Climate Change Influences the Population Density and Suitable Area of Hippotiscus dorsalis (Hemiptera: Pentatomidae) in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
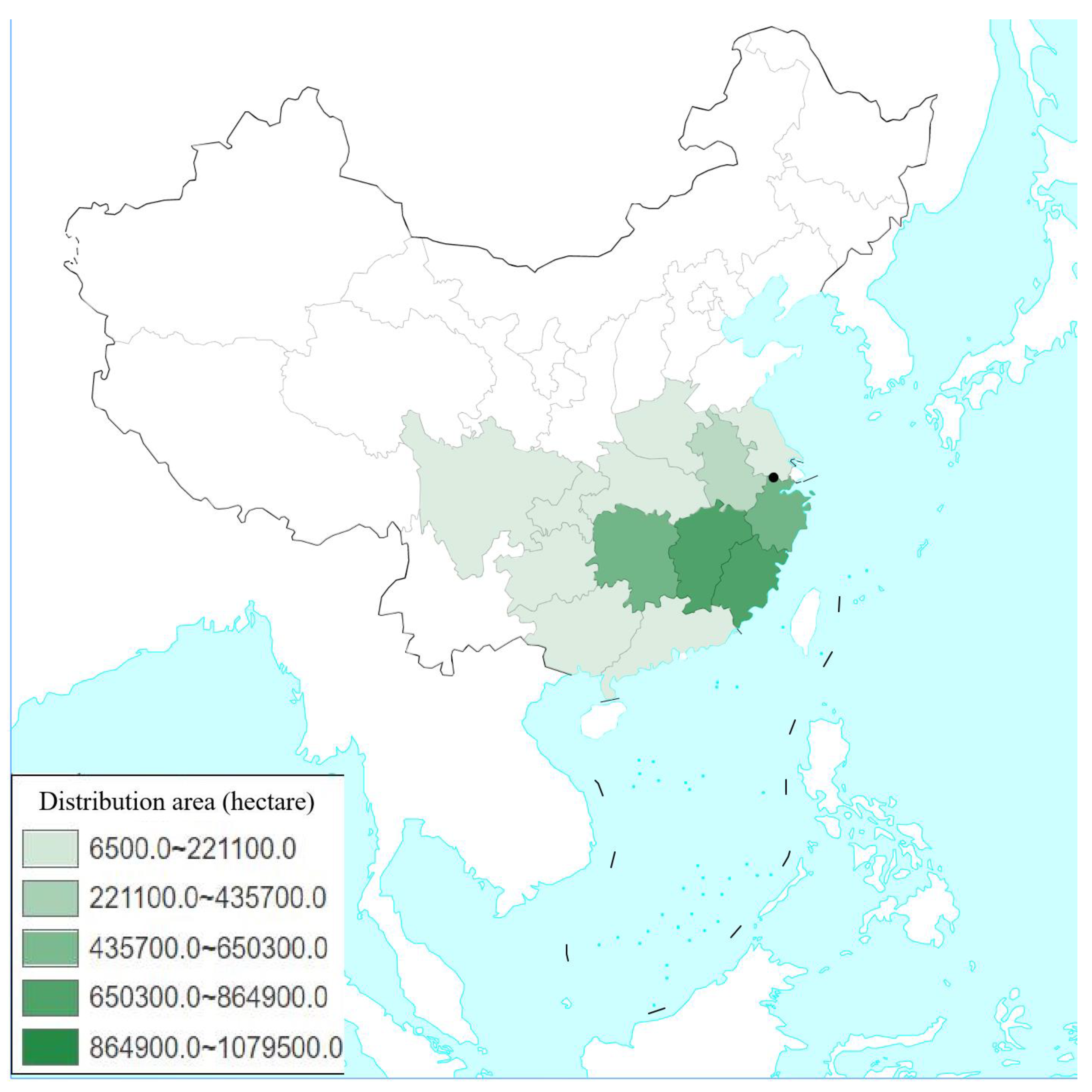
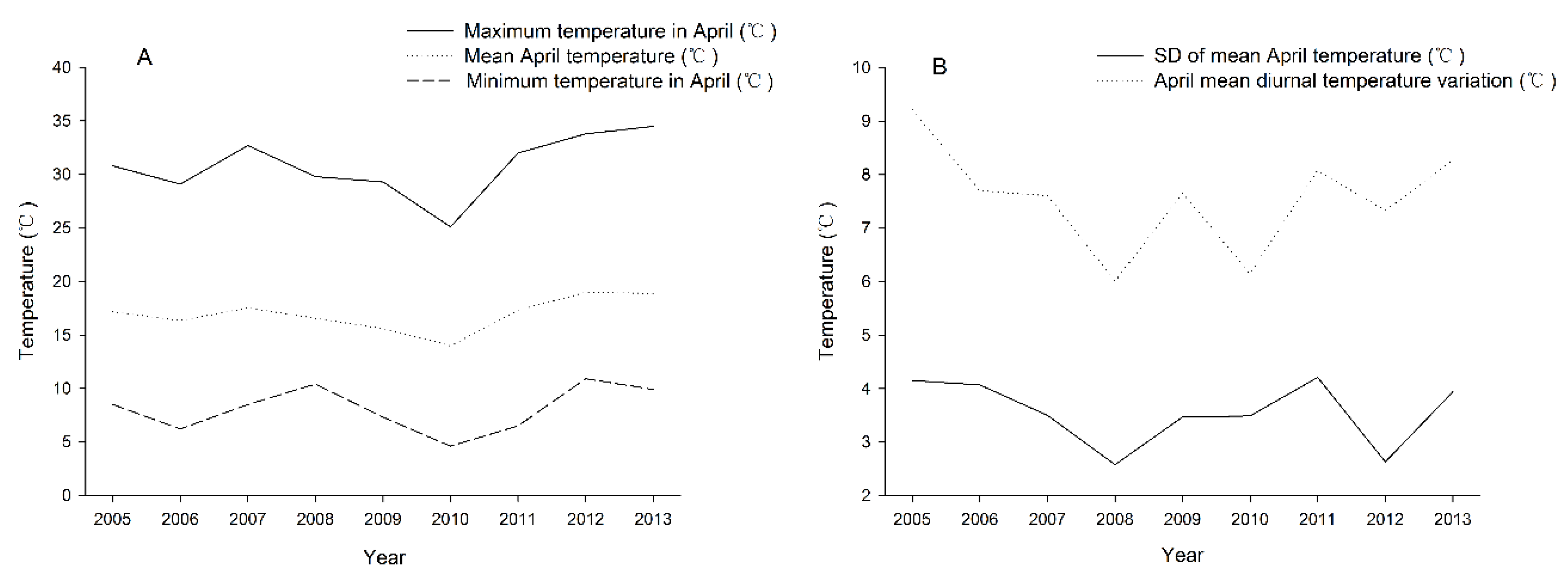
2.1. Damage Investigation of Hippotiscus dorsalis and Climate Data Collection from 2005 to 2013
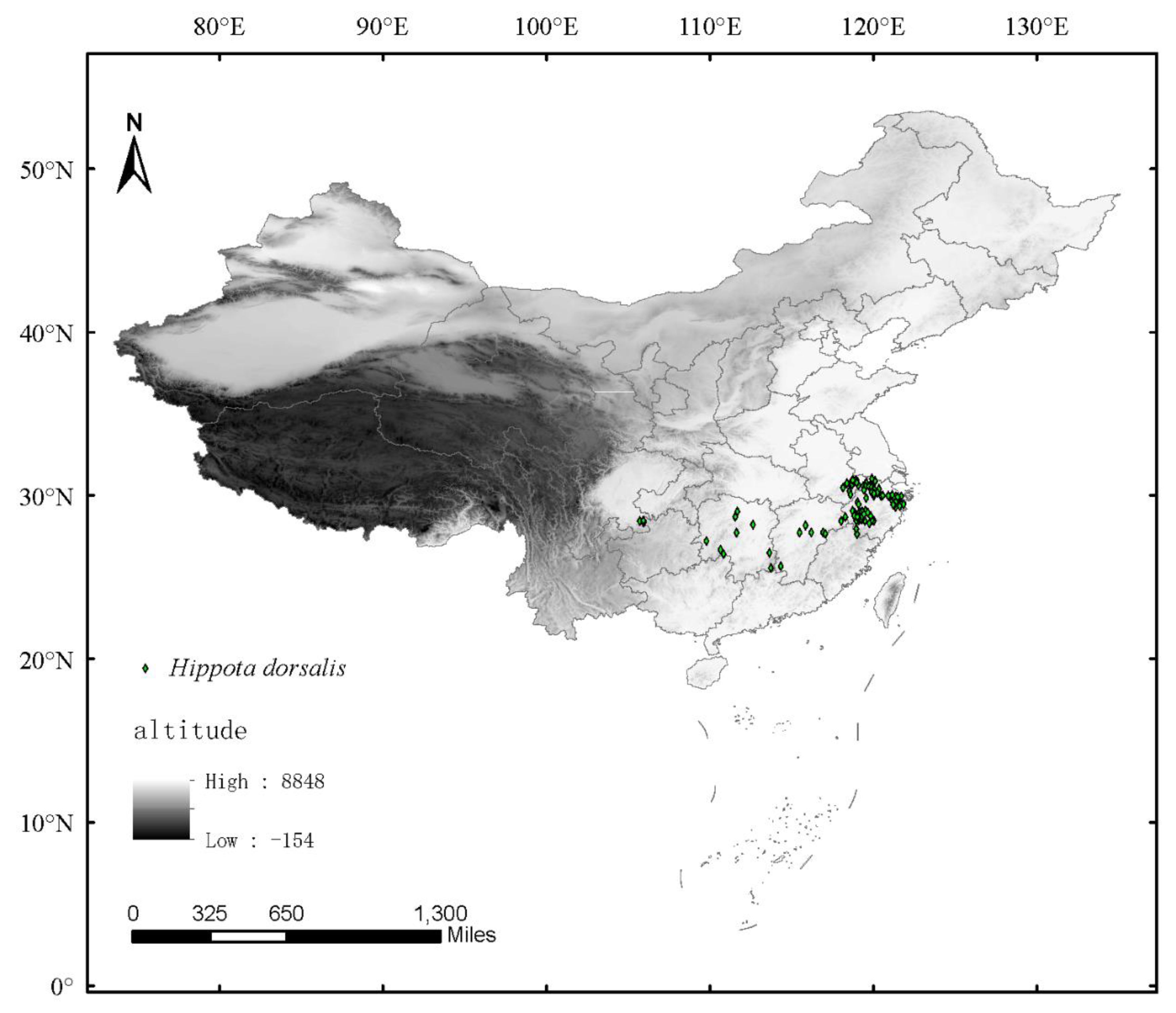
2.2. The Collection of Hippotiscus dorsalis Distribution Data
2.3. Environmental Variables
2.4. Species Distribution Modeling
3. Results
3.1. Correlation Analysis between the Damage Index of H. dorsalis and the Climate Index of the Study Site
3.2. Occurrence Records of H. dorsalis
3.3. Performance and Variables Selection of the MaxEnt Model
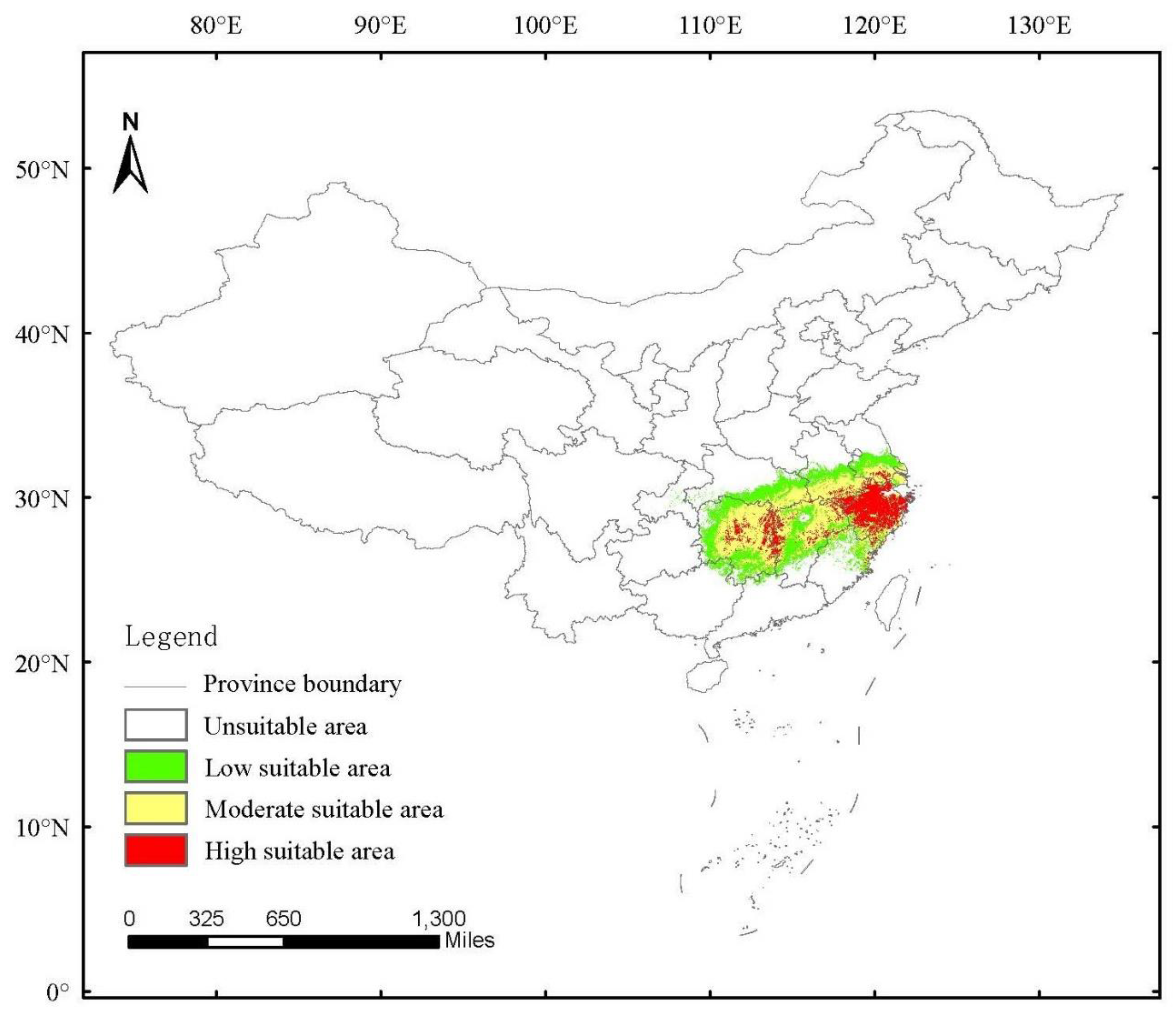
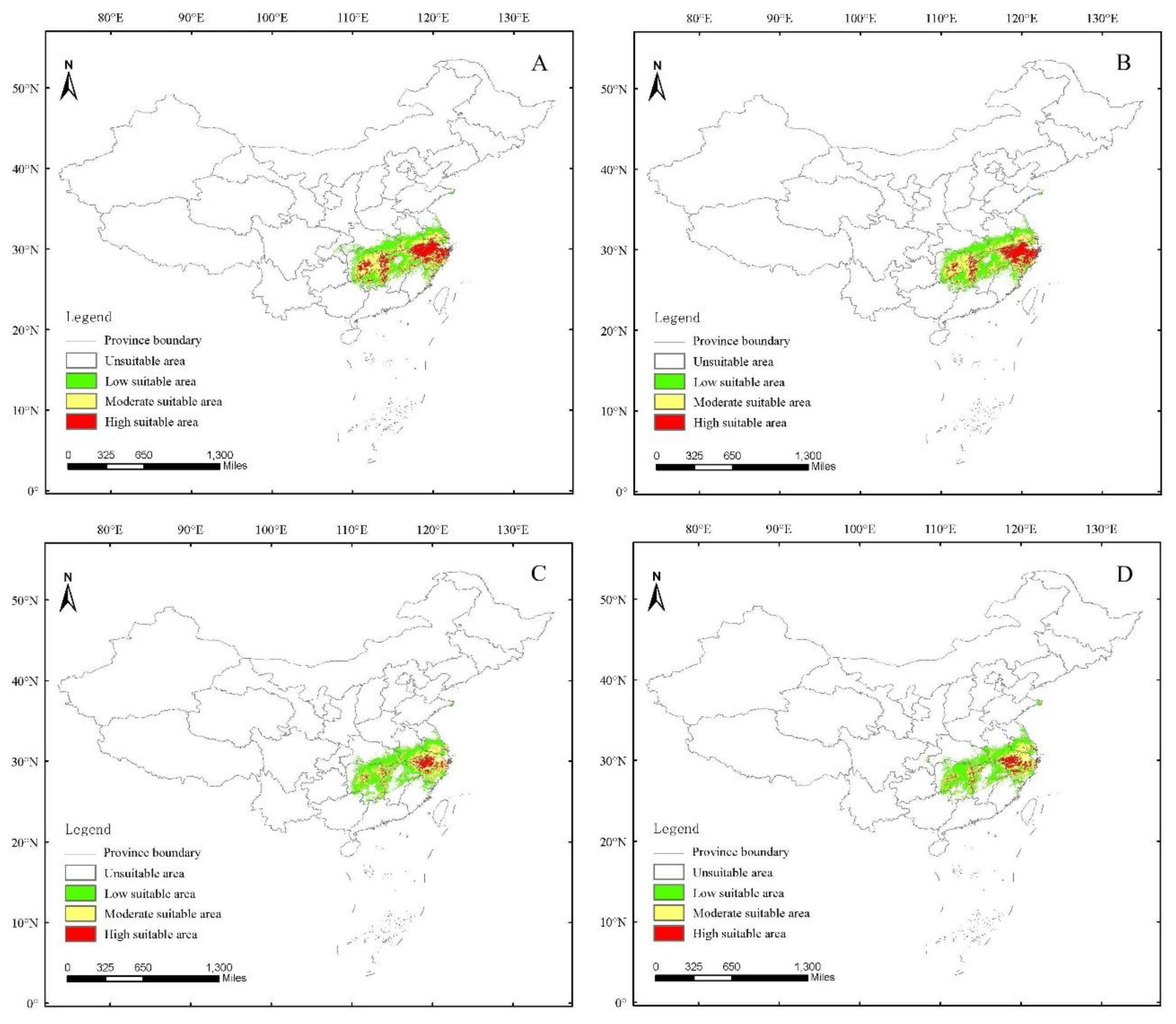
3.4. Current Potential Distribution of H. dorsalis
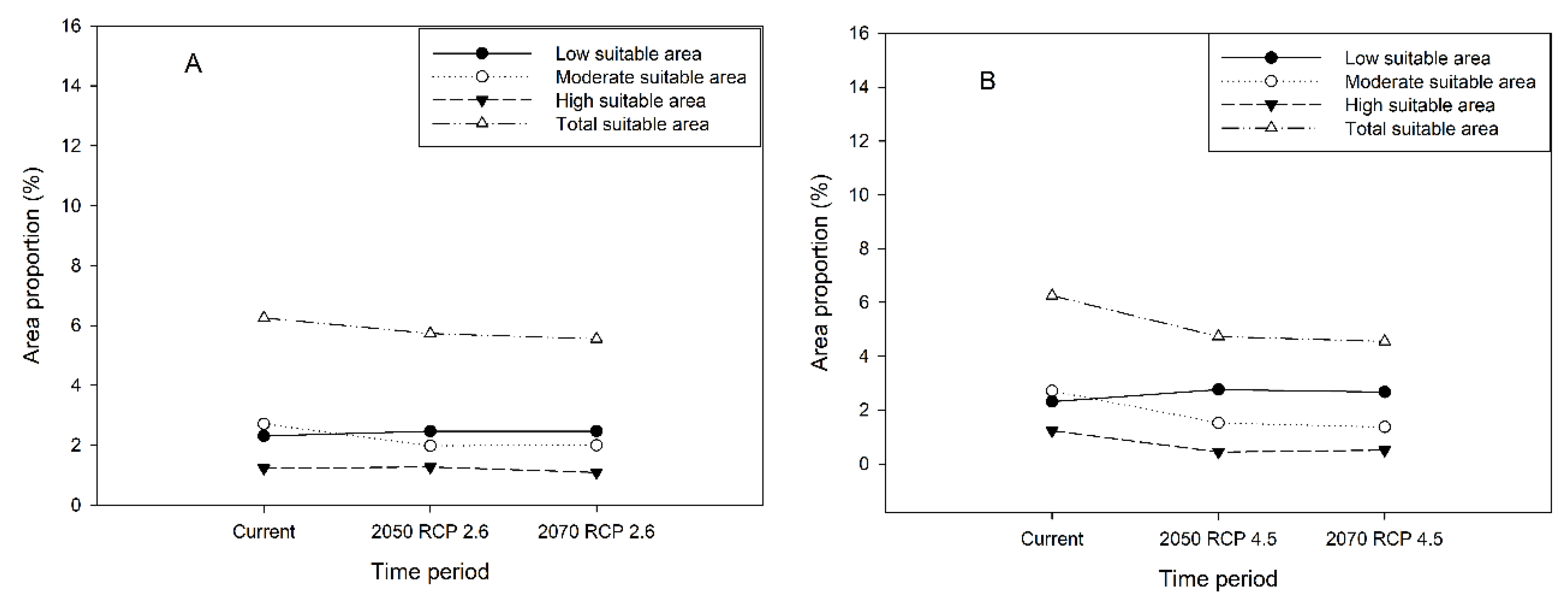
3.5. Future Potential Distribution of H. dorsalis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Z.; Zhang, Q.; Xiang, S.; Li, Y.; Huang, X.; Zhang, Y.; Zhou, X.; Li, Z.; Yao, X.; Li, Q.; et al. Monitoring the severity of Pantana phyllostachysae Chao infestation in moso bamboo forests based on UAV multi-spectral remote sensing feature selection. Forests 2022, 13, 418. [Google Scholar] [CrossRef]
