Simple Summary
Avian haemoproteids are prevalent parasites known to induce pathology or mortality in birds. Culicoides biting midges act as vectors for these parasites. Despite the annual arrival of various haemoproteids to Europe with migrant birds, not all these parasites undergo local transmission. The factors constraining the local spread of these pathogens remain incompletely understood. Our study investigates if the ecological isolation of birds from vectors, either temporally or spatially during the breeding period when transmission occurs, could lead to the cessation of transmission. Biting midges were systematically collected from two distinct habitats between May and September. A total of 1135 parous Culicoides females were identified and examined for the presence of Haemoproteus parasites using both microscopy and molecular tools. Notably, we report the first detection of Haemoproteus asymmetricus sporozoites in the salivary glands of Culicoides festivipennis females. The sporozoites of four Haemoproteus genetic lineages were also identified in Culicoides segnis, C. festivipennis, and C. kibunensis, further validating their status as potential Haemoproteus vectors. While the highest abundance of collected Culicoides females occurred in June, the peak prevalence of Haemoproteus parasites in biting midges was observed in July. Interestingly, the abundance of Culicoides was significantly greater in woodlands compared to reeds throughout the season.
Abstract
Avian haemosporidians (Apicomplexa, Haemosporida) are widespread blood protists, often causing severe haemosporidiosis, pathology, or even mortality in their hosts. Migrant birds regularly bring various haemosporidian parasites from wintering grounds to European breeding areas. Some haemosporidian parasites are prevalent in breeding sites and complete their life cycles in temperate climate zones and can be transmitted, but others do not. The factors altering the spread of these haemosporidians are not fully understood. Culicoides biting midges (Diptera: Ceratopogonidae) play an important role in the transmission of worldwide distributed avian haemosporidian parasites belonging to the genus Haemoproteus, but this information is particularly scarce and insufficient. The key factors limiting the spread of these pathogens in temperate climate zones, which we suspect and aim to study, are the absence of susceptible vectors and the ecological isolation of birds from vectors during the breeding period when transmission occurs. The primary objective of this study was to evaluate how the habitats of biting midges and bird breeding sites influence parasite transmission while also seeking to expand our understanding of the natural vectors for these parasites. Biting midges were collected using UV traps on the Curonian Spit, Lithuania, in different habitats, such as woodland and reeds, from May to September. Parous Culicoides females were identified, dissected, and investigated for the presence of Haemoproteus parasites using both microscopy and PCR-based tools. Among the dissected 1135 parous Culicoides females, the sporozoites of Haemoproteus asymmetricus (genetic lineage hTUPHI01) have been detected for the first time in the salivary glands of Culicoides festivipennis. The sporozoites of four Haemoproteus lineages were detected in Culicoides segnis, C. festivipennis, and Culicoides kibunensis biting midges. PCR-based screening showed that the females of seven Culicoides species were naturally infected with Haemoproteus parasites. The DNA of the parasite of owls, Haemoproteus syrnii (hSTAL2), was detected for the first time in Culicoides punctatus. The highest abundance of collected Culicoides females was in June, but the highest prevalence of Haemoproteus parasites in biting midges was in July. The abundance of Culicoides was higher in the woodland compared with reeds during the season. The acquired findings indicate the varied abundance and diversity of biting midges throughout the season and across distinct habitats. This variability could potentially impact the transmission of Haemoproteus parasites among birds with diverse breeding site ecologies. These outcomes hold the potential to enhance our understanding of the epizootiology of Haemoproteus infections within temperate climatic zones.
1. Background
Outbreaks of new vector-borne haemosporidian infections have been frequently recorded during past decades, calling for research on wildlife pathogens to better understand their epizootiology and the main factors altering the spread of new diseases [1,2,3,4].
Migrant birds regularly bring various haemosporidian parasites from wintering grounds to European breeding areas and serve as reservoirs of potential disease outbreaks [5,6]. Some of these parasites can complete their life cycles in temperate climate zones and can be transmitted there, but others are transmitted only in wintering grounds [7,8,9,10,11]. The factors preventing the spread of these vector-borne infections are not studied sufficiently [11]. The primary factors that plausibly constrain the spread of these pathogens within temperate climatic regions are the lack of susceptible vectors and the ecological segregation of birds from vectors during their reproductive phase. The determination of these constraining factors holds significance due to ongoing climate change, which has caused ecological changes in Europe. Particularly in the southern regions, the expanding presence of hematophagous arthropods (vectors) in novel territories could potentially establish suitable environments for the emergence of hitherto unrecorded avian infections [3,10,12,13,14,15,16]. According to some predictions, an increase in the global temperature by 1 °C will co-occur with a two- to three-fold increase in avian malaria prevalence [17]. It alerts us to the significant changes in the epizootiological situation in temperate zones in the near future. Therefore, the main goal of our study is to investigate the epizootiology and transmission capabilities of Haemoproteus parasites, the factors limiting the spread of these pathogens in temperate climate zones, such as ecological isolation, and the success of transmission between birds and Culicoides females.
The abundance of vectors and the peculiarities of bird biology determine their contact with vectors and influence the prevalence of infection [7,18,19]. Much of the literature is focused on bird movement and the exchange of parasites between regions, but there is insufficient information about vector abundance, diversity, and activity, which would enhance our understanding of the ecology and evolution of host–parasite systems [20].
With the objective of ascertaining the ecological variables that impact the dissemination of parasites and uncovering of novel vector species, we collected female Culicoides specimens from two distinct habitats: a deciduous woodland primarily dominated by alders and a reed-dominated environment. Our investigation encompassed an evaluation of the population density, temporal patterns, and prevalence of avian haemosporidian infections, specifically within parous female specimens. The identification of new natural vectors is important because from more than 1400 Culicoides species described in the world [21], only 6 species have been identified as natural vectors of haemoproteids in Europe [22], and 10 more Culicoides species are known as haemoproteid vectors in the world [8,20].
The results of this study supplemented the list of vectors of haemoproteids in Europe and provided information about the factors influencing the spread of Haemoproteus parasites among birds. Studies focusing on avian Haemoproteus parasites and their vectors, as well as the dynamics of transmission, are still relatively limited. Consequently, these areas remain a significant priority within the field of haemosporidian research [20].
2. Materials and Methods
2.1. Study Site, Collection of Biting Midges
Biting midges were collected in the Curonian Spit, located on the Baltic Sea, Lithuania, from May to September 2021. Four Biogents BG-Pro traps, hung at 1.5 m height, were used in two different habitats: two traps were hung in an old deciduous woodland dominated by alder (Figure 1a) and two traps were hung in reeds near the Curonian lagoon (Figure 1b). The distance between traps in the same habitat was on average 3.5 km, and the distance between traps in different habitats was on average 0.6 km. Additionally, one Onderstepoort 220 V UV trap was used in a woodland habitat at the same height to collect more parous Culicoides females for the detection of sporozoites in salivary glands, thereby targeting natural Haemoproteus vectors [22].

Figure 1.
