôle Des Espaces Artificialisés En Terme de Conservation et de Connectivité. Ph.D. Thesis, Aix-Marseille Université, Marseille, France, 2011. [Google Scholar]
- Pearse, W.D.; Cavender-Bares, J.; Hobbie, S.E.; Avolio, M.L.; Bettez, N.; Roy Chowdhury, R.; Darling, L.E.; Groffman, P.M.; Grove, J.M.; Hall, S.J.; et al. Homogenization of Plant Diversity, Composition, and Structure in North American Urban Yards. Ecosphere 2018, 9, e02105. [Google Scholar] [CrossRef]
- Shwartz, A.; Muratet, A.; Simon, L.; Julliard, R. Local and Management Variables Outweigh Landscape Effects in Enhancing the Diversity of Different Taxa in a Big Metropolis. Biol. Conserv. 2013, 157, 285–292. [Google Scholar] [CrossRef]
- Kowarik, I.; von der Lippe, M. Plant Population Success across Urban Ecosystems: A Framework to Inform Biodiversity Conservation in Cities. J. Appl. Ecol. 2018, 55, 2354–2361. [Google Scholar] [CrossRef]
- Planchuelo, G.; von Der Lippe, M.; Kowarik, I. Untangling the Role of Urban Ecosystems as Habitats for Endangered Plant Species. Landsc. Urban Plan. 2019, 189, 320–334. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from Gardens: Biodiversity Conservation in Urban Environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Bonthoux, S.; Brun, M.; Pietro, F.D.; Greulich, S.; Bouché-Pillon, S. How Can Wastelands Promote Biodiversity in Cities? A Review. Landsc. Urban Plan. 2014, 132, 79. [Google Scholar] [CrossRef]
- Seitz, B.; Buchholz, S.; Kowarik, I.; Herrmann, J.; Neuerburg, L.; Wendler, J.; Winker, L.; Egerer, M. Land Sharing between Cultivated and Wild Plants: Urban Gardens as Hotspots for Plant Diversity in Cities. Urban Ecosyst. 2022, 25, 927–939. [Google Scholar] [CrossRef]
- Aronson, J.; Claeys, F.; Westerberg, V.; Picon, P.; Bernard, G.; Bocognano, J.-M.; de Groot, R. Steps Towards Sustainability and Tools for Restoring Natural Capital: Etang de Berre (Southern France) Case Study. In Sustainability Science: The Emerging Paradigm and the Urban Environment; Weinstein, M.P., Turner, R.E., Eds.; Springer: New York, NY, USA, 2012; pp. 111–138. ISBN 978-1-4614-3188-6. [Google Scholar]
- Haaland, C.; van den Bosch, C.K. Challenges and Strategies for Urban Green-Space Planning in Cities Undergoing Densification: A Review. Urban For. Urban Green. 2015, 14, 760–771. [Google Scholar] [CrossRef]
- Rink, D.; Arndt, T. Investigating Perception of Green Structure Configuration for Afforestation in Urban Brownfield Development by Visual Methods—A Case Study in Leipzig, Germany. Urban For. Urban Green. 2016, 15, 65–74. [Google Scholar] [CrossRef]
- Wintle, B.A.; Kujala, H.; Whitehead, A.; Cameron, A.; Veloz, S.; Kukkala, A.; Moilanen, A.; Gordon, A.; Lentini, P.E.; Cadenhead, N.C.R.; et al. Global Synthesis of Conservation Studies Reveals the Importance of Small Habitat Patches for Biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 909–914. [Google Scholar] [CrossRef]
- Riva, F.; Fahrig, L. The Disproportionately High Value of Small Patches for Biodiversity Conservation. Conserv. Lett. 2022, 15, e12881. [Google Scholar] [CrossRef]
- Jarošík, V.; Konvička, M.; Pyšek, P.; Kadlec, T.; Beneš, J. Conservation in a City: Do the Same Principles Apply to Different Taxa? Biol. Conserv. 2011, 144, 490–499. [Google Scholar] [CrossRef]
- Lizée, M.-H.; Mauffrey, J.-F.; Tatoni, T.; Deschamps-Cottin, M. Monitoring Urban Environments on the Basis of Biological Traits. Ecol. Indic. 2011, 11, 353–361. [Google Scholar] [CrossRef]
- Nielsen, A.B.; van den Bosch, M.; Maruthaveeran, S.; van den Bosch, C.K. Species Richness in Urban Parks and Its Drivers: A Review of Empirical Evidence. Urban Ecosyst. 2014, 17, 305–327. [Google Scholar] [CrossRef]
- Hüse, B.; Szabó, S.; Deák, B.; Tóthmérész, B. Mapping an Ecological Network of Green Habitat Patches and Their Role in Maintaining Urban Biodiversity in and around Debrecen City (Eastern Hungary). Land Use Policy 2016, 57, 574–581. [Google Scholar] [CrossRef]