- Ahmad, I.; Zahid, M. Redescription of the Caystrine genus Praetextatus Distant along with its type species P. typicus Distant (Heteroptera: Pentatomidae) with reference to their unknown characters of metathoracic scent auricle and genitalia and key to its known species and zoogeography and phylogeny. Int. J. Biol. Biotechnol. 2006, 3, 1–5. [Google Scholar]
- Stal, C. Analecta hemipterologica. Berl. Entomol. Z. 1869, 13, 226. [Google Scholar]
- Atkinson, E.T. Notes on Indian Rhynchota: Heteroptera. Asiat. Soc. Beng. 1888, 2, 17–19. [Google Scholar]
- Ahmad, I.; Kamaluddin, S. A revision of the tribe Caystrini Stål (Hemiptera: Pentatomidae: Pentatominae) from Indo-Pakistan subcontinent with description of two new species from Pakistan and their cladistic analysis. Proc. Pak. Congr. Zool. 1989, 9, 169–183. [Google Scholar]
- Ahmad, I.; Zahid, M. Redescription of a serious caystrine stink bug bamboo pest Hippotiscus dorsalis Stal (Hemiptera: Pentatomidae) along with its genus and their phylogenetic relationships. Int. J. Biol. Biotechnol. 2006, 3, 271–275. [Google Scholar]
- Chen, H.Y.; Talamas, E.J.; Pang, H. Notes on the hosts of Trissolcus ashmead (Hymenoptera: Scelionidae) from China. Biodivers. Data J. 2020, 8, 53786. [Google Scholar] [CrossRef]
- Werner, C.; Schmidt, H.P.; Gerten, D.; Lucht, W.; Kammann, C. Biogeochemical potential of biomass pyrolysis systems for limiting global warming to 1.5 degrees C. Environ. Res. Lett. 2018, 13, 044036. [Google Scholar] [CrossRef]