UV light traps used for Culicoides collection in woodland (a) and reeds (b).
Insects were collected over a span of 17 nights, with a collection frequency of 2 nights in September, 3 nights in May, and 4 nights each in June, July, and August. The traps were turned on 1–2 h before sunset and turned off 2–3 h after sunrise. Insects were collected in a water container supplemented with a drop of liquid soap, as described by Bernotienė et al. [23]. The collected insects were transported to the laboratory of the Biological Station of the Nature Research Centre (Juodkrantė). Only parous Culicoides females were sorted according to Dyce [24] and identified according to Gutsevich [25], Glukhova et al. [26], and Mathieu et al. [27]. The material was studied under binocular stereoscopic microscopes Olympus SZ × 10 and Olympus B × 43 (Olympus Corporation, Tokyo, Japan), as described below.
2.2. Microscopic Examination of Preparations
The details of the dissection of parous biting midges were described by Valkiūnas [7] and Žiegytė et al. [22,28]. Briefly, each parous Culicoides female was individually dissected by removing the head and isolating the salivary glands from the breast into a drop of physiological solution on an objective slide. The content of the salivary glands was smeared, air-dried, fixed with absolute methanol, and stained with 4% Giemsa stain [22,28]. All residual parts of Culicoides female were fixed in 96% alcohol for PCR-based investigation. Representative preparations of sporozoites (49651NS–49652NS) were deposited in the Nature Research Centre, Vilnius, Lithuania.
2.3. Molecular Analysis
The genetic material from the remains of each dissected Culicoides female was isolated utilizing the ammonium acetate DNA extraction method [29]. To identify avian haemosporidian parasites within the insects, we employed a nested PCR protocol described by [30,31] with outer primers HaemNFI/HaemNR3 and inner primers HAEMF/HAEMR2 to amplify the mitochondrial DNA cytochrome b (cyt b) gene segment of 479 bp of the parasite (Haemoproteus and Plasmodium spp.). To mitigate the risk of false positives, a negative control (H2O instead of target DNA) was included every 24 samples.
To validate the morphological identification of Culicoides females that tested positive for haemosporidian parasites via PCR, a molecular examination of mitochondrial DNA cytochrome c oxidase subunit 1 (COI) was conducted using primers LCO1490 and HCO2198 [32]. The morphological identification consistently aligned with the PCR-based identification of biting midges, as the obtained sequences exhibited a 99–100% match with corresponding sequences from GenBank.
The DNA fragments from all PCR samples were visualized on 2% agarose gel using MidoriGreen dye (NIPPON Genetics Europe, Düren, Germany). Subsequently, all positive samples underwent sequencing using both forward and reverse primers, employing the Big-Dye® Terminator v3.1 Cycle Sequencing Kit (Thermo Fisher Scientific, Vilnius, Lithuania) and an applied biosystems genetic analyzer 3500. The sequences were edited and aligned using BioEdit software version 7.2.5 [33]. Genetic lineages of the parasites were determined using the ‘Basic Local Alignment Search Tool’ (megablast algorithm) from NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 20 August 2023), and their identification was cross-verified using the MalAvi database BLAST function (http://mbio-serv2.mbioekol.lu.se/Malavi, accessed on 20 August 2023).
2.4. Statistical Analysis
The statistical analysis was carried out using the Statistica 7 software package. The average values of the collected biting midges per night per one trap were calculated and provided with Standard error. The abundances of the midges collected in different habitats were compared using a t-test for dependent samples. A p value of 0.05 or less was considered significant.
3. Results
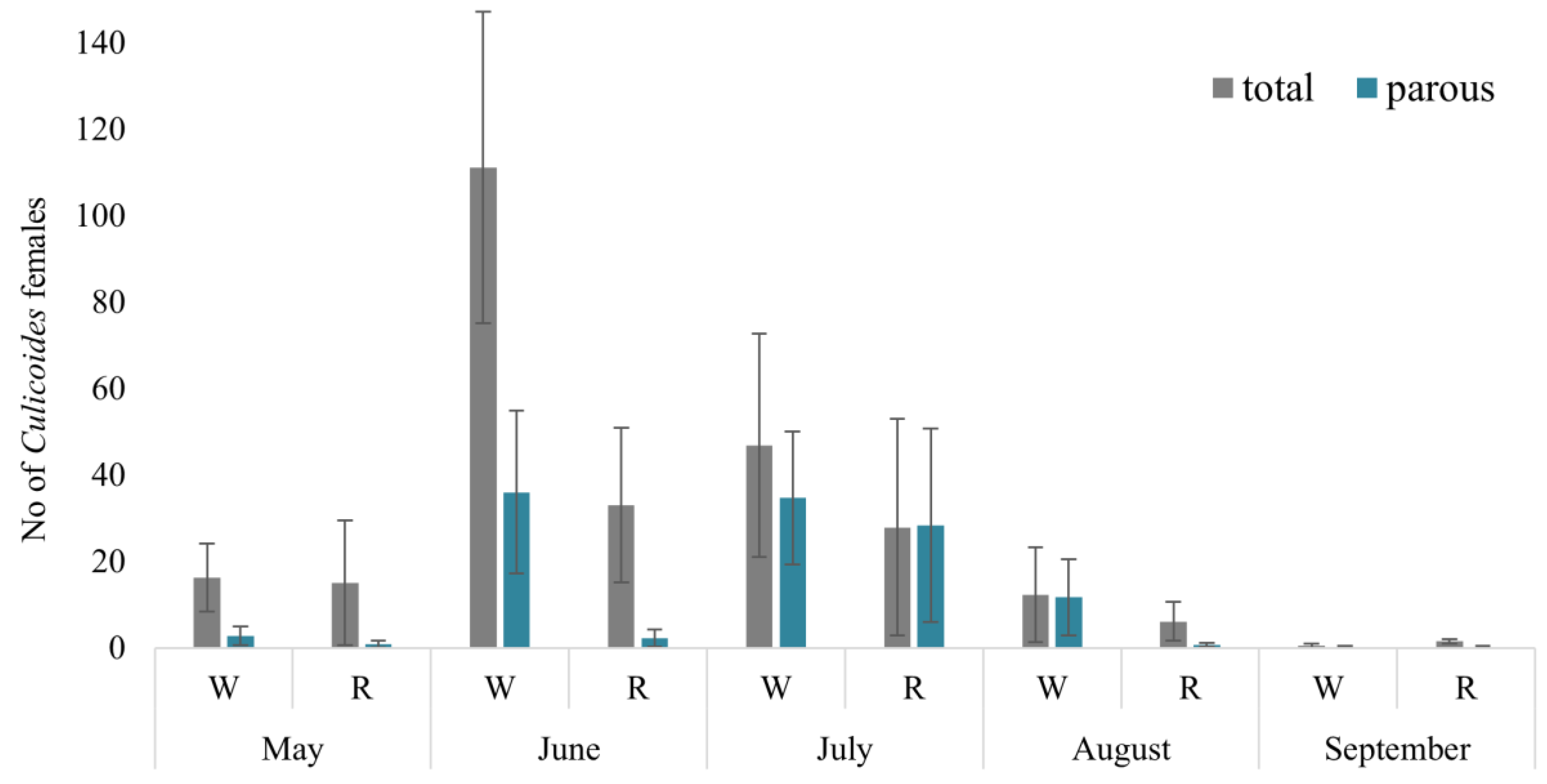
In total, 1900 Culicoides females were collected during May–September 2021 using four Biogents BG-Pro traps in four localities and two habitats. The peak abundance of Culicoides females occurred in June, with woodland areas exhibiting the highest count (reaching up to 273 females, with a mean of 111.1 ± 39 (SE) collected per night per trap). Lower abundances were observed in August in reeds (6.1 ± 4.0) and in both habitats in September (0.5 ± 0.5) (Figure 2). Among all biting midges, 67.7% were collected in woodland habitats, while only 32.3% were found in reeds. Despite utilizing the same collection methodology in both habitats, the abundance of collected Culicoides midges was statistically higher in woodland habitats compared to reeds (t = 2.41, p = 0.023).