- Nature-Based Solutions to Address Global Societal Challenges; Cohen-Shacham, E.; Walters, G.; Janzen, C.; Maginnis, S. (Eds.) IUCN International Union for Conservation of Nature: Gland, Switzerland, 2016; ISBN 978-2-8317-1812-5. [Google Scholar]
- Pauleit, S.; Zölch, T.; Hansen, R.; Randrup, T.B.; Konijnendijk van den Bosch, C. Nature-Based Solutions and Climate Change–Four Shades of Green. In Urban Areas. Theory and Practice of Urban Sustainability Transitions; Springer: Cham, Switzerland, 2017; pp. 29–49. [Google Scholar] [CrossRef]
- Pla-Narbona, C.; Stefanescu, C.; Pino, J.; Cabrero-Sañudo, F.J.; García-Barros, E.; Munguira, M.L.; Melero, Y. Butterfly Biodiversity in the City Is Driven by the Interaction of the Urban Landscape and Species Traits: A Call for Contextualised Management. Landsc. Ecol. 2022, 37, 81–92. [Google Scholar] [CrossRef]
- Tzirkalli, E.; Kadis, C.; Halley, J.M.; Vogiatzakis, I.; Wilson, R.J.; Zografou, K.; Antoniou, A.; Tsintides, T.; Makris, C.; Kati, V. Conservation Ecology of Butterflies on Cyprus in the Context of Natura 2000. Biodivers. Conserv. 2019, 28, 1759–1782. [Google Scholar] [CrossRef]
- Ramírez-Restrepo, L.; MacGregor-Fors, I. Butterflies in the City: A Review of Urban Diurnal Lepidoptera. Urban Ecosyst. 2017, 20, 171–182. [Google Scholar] [CrossRef]
- Hantson, S.; Baz, A. Seasonal Change in Nectar Preference for a Mediterranean Butterfly Community. J. Lepid. Soc. 2013, 67, 134–142. [Google Scholar] [CrossRef]
- Warren, M.S.; Maes, D.; van Swaay, C.A.M.; Goffart, P.; Van Dyck, H.; Bourn, N.A.D.; Wynhoff, I.; Hoare, D.; Ellis, S. The Decline of Butterflies in Europe: Problems, Significance, and Possible Solutions. Proc. Natl. Acad. Sci. USA 2021, 118, e2002551117. [Google Scholar] [CrossRef] [PubMed]
- van Swaay, C.; Warren, M.; Loïs, G. Biotope Use and Trends of European Butterflies. J. Insect Conserv. 2006, 10, 189–209. [Google Scholar] [CrossRef]
- Van Dyck, H.; Van Strien, A.J.; Maes, D.; Van Swaay, C.A. Declines in Common, Widespread Butterflies in a Landscape under Intense Human Use. Conserv. Biol. 2009, 23, 957–965. [Google Scholar] [CrossRef]
- Lizée, M.-H.; Manel, S.; Mauffrey, J.-F.; Tatoni, T.; Deschamps-Cottin, M. Matrix Configuration and Patch Isolation Influences Override the Species–Area Relationship for Urban Butterfly Communities. Landsc. Ecol. 2012, 27, 159–169. [Google Scholar] [CrossRef]
- Lizée, M.-H.; Bourdil, C.; Barthélémy, C.; Deschamps-Cottin, M. Approche Socio-Écologique Des Parcs Publics Marseillais. Relations Entre Gestion et Biodiversité Urbaine. Méditerranée Rev. Géographique Pays Méditerranéens/J. Mediterr. Geogr. 2014, 123, 123–132. [Google Scholar] [CrossRef]
- Ternisien, M.; Deschamps-Cottin, M.; Lizée, M.-H.; March, L.; Robles, C.; Vila, B. How Butterfly Communities Are Structured and Have Changed in Urbanized Areas of Marseille: A 12-Year Monitoring Survey. Urban Ecosyst. 2023, 1–12. [Google Scholar] [CrossRef]
- Deschamps-Cottin, M.; Vila, B.; Robles, C. Le parc urbain des papillons: Un dispositif collaboratif de recherche, formation et diffusion des connaissances sur la biodiversité urbaine. Éducation Relat. l’Environ. Regards–Rech.–Réflexions 2019, 15, 1. [Google Scholar] [CrossRef]
- Maurel, N.; Doux, Y. La Saison des Papillons de Provence. Les Papillons du Jardin et leurs plantes mois par mois; Proserpine: Levallois-Perret, France, 2009; ISBN 978-2-7466-0951-8. [Google Scholar]
- Lafranchis, T.; Jutzeler, D.; Guillosson, J.Y. La Vie des Papillons: Écologie, Biologie et Comportement des Rhopalocères de France; Diatheo: Paris, France, 2015; ISBN 978-2-9521620-6-7. [Google Scholar]
- OpenStreetMap Marseille Region. 2023. Available online: https://www.openstreetmap.org/#map=11/48.5769/7.8642&layers=HLast (accessed on 12 July 2023).