- Klutse, N.A.B.; Ajayi, V.O.; Gbobaniyi, E.O.; Egbebiyi, T.S.; Kouadio, K.; Nkrumah, F.; Quagraine, K.A.; Olusegun, C.; Diasso, U.; Abiodun, B.J.; et al. Potential impact of 1.5 degrees C and 2 degrees C global warming on consecutive dry and wet days over West Africa. Environ. Res. Lett. 2018, 13, 055013. [Google Scholar] [CrossRef]
- Holland, M.M.; Landrum, L.; Bailey, D.; Vavrus, S. Changing seasonal predictability of Arctic summer sea ice area in a warming climate. J. Clim. 2019, 32, 4963–4979. [Google Scholar] [CrossRef]
- Yuan, X.C.; Wei, Y.M.; Wang, B.; Mi, Z.F. Risk management of extreme events under climate change. J. Clean. Prod. 2017, 166, 1169–1174. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Bell, J.R.; Alderson, L.; Izera, D.; Kruger, T.; Parker, S.; Pickup, J.; Shortall, C.R.; Taylor, M.S.; Verrier, P.; Harrington, R. Long-term phenological trends, species accumulation rates, aphid traits and climate: Five decades of change in migrating aphids. J. Anim. Ecol. 2015, 84, 21–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziter, C.; Robinson, E.A.; Newman, J.A. Climate change and voltinism in Californian insect pest species: Sensitivity to location, scenario and climate model choice. Glob. Chang. Biol. 2012, 18, 2771–2780. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Bourougaaoui, A.; Ben Jamâa, M.L.; Robinet, C. Has North Africa turned too warm for a Mediterranean forest pest because of climate change? Clim. Chang. 2021, 165, 46. [Google Scholar] [CrossRef]