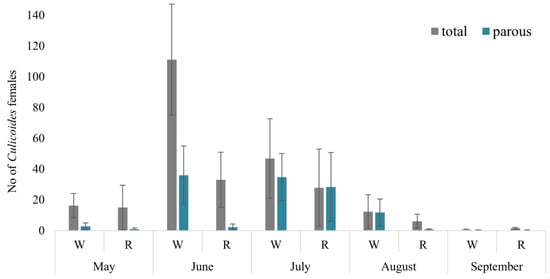
Figure 2.
Mean number of Culicoides females collected per one night with one trap in different habitats. W—woodland, R—reeds.
In total, 857 of the Culicoides midges collected by using four traps were parous and dissected for the salivary gland preparations (Figure 2). Similar to the overall abundance of Culicoides females, the abundance of collected parous females was significantly higher in woodland habitats (72.7% of all collected parous biting midges) compared with the reeds (27.3%; t = 2.65, p = 0.013). The highest abundance of parous Culicoides females was recorded in June and July, with the highest relative abundance (proportion of parous females) ranging from 0 (reeds in May and September) to 72.9% (woodland in July).
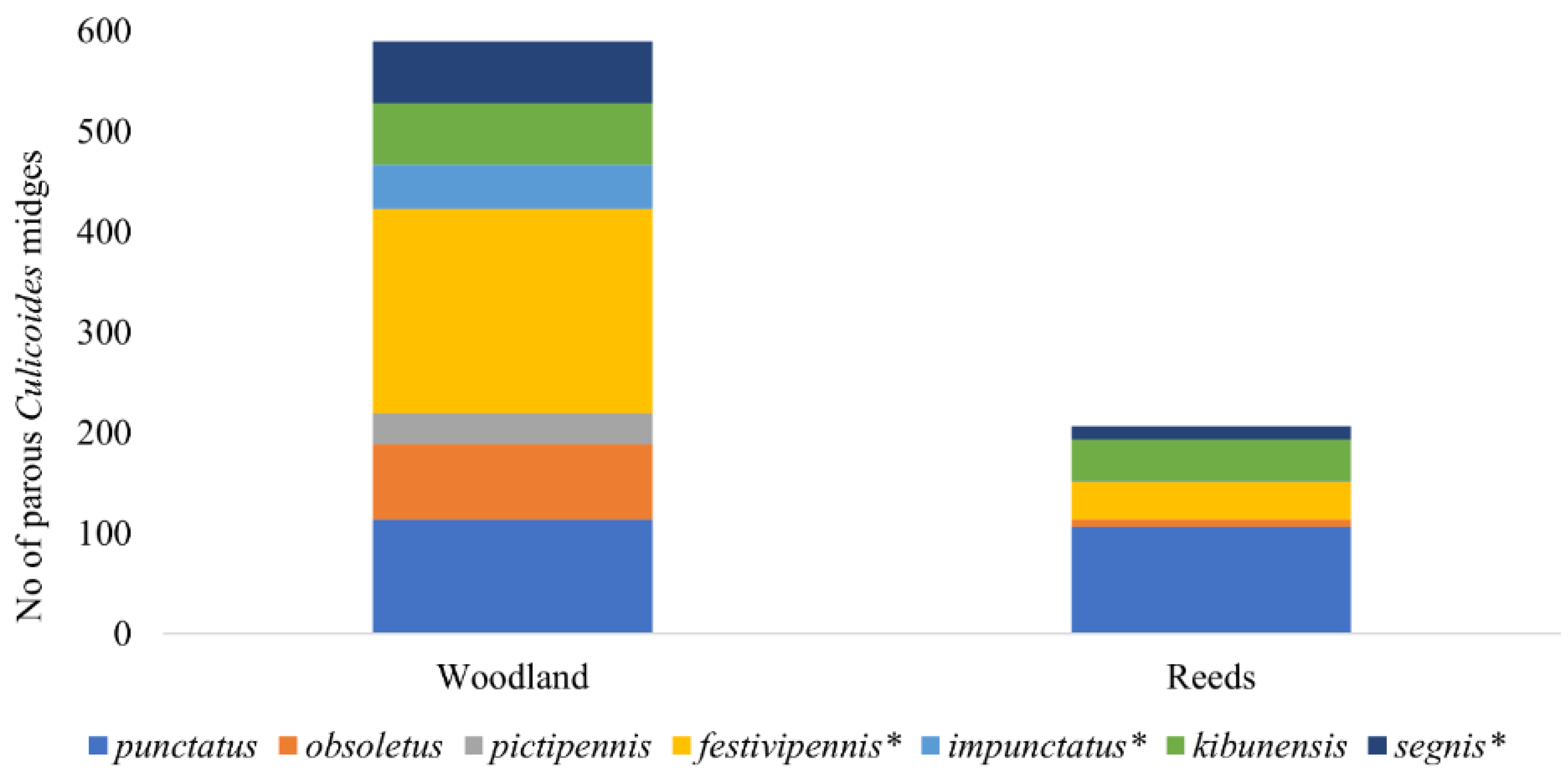
During the investigation, we identified 10 different Culicoides species and the C. obsoletus complex. The dominant Culicoides species were C. punctatus (May and July), C. obsoletus complex (June), C. kibunensis (June), and C. festivipennis (from June to August). No statistical differences have been detected in the abundances of parous females for C. punctatus (n = 218), C. kibunensis (n = 105), and C. obsoletus (n = 83) in different habitats. However, differences in abundance among different habitats have been detected for C. festivipennis (n = 242, p = 0.046), C. segnis (n = 75, p = 0.027), and C. impunctatus (n = 45, p = 0.036) (Figure 3). Culicoides pictipennis was collected only in woodland (n = 33). Only a small number of parous females of Culicoides reconditus, C. pallidicornis, C. nubeculosus, and C. grissescens were collected during the investigation.
