Phenotypic Diversity of a Leafroller Archips podana (Lepidoptera, Tortricidae) Does Not Change along an Industrial Pollution Gradient
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Study Area
2.3. Sampling
2.4. Statistical Analysis
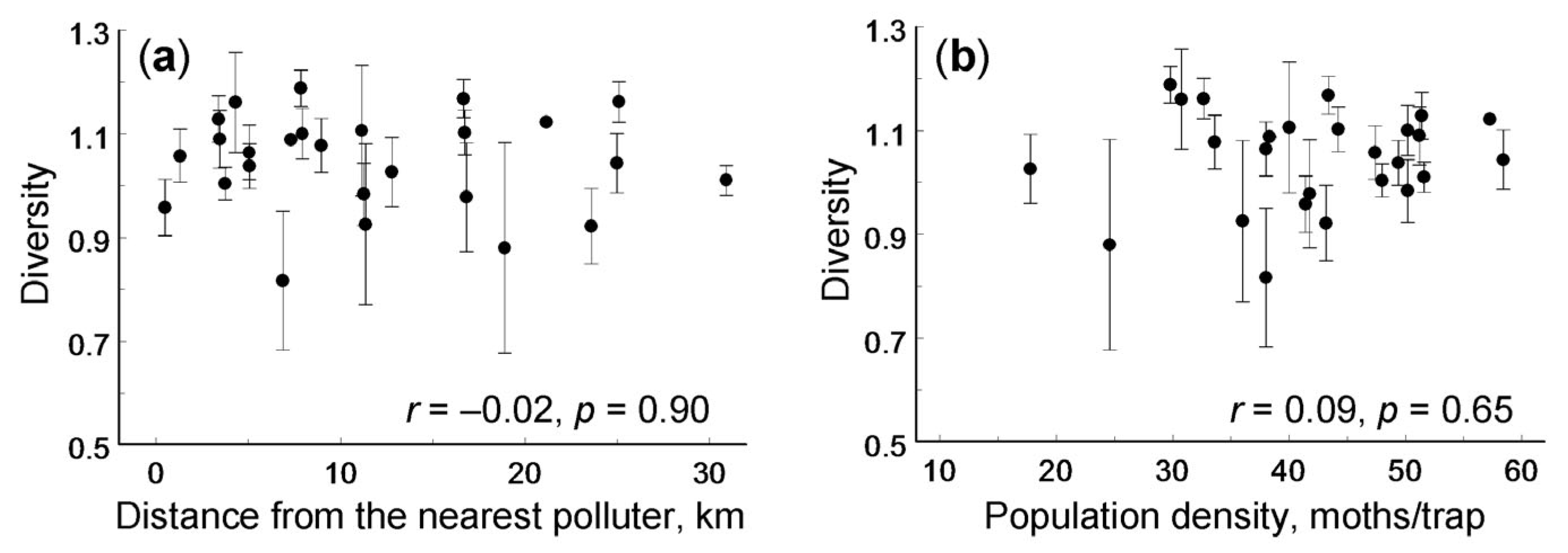
3. Results
4. Discussion
4.1. How Robust Are the Results?
4.2. Potential Drivers of the Phenotypic Structure of Archips podana Populations
4.3. Should Old Data Be Published?
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLean, C.A.; Stuart-Fox, D. Geographic variation in animal colour polymorphisms and its role in speciation. Biol. Rev. 2014, 89, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Forsman, A.; Wennersten, L. Inter-individual variation promotes ecological success of populations and species: Evidence from experimental and comparative studies. Ecography 2016, 39, 630–648. [Google Scholar] [CrossRef]
- Strickland, L.R.; Arias, C.F.; Rodriguez, V.; Johnston, J.S.; McMillan, W.O.; Windsor, D. Inheritance, distribution and genetic differentiation of a color polymorphism in Panamanian populations of the tortoise beetle, Chelymorpha alternans (Coleoptera: Chrysomelidae). Heredity 2019, 122, 558–569. [Google Scholar] [CrossRef]
- Hendry, A.P.; Gotanda, K.M.; Svensson, E.I. Human influences on evolution, and the ecological and societal consequences. Phil. Trans. R. Soc. B 2017, 372, 20160028. [Google Scholar] [CrossRef] [PubMed]
- Catullo, R.A.; Llewelyn, J.; Phillips, B.L.; Moritz, C.C. The potential for rapid evolution under anthropogenic climate change. Curr. Biol. 2019, 29, R996–R1007. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V.; Zvereva, E.L. A second life for old data: Global patterns in pollution ecology revealed from published observational studies. Environ. Pollut. 2011, 159, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Odum, E.P. Trends expected in stressed ecosystems. BioScience 1985, 35, 419–422. [Google Scholar] [CrossRef]
- Rapport, D.J.; Regier, H.A.; Hutchinson, T.C. Ecosystem behavior under stress. Am. Nat. 1985, 125, 617–640. [Google Scholar] [CrossRef]
- Wood, C.W., Jr.; Nash, T.N., III. Copper smelter effluent effects on Sonoran desert vegetation. Ecology 1976, 57, 1311–1316. [Google Scholar] [CrossRef]
- Kozlov, M.V.; Zvereva, E.L.; Zverev, V.E. Impacts of Point Polluters on Terrestrial Biota: Comparative Analysis of 18 Contaminated Areas; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Kozlov, M.V.; Castagneyrol, B.; Zverev, V.; Zvereva, E.L. Recovery of moth and butterfly (Lepidoptera) communities in a polluted region following emission decline. Sci. Total Environ. 2022, 838, 155800. [Google Scholar] [CrossRef]