- Tougou, D.; Musolin, D.L.; Fujisaki, K. Some like it hot! Rapid climate change promotes changes in distribution ranges of Nezara viridula and Nezara antennata in Japan. Entomol. Exp. Appl. 2009, 130, 249–258. [Google Scholar] [CrossRef]
- Batalden, R.V.; Oberhauser, K.; Townsend Peterson, A. Ecological niches in sequential generations of eastern North American Monarch butterflies (Lepidoptera: Danaidae): The ecology of migration and likely climate change implications. Environ. Entomol. 2007, 36, 1365–1373. [Google Scholar] [CrossRef] [Green Version]
- Vinatier, F.; Tixier, P.; Duyck, P.F.; Lescourret, F. Factors and mechanisms explaining spatial heterogeneity: A review of methods for insect populations. Methods Ecol. Evol. 2011, 2, 11–22. [Google Scholar] [CrossRef]
- MacDonald, G.M. Biogeography: Introduction to Space, Time and Life. Prof. Geogr. 2010, 55, 283–285. [Google Scholar]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Griess, V.C.; Padalia, H. Mapping invasion potential using ensemble modelling. A case study on Yushania maling in the Darjeeling Himalayas. Ecol. Modell. 2018, 385, 35–44. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L.; Poulsen, M. Predicting species distributions: Use of climatic parameters in BIOCLIM and its impact on predictions of species’ current and future distributions. Ecol. Modell. 2005, 186, 251–270. [Google Scholar] [CrossRef]
- Booth, T.H. Why understanding the pioneering and continuing contributions of BIOCLIM to species distribution modelling is important. Austral Ecol. 2018, 43, 852–860. [Google Scholar] [CrossRef] [Green Version]