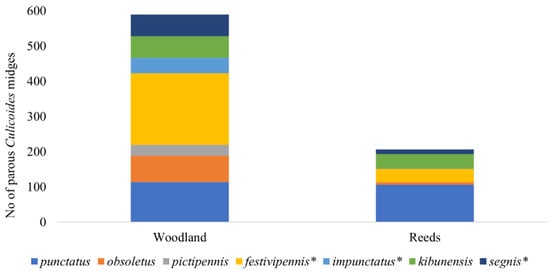
Figure 3.
Number of parous Culicoides females collected in two habitats during the season. Asterisk indicates species whose abundances in different habitats differed significantly.
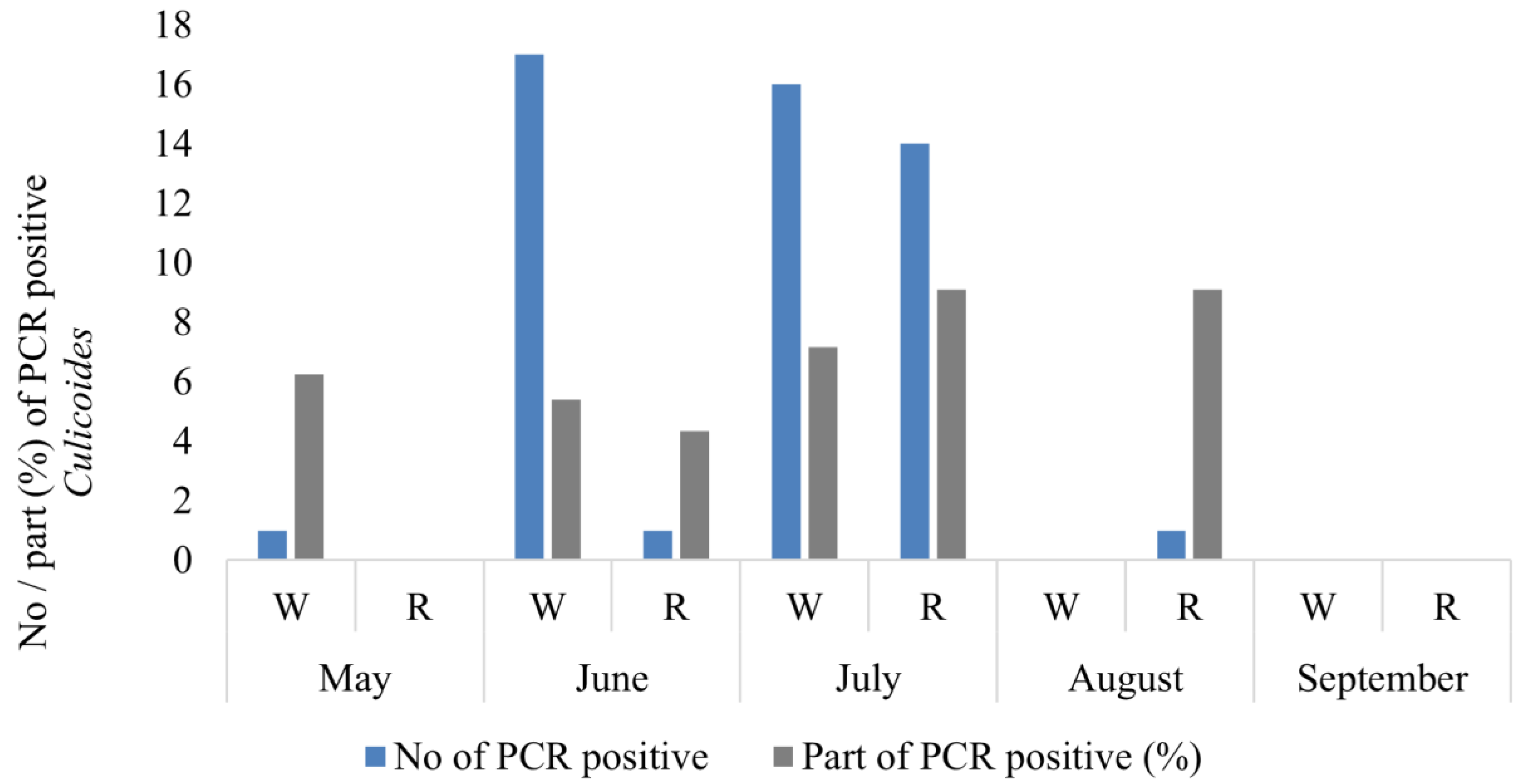
In total, 64 Culicoides females were detected to be PCR-positive for the presence of haemosporidian parasite DNA (Table 1). Thirty-four of these were collected in the woodland, exhibiting 10 genetic lineages of Haemoproteus, while 16 were obtained from reeds featuring 4 lineages (Figure 4, Table 1). A high prevalence of haemosporidian parasites in biting midges was determined in June (18 PCR-positive midges or 5.0% from all parous females), but the highest was determined in July (30 PCR-positive biting midges or 8.3%), while solely one PCR positive female biting midge was identified in both May and August (Figure 4).

Table 1.
Parasites and genetic lineages detected in Culicoides females using PCR.
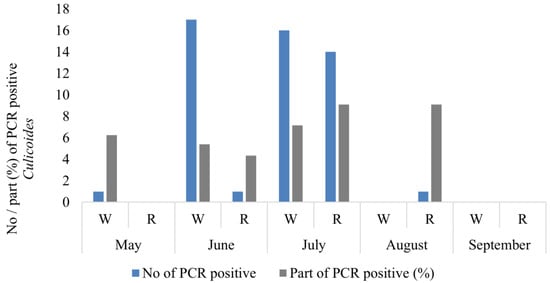
Figure 4.
Number of PCR-positive Culicoides females collected during the warm season in two habitats. Part of PCR-positive (%) Culicoides females from all collected parous females. W—woodland, R—reeds.
An additional 278 parous Culicoides females were collected through the utilization of an Onderstepoort 220 V UV trap deployed within the woodland habitat. This approach was undertaken to enhance the number of studied natural vectors responsible for transmitting Haemoproteus parasites within their natural environment. Fourteen more PCR-positive females (5%) were detected from the material collected using the Onderstepoort trap (Table 1).
June and July were the months with the highest number of Culicoides females with sporozoites detected in salivary glands (5 females collected in June and 9 females collected in July, Table 1). We detected sporozoites in the salivary glands of four PCR-negative C. segnis and one PCR-negative C. kibunensis females.
The DNA of Haemoproteus majoris (genetic lineages hCCF5, hPARUS1, hPHSIB1), Haemoproteus palloris (hWW1), Haemoproteus asymmetricus (hTUPHI01), Haemoproteus minutus (hTURDUS2), Haemoproteus belopolskyi (hHIICT1), Haemoproteus tartakovskyi (hHAWF1, hSISKIN1), and Haemoproteus sp. (hCCF4) was detected in Culicoides females (Table 1). The parasite of owls, Haemoproteus syrnii (hSTAL2), was detected for the first time in C. punctatus. Mixed Haemoproteus infection, detected by double chromatogram peaks, has been detected in C. kibunensis. The DNA of Plasmodium matutinum (genetic lineage pLINN1) was also detected in C. festivipennis, C. impunctatus, C. punctatus, and C. obsoletus biting midges. Detecting Plasmodium DNA in Culicoides females illustrates the possible abortive development of these parasites in non-competent vectors.