- Winchell, K.M.; Losos, J.B.; Verrelli, B.C. Urban evolutionary ecology brings exaptation back info focus. Trends Ecol. Evol. 2023, 38, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.M. Records of industrial melanism in British moths. Biol. J. Linn. Soc. 2018, 125, 862–866. [Google Scholar] [CrossRef]
- Kettlewell, H.B.D. The Evolution of Melanism. The Study of a Recurring Necessity; Oxford University Press: Oxford, UK, 1973. [Google Scholar]
- Majerus, M.E.N. Melanism: Evolution in Action; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Saccheri, I.J.; Rousset, F.; Watts, P.C.; Brakefield, P.M.; Cook, L.M. Selection and gene flow on a diminishing cline of melanic peppered moths. Proc. Natl. Acad. Sci. USA 2008, 105, 16212–16217. [Google Scholar] [CrossRef]
- Muggleton, J.; Lonsdale, D.; Benham, B.R. Melanism in Adalia bipunctata L. (Col., Coccinellidae) and its relationship to atmospheric pollution. J. Appl. Ecol. 1975, 12, 451–464. [Google Scholar] [CrossRef]
- Heliövaara, K.; Väisänen, R. Insects and Pollution; CRC: Boca Raton, FL, USA, 1993. [Google Scholar]
- Belskaya, E.; Gilev, A.; Belskii, E. Ant (Hymenoptera, Formicidae) diversity along a pollution gradient near the Middle Ural copper smelter, Russia. Environ. Sci. Pollut. Res. 2017, 24, 10768–10777. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.V. Pollution impact on insect biodiversity in boreal forests. In Disturbance and Recovery in Arctic Lands: An Ecological Perspective, Proceedings of the NATO Advanced Research Workshop on Disturbance and Recovery of Arctic Terrestrial Ecosystems, Rovaniemi, Finland, 24–30 September 1995; Crawford, R.M.M., Ed.; NATO ASI Series, Partnership Subseries 2, Environment; Kluwer: Dordrecht, The Netherlands, 1997; Volume 25, pp. 213–250. [Google Scholar]
- Zvereva, E.L.; Hunter, M.D.; Zverev, V.; Kruglova, O.Y.; Kozlov, M.V. Climate warming leads to decline in frequencies of melanic individuals in subarctic leaf beetle populations. Sci. Total Environ. 2019, 673, 237–244. [Google Scholar] [CrossRef]
- Zverev, V.; Kozlov, M.V. Decline of Eulia ministrana (Lepidoptera: Tortricidae) in polluted habitats is not accompanied by phenotypic stress responses. Insect Sci. 2021, 28, 1482–1490. [Google Scholar] [CrossRef]