- Google Earth Butterfly Urban Park (43°20′14.92″ N/ 5°24′0.52″ E). Landsat/Copernicus Data. 2. 2023. Available online: https://earth.google.com/web/ (accessed on 12 July 2023).
- Pollard, E. Temperature, Rainfall and Butterfly Numbers. J. Appl. Ecol. 1988, 25, 819–828. [Google Scholar] [CrossRef]
- Altermatt, F. Temperature-Related Shifts in Butterfly Phenology Depend on the Habitat. Glob. Change Biol. 2012, 18, 2429–2438. [Google Scholar] [CrossRef]
- Dozières, A.; Valarcher, J.; Clément, Z. Papillons Des Jardins, Des Prairies et Des Champs. Guide de Terrain Pour les Observatoires de Sciences Participatives. 2017. p. 60. Available online: https://www.sciences-participatives-au-jardin.org/files/Guide-papillons.pdf (accessed on 9 June 2023).
- Lafranchis, T. Papillons De France. Guide De Détermination Des Papillons Diurnes (Rhopalocères, Zygènes Et Hétérocères Diurnes)—Tristan Lafranchis; Diatheo: Paris, France, 2015; ISBN 978-2-9521620-5-0. [Google Scholar]
- Hutcheson, K. A Test for Comparing Diversities Based on the Shannon Formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, V4.0.3,Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 2 February 2023).
- Oksanen, J.; Blanchet, G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’. 2020. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 2 February 2023).
- Baselga, A.; Orme, C.D.L. Betapart: An R Package for the Study of Beta Diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Clarke, K.R. Non-Parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species:The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Roberts, D. Package ‘Labdsv’ 2022. 2023. Available online: https://cran.r-project.org/web/packages/labdsv/index.html (accessed on 2 September 2023).
- Sawchik, J.; Dufrêne, M.; Lebrun, P. Distribution patterns and indicator species of butterfly assemblages of wet meadows in southern Belgium. Belg. J. Zool. 2005, 135, 43. Available online: http://hdl.handle.net/2078.1/38751 (accessed on 2 September 2023).
- Fileccia, V.; Santorsola, S.; Arpaia, S.; Manachini, B. Seasonal Patterns in Butterfly Abundance and Species Diversity in Five Characteristic Habitats in Sites of Community Importance in Sicily (Italy). Bull. Insectology 2015, 68, 91–102. [Google Scholar]
- Bence, S.; Richaud, S. Atlas Des Papillons de Jour et Zygènes: Provence-Alpes-Côte d’Azur 2020; Le Naturographe: Gap, France, 2021; ISBN 9782956054917. [Google Scholar]
- Colom, P.; Ninyerola, M.; Pons, X.; Traveset, A.; Stefanescu, C. Phenological Sensitivity and Seasonal Variability Explain Climate-Driven Trends in Mediterranean Butterflies. Proc. R. Soc. B Biol. Sci. 2022, 289, 20220251. [Google Scholar] [CrossRef]
- Flo, V.; Bosch, J.; Arnan, X.; Primante, C.; González, A.M.M.; Barril-Graells, H.; Rodrigo, A. Yearly Fluctuations of Flower Landscape in a Mediterranean Scrubland: Consequences for Floral Resource Availability. PLoS ONE 2018, 13, e0191268. [Google Scholar] [CrossRef]
- Bosch, J.; Retana, J.; Cerdá, X. Flowering Phenology, Floral Traits and Pollinator Composition in a Herbaceous Mediterranean Plant Community. Oecologia 1997, 109, 583–591. [Google Scholar] [CrossRef]