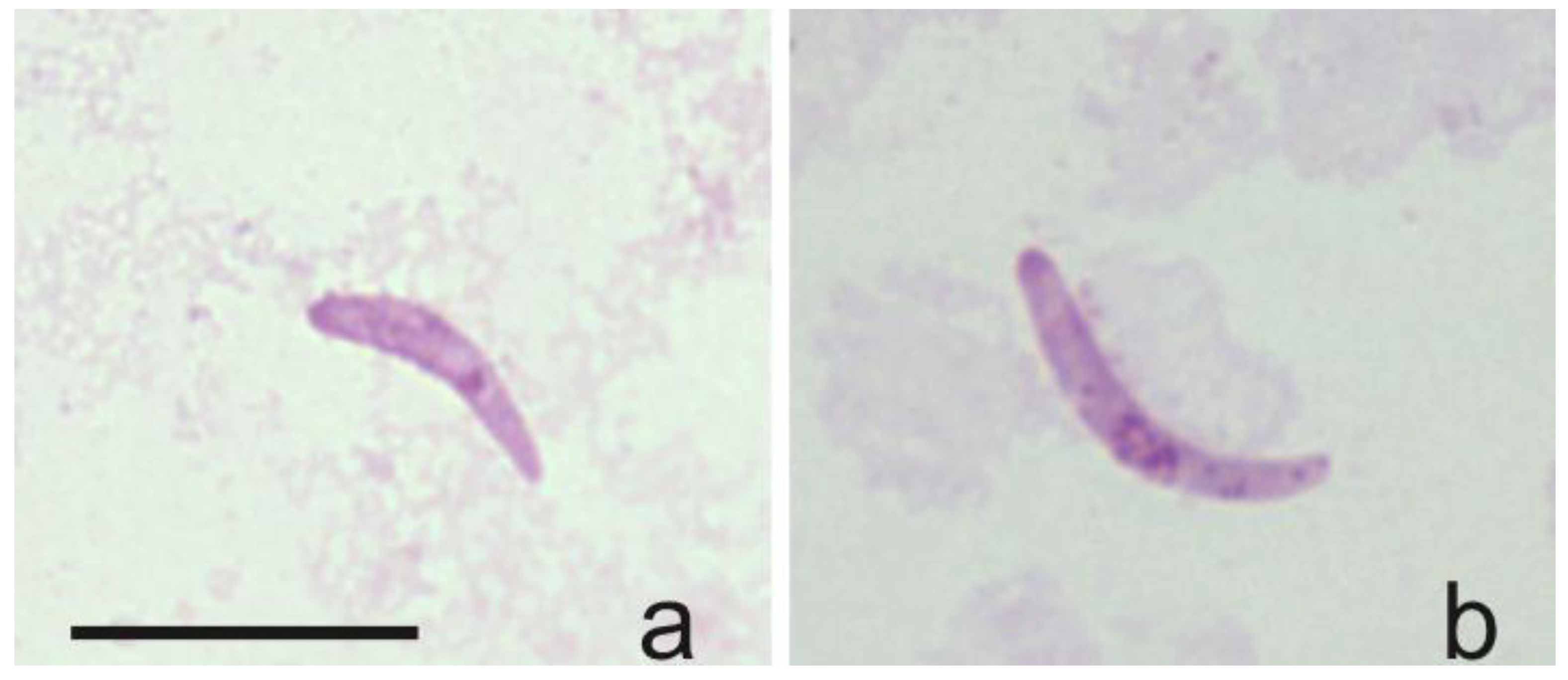
The DNA and sporozoites of H. asymmetricus (hTUPHI01) were detected for the first time in C. festivipennis biting midges’ salivary glands (Table 1, Figure 5a,b), showing that this Culicoides species can be considered as a natural vector of H. asymmetricus. Sporozoites originating from H. majoris, H. asymmetricus, H. tartakovskyi, and H. minutus (belonging to genetic lineages hPHSIB1, hTUPHI01, hHAWF1, hTURDUS2, respectively) were identified within biting midges of the species C. segnis, C. festivipennis, and C. kibunensis (Table 1).
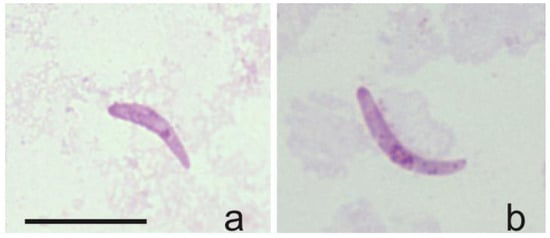
Figure 5.
Sporozoites detected in salivary glands of two (a,b) individual Culicoides festivipennis biting midges. Genetic lineage was identified as Haemoproteus asymmetricus (hTUPHI01).
4. Discussion
We determined that the highest abundance of Culicoides females occurs in June, with an average of 72 ± 44 Culicoides females collected per night using one trap. Conversely, the lowest abundances were recorded in August (9.2 ± 5.1) and September (0.9 ± 0.5) in the investigated area. This seasonal pattern, characterized by the highest abundance of collected biting midges in June, aligns with findings from prior investigations [34]. This consistent trend has also been noted by other authors conducting research in the Curonian spit [26,35,36,37]. Interestingly, the highest abundance of parous Culicoides females was recorded in July (Figure 2), not at the same time as the total Culicoides abundance (June), and the highest relative abundance (the proportion of parous females from all females) was the highest in July (56.5 ± 6.9% and up to 72.9% in woodland habitat). The lowest relative abundance of parous Culicoides females was detected in May (7.61 ± 3.3%) and September (9.38 ± 6.0%) when no parous females were collected in two out of four traps). Biting midge females live 2–4 weeks [26], so the highest proportion of parous females, which had already had at least one gonotrophic cycle, may appear later in comparison with the highest total abundance of Culicoides females. July had the highest number of Culicoides females with sporozoites detected in salivary glands (30 out of 64). This information is important for scientists elucidating the natural vectors of haemosporidian parasites. Our results show that July is the best month for this kind of investigation as it can be the month of the most active transmission of haemosporidian parasites of some species.