- Zvereva, E.L.; Serebrov, V.; Glupov, V.; Dubovsky, I. Activity and heavy metal resistance of non-specific esterases in leaf beetle Chrysomela lapponica from polluted and unpolluted habitats. Comparat. Biochem. Physiol. C 2003, 135, 383–391. [Google Scholar] [CrossRef]
- Merritt, T.J.S.; Bewick, A. Genetic diversity in insect metal tolerance. Front. Genet. 2017, 8, 172. [Google Scholar] [CrossRef]
- McCulloch, G.A.; Waters, J.M. Rapid adaptation in a fast-changing world: Emerging insights from insect genomics. Glob. Change Biol. 2023, 29, 943–954. [Google Scholar] [CrossRef]
- Safonkin, A.F. The influence of food change on the development of Archips podana Sc. (Lepidoptera: Tortricidae), a polyphagous insect. Russ. J. Ecol. 2000, 31, 203–206. [Google Scholar] [CrossRef]
- Safonkin, A.F.; Triseleva, T.A. Phenotypic composition of a laboratory population of large fruit-tree tortrix Archips podana (Lepidoptera, Tortricidae) on different plants. In Biodiversity and the Role of Zoocenosis in Natural and Anthropogenic Ecosystems: Proceedings of the II International Scientific Conference; Dnepropetrovsk National University: Dnepropetrovsk, Ukraine, 2003; pp. 162–163. [Google Scholar]
- Jiang, N.J.; Chang, H.; Weißflog, J.; Eberl, F.; Veit, D.; Weniger, K.; Hansson, B.S.; Knaden, M. Ozone exposure disrupts insect sexual communication. Nat. Commun. 2023, 14, 1186. [Google Scholar] [CrossRef] [PubMed]
- Henneken, J.; Jones, T.M. Pheromones-based sexual selection in a rapidly changing world. Curr. Opin. Insect Sci. 2017, 24, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Riemer, J.; Whittaker, J.B. Air pollution and insect herbivores: Observed interactions and possible mechanisms. In Insect-Plant Interactions; Bernays, E.A., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 73–105. [Google Scholar]
- Meléndez-Jaramillo, E.; Cantú-Ayala, C.M.; Treviño-Garza, E.J.; Sánchez-Reyes, U.J.; Herrera-Fernández, B. Composition and diversity of butterflies (Lepidoptera, Papilionoidea) along an atmospheric pollution gradient in the Monterrey Metropolitan Area, Mexico. ZooKeys 2021, 1037, 73–103. [Google Scholar] [CrossRef] [PubMed]
- Litvinova, A.N. On feeding specialisation of leafrollers (Lepidoptera, Tortricidae)– fruit-tree tortrix (Archips podana Scop.) and others. In Problems of Entomology; Lopatin, I.K., Ed.; Belarus State University: Minsk, Russia, 1974; pp. 97–112. (In Russian) [Google Scholar]
- Bradley, J.D.; Tremewan, W.G.; Smith, A. British Tortricoid Moths. Cochylidae and Tortricidae: Tortricinae; The Ray Society: London, UK, 1973. [Google Scholar]