- Giusti, M.; Bo, M.; Angiolillo, M.; Cannas, R.; Cau, A. Habitat preference of Viminella flagellum (Alcyonacea: Ellisellidae) in relation to bathymetric variables in southeastern Sardinian waters. Cont. Shelf Res. 2017, 138, 41–45. [Google Scholar] [CrossRef]
- Palfreyman, J.; Graham-Brown, J.; Caminade, C.; Gilmore, P.; Otranto, D. Predicting the distribution of Phortica variegata and potential for Thelazia callipaeda transmission in Europe and the United Kingdom. Parasit. Vectors 2018, 11, 272. [Google Scholar] [CrossRef]
- Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Elith, J.; Gordon, A.; Kujala, H.; Lentini, P.E.; Mc Carthy, M.A.; Tingley, R.; Wintle, B.A. Is my species distribution model fit for purpose? Matching data and models to applications. Glob. Ecol. Biogeogr. 2015, 24, 276–292. [Google Scholar] [CrossRef]
- Heads, M. The relationship between biogeography and ecology: Envelopes, models, predictions: Biogeography and ecology. Biol. J. Linn. Soc. 2015, 115, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Ding, C.Q. Effects of sample size, sample accuracy and environmental variables on predictive performance of MaxEnt model. Pol. J. Ecol. 2016, 64, 303–312. [Google Scholar] [CrossRef]
- Halvorsen, R. A strict maximum likelihood explanation of MAXENT and some implications for distribution modelling. Sommerfeltia 2013, 36, 1–132. [Google Scholar] [CrossRef]