Birds of many species in Lithuania have offspring in June during the highest activity of Culicoides biting midges, so the observed overlapping of vector activity and the appearance of birds’ offspring should favor the transmission of infections to juvenile birds. However, June might be the month when biting midges have their first blood meal and are free of parasites. For instance, there are no records of H. nucleocondensus local transmission, a parasite of great reed warbler (Acrocephalus arundinaceus) in juvenile birds in northern Europe, although locally abundant Culicoides species could serve as vector candidates for H. nucleocondensus transmission [38,39]. The maximum hatching and fledging period of great read warblers occurs in June and vector abundance, according to our investigations, is high at that time [39]. However, this situation may change with changes in the arrival times of migrant birds due to climate change [40] or the shifting of the time to earlier months of the biggest abundance of biting midges. We have observed variations in the population density of female Culicoides in two distinct habitats, with reeds being less conducive to these insects comparing to woodland. This factor might additionally contribute to the disruption of parasite transmission. For example, great read warblers breed in reeds, where the abundance of Culicoides females and especially parous females is lower compared with the woodland habitats. This discordance in habitats might prevent the transmission of some infections from adult birds to juveniles. Most of the biting midges—67.7%—have been collected in woodland and 32.3% have been collected in reeds; 34 PCR-positive biting midges were collected in the woodland and 16 were collected in reeds (Figure 4). It is known that Culicoides midges are more abundant in moist woodlands and grassy groves, which serve as suitable habitats for both adult Culicoides biting midges [26,41,42] and their larval development. This correlation may be attributed to the conducive conditions for the breeding of Culicoides larvae in such environments. According to certain studies [43], the biting midges of some species (e.g., C. impunctatus) typically fly an average distance of 75 m from their breeding areas. However, over several days, females of other Culicoides species can cover longer distances, reaching approximately 2 km [44]. Only one C. impunctatus female was collected in reeds, while 44 females were collected in the woodland (Figure 3) during our investigation. Reeds can be unattractive to biting midges because they are exposed to more wind and have less favorable microclimate for a tiny fly to hide in.
In our study, the dominant Culicoides species were the same in both habitats. These species (C. punctatus, C. obsoletus complex, C. kibunensis, C. festivipennis) are also known to be among the dominant Culicoides species at other localities in Lithuania and in other countries in Europe [22,34,42,45]. However, based on our data, the abundance of certain Culicoides species exhibited significant variation across different habitats (Figure 3). Specifically, known vectors of Haemoproteus such as C. segnis, C. impunctatus [22,36], and C. pictipennis (the latter only was found in woodland) demonstrated distinct habitat preferences. It is noteworthy that the prevalent parasites identified within Culicoides females align predominantly with those associated with the avian genera of forest habitats, such as Turdus (hTUPHI01, hTURDUS2), Carduelis (hSISKIN1), Parus (hPARUS1), or Fringilla (hCCF5). This observation implies that instances of detecting positive midges in reed habitats infected with the hPARUS1 parasite likely stem from movement originating in the nearby forests. This is substantiated by the necessity for these midges to have fed upon forest birds, like Parus majoris, to contract parasites from this specific lineage.
The DNA and sporozoites of H. asymmetricus (hTUPHI01) were detected for the first time in C. festivipennis biting midges’ salivary glands (Figure 5). This parasite was described by Valkiūnas et. al. [46] and was predominantly reported in song thrush Turdus philomelos and was closely related with H. minutus (hTURDUS2), which can be found in the black bird Turdus merula [47]. Previously, two vector species of H. asymmetricus (hTUPHI01) were reported: C. kibunensis [28] and C. segnis [48]. We also detected sporozoites of this parasite in C. segnis and C. kibunensis during our study. Moreover, our study shows that C. festivipennis is also a vector of this avian parasite, and this is a new natural vector of Haemoproteus parasites in Europe.
The sporozoites of H. majoris, H. asymmetricus, H. tartakovskyi, and H. minutus (hPHSIB1, hTUPHI01, hHAWF1, and hTURDUS2, respectively) were detected in C. segnis, C. pictipennis, and C. kibunensis biting midges. Haemoproteus majoris (hPHSIB1) sporozoites have already been detected in C. segnis [48] and our investigation can confirm this. The parasites of other genetic lineages of H. majoris can complete sporogony in C. segnis (hCCF5) [22] and C. impunctatus (hPARUS1) [49] females.
Haemoproteus tartakovskyi was known to be transmitted by C. impunctatus [36] and C. segnis (hHAWF1) [22], and in laboratory this parasite is known to form sporozoites in C. nubeculosus (hSISKIN1) [50]. We detected sporozoites of hHAWF1 in wild-caught C. segnis during this study.
Haemoproteus minutus (hTURDUS2) sporozoites were detected and confirmed by PCR in wild-caught C. impunctatus [51], C. kibunensis [28], C. segnis [48], and in laboratory-reared C. nubeculosus [52]. Similarly, our study confirmed that this parasite can complete sporogony in a new vector C. kibunensis. It can also be mentioned that the females of some Culicoides species (C. pictipennis, C. segnis, C. impunctatus) which were caught that were significantly more abundant in woodland compared with reeds are known as vectors of many Haemosporidian parasites, as detected by [22,48].
In summary, our findings suggest that the factors influencing the disruption of parasite transmission in certain bird species may be linked to vector biology. This includes variations in the timing of vector activity compared to bird hatching and fledging, differences in vector abundance and species diversity in various habitats, and a mismatch between the habitats where some bird species usually nest. We enhanced the understanding of the Culicoides species’ role as a vector for Haemoproteus parasites, contributing novel insights into the principal Culicoides species responsible for transmitting haemoproteids in Northern Europe.
5. Conclusions
The sporozoites of Haemoproteus asymmetricus (genetic lineage hTUPHI01) have been detected for the first time in the salivary glands of Culicoides festivipennis. The sporozoites of four Haemoproteus species were detected in Culicoides segnis, C. festivipennis, and Culicoides kibunensis biting midges. PCR-based screening showed that the females of seven Culicoides species were naturally infected with Haemoproteus parasites. The DNA of the parasite of owls, Haemoproteus syrnii (hSTAL2), was detected for the first time in Culicoides punctatus, which indicates that the biting midges of this species naturally take bloodmeal on owls.
The highest abundance of collected Culicoides females was in June, but the prevalence of Haemoproteus parasites in biting midges was the highest in July. The abundance of Culicoides was significantly higher in the woodland compared with reeds during the season. The acquired findings indicate the varied abundance and diversity of biting midges throughout the season and across distinct habitats. This variability could potentially impact the transmission of Haemoproteus parasites among birds with diverse breeding ecologies.
Author Contributions
Experimental conception and design: R.Ž., V.P. and R.B.; biting midge fieldwork: R.Ž., V.P. and R.B.; biting midge dissection, preparation of specimens, molecular analysis: R.Ž. and R.B.; microscopy of preparation of salivary glands R.Ž.; paper writing: R.Ž., R.B. and V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants (No. S-MIP-20-25, Rita Žiegyte; No. S-MIP-22-52, Vaidas Palinauskas) from the Research Council of Lithuania.
Data Availability Statement
The data presented in this study are available upon email inquiry.