- Kozlov, M.V.; Haukioja, E. Density and size of Archips podana (Lepidoptera, Tortricidae) males in an air pollution gradient as revealed by pheromone traps. Environ. Entomol. 1993, 22, 438–444. [Google Scholar] [CrossRef]
- Sterling, P.; Parsons, M.; Lewington, R. Field Guide to the Micromoths of Great Britain and Ireland; British Wildlife Publishing: Gillingham, UK, 2012. [Google Scholar]
- Kuznetzov, V.I. Family Tortricidae (Olethreutidae, Cochylidae)–leafrollers. In Keys to the Insects of the European Part of the USSR; Lepidoptera, pt 1; Medvedev, G.S., Ed.; Nauka: Leningrad, Russia, 1978; Volume 4, pp. 193–680. (In Russian) [Google Scholar]
- Ivanova, T.V.; Mõttus, E. Heterogenity of geographical population of Archips podana. In Chemical Communication of Animals. Theory and Practice; Sokolov, V.E., Ed.; Nauka: Moscow, Russia, 1986; pp. 79–82. (In Russian) [Google Scholar]
- Kozlov, M.V.; Motorkin, M.E. Geographical variability of the leafroller, Archips podana (Lepidoptera, Tortricidae). Entomol. Rev. 1990, 69, 57–65. [Google Scholar]
- Safonkin, A.F. Polymorphism and ecological plasticity of leaf roller Archips podana Sc. (Lepidoptera, Tortricidae). J. Fundam. Biol. 1990, 51, 393–400. (In Russian) [Google Scholar]
- Liblikas, I.; Mõttus, E.; Nikolaeva, Z.V.; Ojarand, A.; Borg-Karlson, A.K.; Ovsyannikova, E.I.; Grichanov, I.Y.; Ivanova, T.V.; Yemelyanov, V.A. Variability of genitalia and pheromone communication channels of Archips podana (Scopoli) (Lepidoptera: Tortricidae). Proc. Estonian Acad. Sci. Biol. Ecol. 2004, 53, 75–87. [Google Scholar]
- Safonkin, A.F.; Kulikov, A.M. Genetic basis of heterogeneity for morphological and behavioral characters in a population of Archips podana Scop. (Lepidoptera: Tortricidae). Russ. J. Genet. 2001, 37, 240–246. [Google Scholar] [CrossRef]
- Berlyand, M.E. (Ed.) Annual Report of Ambient Air Pollution in Cities and Industrial Centres of the Russian Federation. Emissions of Pollutants: 1991; Voeikov Main Geophysical Observatory: St. Petersburg, Russia, 1992. (In Russian) [Google Scholar]
- Cherkasov, M.S. The Industrial Lipetsk; Lipetsk Publishing House: Lipetsk, Russia, 1959. (In Russian) [Google Scholar]
- Zaitsev, G.A.; Dubrovina, O.A.; Kulagin, A.Y.; Shainurov, R.I. Cadmium and zinc migration in Scots pine stands growing in contaminated areas from metallurgical plant emissions. Int. J. Environ. Sci. Technol. 2021, 18, 3625–3634. [Google Scholar] [CrossRef]