- Manachini, B.; Bazan, G.; Schicchi, R. Potential Impact of Genetically Modified Lepidoptera-Resistant Brassica Napus in Biodiversity Hotspots: Sicily as a Theoretical Model. Insect Sci. 2018, 25, 562–580. [Google Scholar] [CrossRef] [PubMed]
- Szigeti, V.; Kőrösi, Á.; Harnos, A.; Kis, J. Temporal Changes in Floral Resource Availability and Flower Visitation in a Butterfly. Arthropod-Plant Interact. 2018, 12, 177–189. [Google Scholar] [CrossRef]
- Nakahama, N.; Hayamizu, M.; Iwasaki, K.; Nitta, N. Management and Landscape of Shelterbelts Contribute to Butterfly and Flowering Plant Diversity in Northern Japan. Ecol. Res. 2022, 37, 780–790. [Google Scholar] [CrossRef]
- Waltz, A.E.M.; Wallace Covington, W. Ecological Restoration Treatments Increase Butterfly Richness and Abundance: Mechanisms of Response. Restor. Ecol. 2004, 12, 85–96. [Google Scholar] [CrossRef]
- Kurylo, J.S.; Threlfall, C.G.; Parris, K.M.; Ossola, A.; Williams, N.S.G.; Evans, K.L. Butterfly Richness and Abundance along a Gradient of Imperviousness and the Importance of Matrix Quality. Ecol. Appl. 2020, 30, e02144. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, C.; Sun, Z.; She, J.; Yin, L.; Bian, Q.; Han, W. Microhabitat Preferences of Butterflies in Urban Parks: Both Vegetation Structure and Resources Are Decisive. Urban For. Urban Green. 2022, 71, 127552. [Google Scholar] [CrossRef]
- Kitahara, M.; Sei, K. A Comparison of the Diversity and Structure of Butterfly Communities in Semi-Natural and Human-Modified Grassland Habitats at the Foot of Mt. Fuji, Central Japan. Biodivers. Conserv. 2001, 10, 331–351. [Google Scholar] [CrossRef]
- Bergerot, B.; Merckx, T.; Van Dyck, H.; Baguette, M. Habitat Fragmentation Impacts Mobility in a Common and Widespread Woodland Butterfly: Do Sexes Respond Differently? BMC Ecol. 2012, 12, 5. [Google Scholar] [CrossRef]
- Bubová, T.; Vrabec, V.; Kulma, M.; Nowicki, P. Land Management Impacts on European Butterflies of Conservation Concern: A Review. J. Insect Conserv. 2015, 19, 805–821. [Google Scholar] [CrossRef]
- Smallidge, P.J.; Leopold, D.J. Vegetation Management for the Maintenance and Conservation of Butterfly Habitats in Temperate Human-Dominated Landscapes. Landsc. Urban Plan. 1997, 38, 259–280. [Google Scholar] [CrossRef]
- Aguilera, G.; Ekroos, J.; Persson, A.S.; Pettersson, L.B.; Öckinger, E. Intensive Management Reduces Butterfly Diversity over Time in Urban Green Spaces. Urban Ecosyst. 2019, 22, 335–344. [Google Scholar] [CrossRef]
- Wang, W.-L.; Suman, D.O.; Zhang, H.-H.; Xu, Z.-B.; Ma, F.-Z.; Hu, S.-J. Butterfly Conservation in China: From Science to Action. Insects 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel ecosystems: Theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 2006, 15, 1–7. [Google Scholar] [CrossRef]
- Curtis, R.J.; Brereton, T.M.; Dennis, R.L.H.; Carbone, C.; Isaac, N.J.B. Butterfly Abundance Is Determined by Food Availability and Is Mediated by Species Traits. J. Appl. Ecol. 2015, 52, 1676–1684. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Hodgson, J.G.; Grenyer, R.; Shreeve, T.G.; Roy, D.B. Host Plants and Butterfly Biology. Do Host-Plant Strategies Drive Butterfly Status? Ecol. Entomol. 2004, 29, 12–26. [Google Scholar] [CrossRef]
| BUPbefore | BUPafter | WAS | p-Value 1 | ||
|---|---|---|---|---|---|
| Total species richness | 25 | 42 | 46 | ||