Acknowledgments
The authors are grateful to the Curonian Spit National Park administration for opportunities to conduct scientific research in this area.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marzal, A.; Longoria, L.G.; Cárdenas Callirgos, J.M.; Sehgal, R.N.M. Invasive avian malaria as an emerging parasitic disease in native birds of Peru. Biol. Invasions 2015, 17, 39–45. [Google Scholar] [CrossRef]
- Waldenström, J.; Bensch, S.; Kiboi, S.; Hasselquist, D.; Ottosson, U. Cross-species infection of blood parasites between resident and migratory songbirds in Africa. Mol. Ecol. 2002, 11, 1545–1554. [Google Scholar] [CrossRef]
- Dinhopl, N.; Nedorost, N.; Mostegl, M.M.; Weissenbacher-Lang, C.; Weissenböck, H. In situ hybridization and sequence analysis reveal an association of Plasmodium spp. with mortalities in wild passerine birds in Austria. Parasitol. Res. 2015, 114, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Dinhopl, N.; Meike, M.; Mostegl, M.M.; Richter, B.; Nedorost, N.; Maderner, A.; Fragner, K.; Weissenböck, H. Application of in-situ hybridization for the detection and identification of avian malaria parasites in paraffin wax-embedded tissues from captive penguins. Avian Pathol. 2011, 40, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Ricklefs, R.E.; Medeiros, M.; Ellis, V.A.; Svensson-Coelho, M.; Blake, J.G.; Loiselle, B.A.; Soares, L.; Fecchio, A.; Outlaw, D.; Marra, P.P.; et al. Avian migration and the distribution of malaria parasites in New World passerine. J. Biogeogr. 2017, 44, 1113–1123. [Google Scholar] [CrossRef]
- Marzal, A.; Ricklefs, R.E.; Valkiūnas, G.; Albayrak, T.; Arriero, E.; Bonneaud, C.; Czirjak, G.A.; Ewen, J.; Hellgren, O.; Horakova, D.; et al. Diversity, loss, and gain of malaria parasites in a globally invasive bird. PLoS ONE 2011, 6, e21905. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0415300971. [Google Scholar]
- Atkinson, C.T.; Thomas, N.J.; Hunter, D.B. Parasitic Diseases of Wild Birds; Wiley Blackwell: Hoboken, NJ, USA, 2008; ISBN 978-0-813-82081-1. [Google Scholar]
- Palinauskas, V.; Žiegytė, R.; Iezhova, T.A.; Ilgūnas, M.; Bernotienė, A.; Valkiūnas, G. Description, molecular characterization, diagnostics and life cycle of Plasmodium elongatum (lineage pERIRUB01), the virulent avian malaria parasite. Int. J. Parasitol. 2016, 46, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Palinauskas, V.; Žiegytė, R.; Ilgūnas, M.; Iezhova, T.A.; Bernotienė, R.; Bolshakov, C.; Valkiūnas, G. Description of the first cryptic avian malaria parasite, Plasmodium homocircumflexum n. sp., with experimental data on its virulence and development in avian hosts and mosquitoes. Int. J. Parasitol. 2015, 45, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Žiegytė, R.; Palinauskas, V.; Bernotienė, R.; Bukauskaitė, D.; Ilgūnas, M.; Dimitrov, D.; Iezhova, T.A. Complete sporogony of Plasmodium relictum (lineage pGRW4) in mosquitoes Culex pipiens pipiens, with implications on avian malaria epidemiology. Parasitol. Res. 2015, 144, 3075–3085. [Google Scholar] [CrossRef]
- Howe, L.; Castro, I.C.; Schoener, E.R.; Hunter, S.; Barraclough, R.K.; Alley, M.R. Malaria parasites (Plasmodium spp.) infecting introduced, native and endemic New Zealand birds. Parasitol. Res. 2012, 110, 913–923. [Google Scholar] [CrossRef]
- Hammers, M.; Komdeur, J.; Kingma, S.A.; Hutchings, K.; Fairfield, E.A.; Gilroy, D.L.; Richardson, D.S. Age-specific haemosporidian infection dynamics and survival in Seychelles warblers. Sci. Rep. 2016, 6, 29720. [Google Scholar] [CrossRef]
- Rojo, M.A.; Hernandez, M.A.; Campos, F.; Santamaria, T.; Dias, S.; Casanueva, P. The Iberian Peninsula is an area of infection by Haemoproteus payevskyi and Haemoproteus nucleocondensus for the white-throated Dipper Cinclus cinclus. Ardeola 2015, 62, 373–382. [Google Scholar] [CrossRef]
- Harvell, C.D.; Mitchell, C.E.; Ward, J.R.; Altizer, S.; Dobson, A.P.; Ostfeld, R.S.; Samuel, M.D. Climate Warming and Disease Risks for Terrestrial and Marine Biota. Science 2002, 296, 2158. [Google Scholar] [CrossRef]
- Montoya, J.M.; Raffaelli, D. Climate change, biotic interactions and ecosystem services. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2013–2018. [Google Scholar] [CrossRef]
- Garamszegi, Z. Climate change increases the risk of malaria in birds. Glob. Chang. Biol. 2011, 17, 1751–1759. [Google Scholar] [CrossRef]
- Hasselquist, D.; Nilsson, J.A. Maternal transfer of antibodies in vertebrates: Trans-generational effects on offspring immunity. Philos. Trans. R. Soc. B 2009, 364, 51–60. [Google Scholar] [CrossRef]
- Chernetsov, N. Habitat distribution during the post-breeding and post-fledging period in the reed warbler Acrocephalus scirpaceus and sedge warbler A. schoenobaenus depends on food abundance. Ornis Svec. 1998, 8, 77–82. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Marzal, A. (Eds.) Avian Malaria and Related Parasites in the Tropics; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Borkent, A.; Dominiak, P. Catalog of the Biting Midges of the World (Diptera: Ceratopogonidae). Zootaxa 2020, 4787, 1–377. [Google Scholar] [CrossRef]
- Žiegytė, R.; Bernotienė, R.; Palinauskas, V. Culicoides segnis and Culicoides pictipennis biting midges (Diptera, Ceratopogonidae), new reported vectors of Haemoproteus parasites. Microorganisms 2022, 10, 898. [Google Scholar] [CrossRef]
- Bernotienė, R.; Žiegytė, R.; Vaitkutė, G.; Valkiūnas, G. Identification of a new vector species of avian haemoproteids, with a description of methodology for the determination of natural vectors of haemosporidian parasites. Parasites Vectors 2019, 12, 307. [Google Scholar] [CrossRef]
- Dyce, A.L. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Aust. J. Entomol. 1969, 8, 11–15. [Google Scholar] [CrossRef]
- Gutsevich, A.V. The bloodsucking midges (Ceratopogonidae), in the fauna of the USSR. Dipteran insects. Nauka Press 1973, 5, 3. (In Russian) [Google Scholar]
- Glukhova, V.M.; Valkiūnas, G. On the fauna and ecology of biting midges (Ceratopogonidae: Culicoides) in the Kuršiu Nerija, the methods of their collection from the birds and experimental infection with Haemoproteids (Haemosporidia: Haemoproteidae). Ekologija 1993, 2, 68–73. [Google Scholar]