- Sedykh, V.A.; Kurolap, S.A. Assessment of aerotechnogenic pollution of the city of Lipetsk with using geoinformation mapping of the territory. In Geoinformation Mapping in the Regions of Russia: Materials of the XI All-Russian Scientific and Practical Conference (Voronezh, Russia, 23–24 November 2020); Nesterov, Y.A., Ed.; Digital Polygraphy: Voronezh, Russia, 2020; pp. 309–316. (In Russian) [Google Scholar]
- Zaytsev, G.A.; Kulagin, A.Y.; Urazgildin, R.V.; Dubrovina, O.A.; Logvinov, K.V.; Afanasov, N.A.; Chaban, A.N.; Shaynurov, R.I.; Tagirova, O.V.; Amineva, K.Z. Relative vital condition of woody plantings under industrial pollution. Bull. Ufa Sci. Centre Russ. Acad. Sci. 2017, 2, 63–68. [Google Scholar]
- Kurbakov, D.N.; Kuznetsov, V.K.; Andreeva, N.V.; Sidorova, E.V. Influence of metallurgical production on snow cover contamination. In Nuclear and Physical Investigations and Technologies in Agriculture. (To the 50th Anniversary of Russian Institute of Radiology and Agroecology), Proceedings of the International Research and Practice Conference Obninsk, Obninsk, Russia, 16–18 September 2020; Shubina, O.A., Ed.; Russian Institute of Radiology and Agroecology: Obninsk, Russia, 2020; pp. 270–273, (In Russian, English Summary). [Google Scholar]
- Salemaa, M.; Uotila, T. Seed bank composition and seedling survival in forest soil polluted with heavy metals. Basic Appl. Ecol. 2001, 2, 251–263. [Google Scholar] [CrossRef]
- Kapusta, P.; Sobczyk, L. Effects of heavy metal pollution from mining and smelting on enchytraeid communities under different land management and soil conditions. Sci. Total Environ. 2015, 536, 517–526. [Google Scholar] [CrossRef]
- Zar, J.H. Statistical Analysis, 2nd ed.; Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA, 1984. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Jennions, M.D.; Møller, A.P. A survey of the statistical power of research in behavioral ecology and animal behavior. Behav. Ecol. 2003, 14, 438–445. [Google Scholar] [CrossRef]
- Smith, D.R.; Hardy, I.C.; Gammell, M.P. Power rangers: No improvement in the statistical power of analyses published in animal behaviour. Anim. Behav. 2011, 81, 347–352. [Google Scholar] [CrossRef]
- Yang, Y.; Sánchez-Tójar, A.; O’Dea, R.E.; Noble, D.W.A.; Koricheva, J.; Jennions, M.D.; Parker, T.H.; Lagisz, M.; Nakagawa, S. Publication bias impacts on effect size, statistical power, and magnitude (Type M) and sign (Type S) errors in ecology and evolutionary biology. BMC Biol. 2023, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Oke, T.R. City size and urban heat island. Atmos. Environ. 1973, 7, 769–779. [Google Scholar] [CrossRef]