| Mean species richness | June | 6.00 ± 3.38 a | 15.30 ± 3.83 b | 16.90 ± 3.64 b | *** |
| July | 8.60 ± 2.97 | 11.70 ± 3.53 | 11.50 ± 4.60 | ns | |
| August | 6.25 ± 3.40 | 8.30 ± 2.67 | 9.90 ± 2.91 | ns | |
| Pooled | 6.82 ± 2.68 a | 11.77 ± 3.68 b | 12.78 ± 3.88 b | *** | |
| Mean abundance | June | 33.75 ± 22.47 | 50.50 ± 26.10 | 56.20 ± 13.80 | ns 2 |
| July | 20.80 ± 11.12 a | 32.70 ± 12.32 ab | 38.60 ± 14.79 b | * | |
| August | 12.00 ± 5.42 a | 21.20 ± 12.05 ab | 29.90 ± 14.31 b | * | |
| Pooled | 24.82 ± 15.79 a | 34.80 ± 13.92 ab | 41.66 ± 14.37 b | * | |
| Mean diversity (Shannon index) | June | 1.15 ± 0.63 a | 2.34 ± 0.28 b | 2.44 ± 0.30 b | *** |
| July | 1.86 ± 0.30 | 2.12 ± 0.29 | 1.91 ± 0.56 | ns | |
| August | 1.45 ± 0.74 | 1.78 ± 0.34 | 1.92 ± 0.39 | ns | |
| Pooled | 1.43 ± 0.51 a | 2.08 ± 0.29 ab | 2.08 ± 0.42 b | * | |
| Pielou index | June | 0.61 ± 0.28 a | 0.87 ± 0.03 b | 0.87 ± 0.06 b | * |
| July | 0.89 ± 0.06 | 0.88 ± 0.06 | 0.79 ± 0.13 | ns | |
| August | 0.84 ± 0.11 | 0.88 ± 0.12 | 0.85 ± 0.11 | ns | |
| Pooled | 0.75 ± 0.17 | 0.87 ± 0.06 | 0.83 ± 0.09 | ns |
| Species | IndVal | Relative Species Frequency | p-Value | ||||
|---|---|---|---|---|---|---|---|
| BUPbefore | BUPafter | WAS | BUPbefore | BUPafter | WAS | ||
| (2010–2012) | (2018–2022) | (2018–2022) | (2010–2012) | (2018–2022) | (2018–2022) | ||
| Melanargia occitanica | 0.800 | 0.8 | * | ||||
| Euchloe crameri | 0.033 | 0.833 | 0.2 | 1 | ** | ||
| Pontia daplidice | 0.114 | 0.714 | 0.4 | 1 | * | ||
| Pieris napi | 0.714 | 0.114 | 1 | 0.4 | * | ||
| Gonepteryx cleopatra | 0.355 | 0.556 | 0.8 | 1 | * | ||
| Melitaea didyma | 0.356 | 0.556 | 0.8 | 1 | * | ||
| Vanessa cardui | 0.625 | 0.225 | 1 | 0.6 | * | ||
| Pyronia bathseba | 0.072 | 0.026 | 0.652 | 0.3 | 0.2 | 1 | * |
| Pyronia cecilia | 0.048 | 0.429 | 0.429 | 0.3 | 1 | 1 | * |
| Thymelicus acteon | 0.048 | 0.429 | 0.429 | 0.3 | 1 | 1 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deschamps-Cottin, M.; Jacek, G.; Seguinel, L.; Le Champion, C.; Robles, C.; Ternisien, M.; Duque, C.; Vila, B. A 12-Year Experimental Design to Test the Recovery of Butterfly Biodiversity in an Urban Ecosystem: Lessons from the Parc Urbain des Papillons. Insects 2023, 14, 780. https://doi.org/10.3390/insects14100780
Deschamps-Cottin M, Jacek G, Seguinel L, Le Champion C, Robles C, Ternisien M, Duque C, Vila B. A 12-Year Experimental Design to Test the Recovery of Butterfly Biodiversity in an Urban Ecosystem: Lessons from the Parc Urbain des Papillons. Insects. 2023; 14(10):780. https://doi.org/10.3390/insects14100780
Chicago/Turabian StyleDeschamps-Cottin, Magali, Guillaume Jacek, Louise Seguinel, Clémentine Le Champion, Christine Robles, Mélanie Ternisien, Chloé Duque, and Bruno Vila. 2023. "A 12-Year Experimental Design to Test the Recovery of Butterfly Biodiversity in an Urban Ecosystem: Lessons from the Parc Urbain des Papillons" Insects 14, no. 10: 780. https://doi.org/10.3390/insects14100780
APA StyleDeschamps-Cottin, M., Jacek, G., Seguinel, L., Le Champion, C., Robles, C., Ternisien, M., Duque, C., & Vila, B. (2023). A 12-Year Experimental Design to Test the Recovery of Butterfly Biodiversity in an Urban Ecosystem: Lessons from the Parc Urbain des Papillons. Insects, 14(10), 780. https://doi.org/10.3390/insects14100780