- Mathieu, B.; Cêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the Western Palaearctic region. Parasites Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Žiegytė, R.; Platonova, E.; Kinderis, E.; Mukhin, A.; Palinauskas, V.; Bernotienė, R. Culicoides biting midges involved in transmission of haemoproteids. Parasites Vectors 2021, 14, 27. [Google Scholar] [CrossRef]
- Richardson, D.S.; Jury, F.L.; Blaakmeer, K.; Komdeur, J.; Burke, T. Parentage assignment and extra group paternity in a cooperative breeder: The Seychelles warbler (Acrocephalus sechellensis). Mol. Ecol. 2001, 10, 2263–2273. [Google Scholar] [CrossRef]
- Bensch, S.; Stjenman, M.; Hasselquist, D.; Ostman, O.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. R. Soc. 2000, 276, 1583–1589. [Google Scholar] [CrossRef]
- Hellgren, O.; Waldenstrom, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Hall, T.A. A user-friendly biological sequence alignment editor and analysis program for Windows 98/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Bernotienė, R.; Bartkevičienė, G.; Bukauskaitė, D. The flying activity of biting midges (Ceratopogonidae: Culicoides) in Verkiai Regional Park, southeastern Lithuania. Parasites Res. 2021, 120, 2323–2332. [Google Scholar] [CrossRef]
- Liutkevičius, G. The new data on the epidemiology of bird haemoproteids (Haemosporida: Haemoproteidae) on the Curonian Spit. Acta Zool. Lithuan 2000, 2, 72–77. [Google Scholar] [CrossRef]
- Valkiūnas, G.; Liutkevičius, G.; Iezhova, T.A. Complete development of three species of Haemoproteus (Haemosporida, Haemoproteidae) in the biting midge Culicoides impunctatus (Diptera, Ceratopogonidae). J. Parasitol. 2002, 88, 864–868. [Google Scholar] [CrossRef]
- Trukhan, M.N.; Tereshkina, N.V.; Liutkevičius, G. Peculiarities of the range of species and the ecology of midges (Diptera, Ceratopogonidae) on the Curonian spit. Vesci Nacyanalnaj Akad. Navuk Belarusi 2003, 2, 88–91. [Google Scholar]
- Žiegytė, R.; Platonova, E.; Bernotienė, R.; Valkiūnas, G.; Palinauskas, V. Complete sporogony of the blood parasite Haemoproteus nucleocndensus in common biting midges: Why is its transmission interrupted in Europe? Parasitology 2020, 147, 593–600. [Google Scholar] [CrossRef]
- Hasselquist, D.; Ostman, O.; Waldenstrom, J.; Bensch, S. Temporal patterns of occurrence and transmission of the blood parasite Haemoproteus payevskyi in the great reed warbler Acrocephalus arundinaceus. J. Ornithol. 2007, 148, 401–409. [Google Scholar] [CrossRef]
- Fuller, T.; Bensch, S.; Müller, I.; Novembre, J.; Pérez-Tris, J.; Ricklefs, R.E.; Smith, T.B.; Waldenstrom, J. The ecology of emerging infectious diseases in migratory birds: An assessment of the role of climate change and priorities for future research. EcoHealth 2012, 9, 80–88. [Google Scholar] [CrossRef]
- Kameke, D.; Kampen, H.; Wacker, A.; Werner, D. Field studies on breeding sites of Culicoides Laatreille (Diptera: Ceratopogonidae) in agriculturally used and natural habitats. Sci. Rep. 2021, 11, 10007. [Google Scholar] [CrossRef]
- González, M.A.; Goiri, F.; Prosser, S.W.J.; Cevidanes, A.; Hernándz-Triana, L.M.; Barandika, J.F.; Hebert, P.D.N.; Garcia-Perez, A.L. Culicoides species community composition and feeding preferences in two aquatic ecosystems in northern Spain. Parasites Vectors 2022, 15, 199. [Google Scholar] [CrossRef]
- Kettle, D.S. The spatial distribution of Culicoides impunctatus Goet under woodland and moorland conditions and its flight range through woodland. Bull. Entomol. Res. 1951, 42, 239–291. [Google Scholar] [CrossRef]
- Lillie, T.H.; Marquardt, W.C.; Jones, R.H. The flight range of Culicoides variipennis (Diptera: Ceratopogonidae). Can. Entomol. 2012, 113, 419–426. [Google Scholar] [CrossRef]
- Santiago-Alarcon, D.; Havelka, P.; Pineda, E.; Segelbacher, G.; Schaefer, H.M. Urban forests as hubs for novel zoonosis: Blood meal analysis, seasonal variation in Culicoides (Diptera: Ceratopogonidae) vectors, and avian haemosporidians. Parasitology 2013, 140, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Duc, M.; Iezhova, T.A. Description of Haemoproteus asymmetricus n. sp. (Haemoproteidae), with remarks on predictability of the DNA haplotype networks in haemosporidian parasite taxonomy research. Acta Trop. 2021, 218, 105905. [Google Scholar] [CrossRef] [PubMed]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Bakonyi, T.; Weissenbock, H. Geographic and host distribution of haemosporidian parasite lineages from birds of the family Turdidae. Malar. J. 2020, 19, 335. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Hernandez-Lara, C.; Duc, M.; Valavičiūtė-Pocienė, K.; Bernotienė, R. What can haemosporidian lineages found in Culicoides biting midges tell us about their feeding preferences? Diversity 2022, 14, 957. [Google Scholar] [CrossRef]
- Žiegytė, R.; Markovets, M.Y.; Bernotienė, R.; Mukhin, A.; Iezhova, T.A.; Valkiūnas, G.; Palinauskas, V. The widespread biting midge Culicoides impunctatus (Ceratopogonidae) is susceptible to infection with numerous Haemoproteus (Haemoproteidae) species. Parasites Vectors 2017, 10, 397. [Google Scholar] [CrossRef]
- Žiegytė, R.; Bernotienė, R.; Palinauskas, V.; Valkiūnas, G. Haemoproteus tartakovskyi (Haemoproteidae): Complete sporogony in Culicoides nubeculosus (Ceratopogonidae), with implications for avian haemoproteid experimental research. Exp. Parasitol. 2016, 160, 17–22. [Google Scholar] [CrossRef]
- Žiegytė, R.; Palinauskas, V.; Bernotienė, R.; Iezhova, T.A.; Valkiūnas, G. Haemoproteus minutus and Haemoproteus belopolskyi (Haemoproteidae): Complete sporogony in the biting midge Culicoides impunctatus (Ceratopogonidae), with implications on epidemiology of haemoproteosis. Exp. Parasitol. 2014, 145, 74–79. [Google Scholar] [CrossRef]
- Bukauskaitė, D.; Iezhova, T.A.; Ilgūnas, M.; Valkiūnas, G. High susceptibility of the laboratory-reared biting midges Culicoides nubeculosus to Haemoproteus infections, with review on Culicoides species that transmit avian haemoproteids. Parasitology 2019, 146, 333–341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).