- Belskii, E.; Belskaya, E. Thermal effect of the Middle Ural copper smelter (Russia) and growth of birch leaves. Environ. Sci. Pollut. Res. 2021, 28, 26064–26072. [Google Scholar] [CrossRef]
- de Jong, P.W.; Brakefield, P.M. Climate and change in clines for melanism in the two-spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Proc. R. Soc. B—Biol. Sci. 1998, 265, 39–43. [Google Scholar] [CrossRef]
- Umina, P.A.; Weeks, A.R.; Kearney, M.R.; McKechnie, S.W.; Hoffmann, A.A. A rapid shift in a classic clinal pattern in Drosophila reflecting climate change. Science 2005, 308, 691–693. [Google Scholar] [CrossRef]
- Anufrieva, A.F.; Zagainova, M.S.; Ivleva, T.P.; Lyubushkina, T.N.; Smirnova, I.V. Annual Report: The State of Ambient Air Pollution in Cities of Russia in 2021; Voeikov Main Geophysical Observatory: St. Petersburg, Russia, 2022. (In Russian) [Google Scholar]
- Jennions, M.D.; Møller, A.P. Relationships fade with time: A meta-analysis of temporal trends in publication in ecology and evolution. Proc. R. Soc. B—Biol. Sci. 2002, 269, 43–48. [Google Scholar] [CrossRef]
- Koricheva, J.; Kulinskaya, E. Temporal instability of evidence base: A threat to policy making? Trends Ecol. Evol. 2019, 34, 895–902. [Google Scholar] [CrossRef]
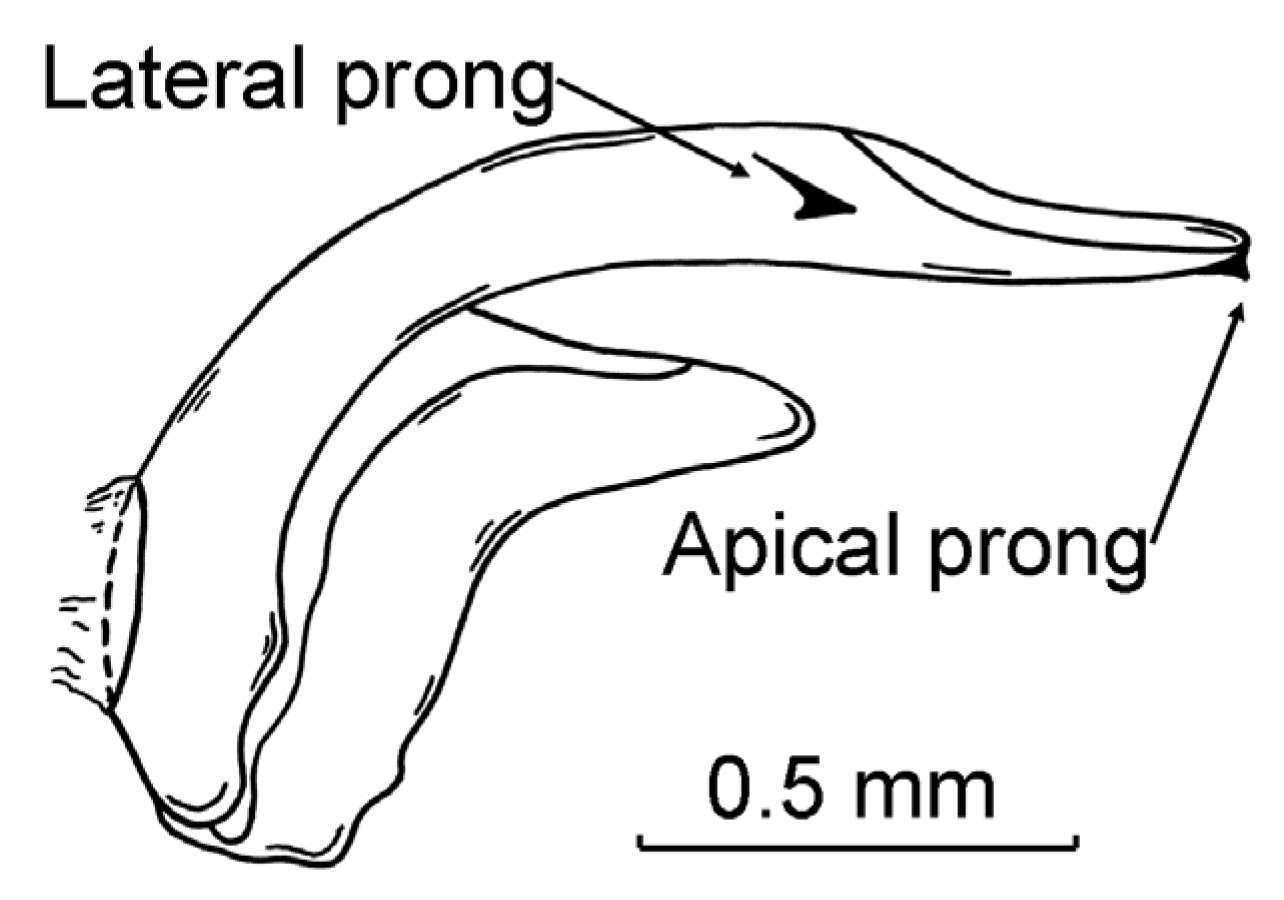
| Morph Characteristics | Distance to Polluter | Population Abundance | ||
|---|---|---|---|---|
| r | p | r | p | |
| No prongs | 0.28 | 0.17 | −0.18 | 0.38 |
| Apical prong(s) only | 0.08 | 0.71 | −0.31 | 0.12 |
| Lateral prong(s) only | −0.03 | 0.88 | 0.26 | 0.20 |
| Both prongs | −0.13 | 0.52 | 0.07 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozlov, M.V. Phenotypic Diversity of a Leafroller Archips podana (Lepidoptera, Tortricidae) Does Not Change along an Industrial Pollution Gradient. Insects 2023, 14, 927. https://doi.org/10.3390/insects14120927
Kozlov MV. Phenotypic Diversity of a Leafroller Archips podana (Lepidoptera, Tortricidae) Does Not Change along an Industrial Pollution Gradient. Insects. 2023; 14(12):927. https://doi.org/10.3390/insects14120927
Chicago/Turabian StyleKozlov, Mikhail V. 2023. "Phenotypic Diversity of a Leafroller Archips podana (Lepidoptera, Tortricidae) Does Not Change along an Industrial Pollution Gradient" Insects 14, no. 12: 927. https://doi.org/10.3390/insects14120927
APA StyleKozlov, M. V. (2023). Phenotypic Diversity of a Leafroller Archips podana (Lepidoptera, Tortricidae) Does Not Change along an Industrial Pollution Gradient. Insects, 14(12), 927. https://doi.org/10.3390/insects14120927