- Tognelli, M.F.; Roig-Junent, S.A.; Marvaldi, A.E.; Flores, G.E.; Lobo, J.M. An evaluation of methods for modelling distribution of Patagonian insects. Rev. Chil. Hist. Nat. 2009, 82, 347–360. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.P.; Gonzalez, I. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with MAXENT. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Narouei-Khandan, H.A.; Halbert, S.E.; Worner, S.P.; van Bruggen, A.H.C. Global climate suitability of citrus huanglongbing and its vector, the Asian citrus psyllid, using two correlative species distribution modeling approaches, with emphasis on the USA. Eur. J. Plant Pathol. 2016, 144, 655–670. [Google Scholar] [CrossRef]
- Hernández-Baz, F.; Romo, H.; González, J.M.; de Jesús, M.; Hernández, M.; Gámez Pastrana, R. Maximum entropy niche-based modeling (Maxent) of potential geographical distribution of Coreura albicosta (Lepidoptera: Erebidae: Ctenuchina) in Mexico. Fla. Entomol. 2016, 99, 376–380. [Google Scholar] [CrossRef] [Green Version]
- Bosso, L.; Di Febbraro, M.; Cristinzio, G.; Zoina, A.; Russo, D. Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin. Biol. Invasions 2016, 18, 1759–1768. [Google Scholar] [CrossRef]
- Gorshkov, A.; Novikova, O. Estimating the validity of the recognition results of earthquake-prone areas using the ArcMap. Acta Geophys. 2018, 66, 843–853. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Popes, M. A checklist for maximizing reproducibility of ecological niche models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef] [Green Version]
- Stocker, T.F.; Qin, D.; Plattner, G.K.; Tignor, M.; Allen, S.K.; Boschung, J. IPCC. Climate change 2013: The physical science basis. In Anthropogenic and Natural Radiative Forcing; Cambridge University Press: Cambridge, UK, 2014; pp. 710–719. [Google Scholar]
- Ning, H.; Tang, M.; Chen, H. Impact of climate change on potential distribution of Chinese White Pine Beetle Dendroctonus armandi in China. Forests 2021, 12, 544. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef] [Green Version]
- Bean, W.T.; Stafford, R.; Brashares, J.S. The effects of small sample size and sample bias on threshold selection and accuracy assessment of species distribution models. Ecography 2012, 35, 250–258. [Google Scholar] [CrossRef]
- Phillips, S.J.; Schapire, R.E.; Dudík, M. A maximum entropy approach to species distribution modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AB, Canada, 4–8 July 2004; ACM Press: New York, NY, USA, 2004; pp. 655–662. [Google Scholar]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with MAXENT: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Mc Pherson, J.M.; Jetz, W.; Rogers, D.J. The effects of species’ range sizes on the accuracy of distribution models: Ecological phenomenon or statistical artefact? J. Appl. Ecol. 2004, 41, 811–823. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B. Niche properties and geographical extent as predictors of species sensitivity to climate change. Glob. Ecol. Biogeogr. 2005, 14, 347–357. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2010, 43, 1223–1232. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [Green Version]
- França, S.; Cabral, H.N. Distribution models of estuarine fish species: The effect of sampling bias, species ecology and threshold selection on models’ accuracy. Ecol. Inform. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Ning, H.; Tang, M.; Chen, H. Mapping invasion potential of the pest from Central Asia, Trypophloeus klimeschi (Coleoptera: Curculionidae: Scolytinae), in the shelter forests of Northwest China. Insects 2021, 12, 242. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Q.; Huang, J.P.; Jia, J.Y.; Zhu, T.F.; Hong, T.; Su, J. The functional microbiota of on- and off-year moso bamboo (Phyllostachys edulis) influences the development of the bamboo pest Pantana phyllostachysae. BMC Plant Biol. 2022, 22, 307. [Google Scholar] [CrossRef]
- Kistner, E.J. Climate change impacts on the potential distribution and abundance of the brown marmorated stink bug (Hemiptera: Pentatomidae) with special reference to North America and Europe. Environ. Entomol. 2017, 46, 1212–1224. [Google Scholar] [CrossRef] [PubMed]
- Kiritani, K. Predicting impact of global warming on population dynamics and distribution of arthropods in Japan. Popul. Ecol. 2006, 48, 5–12. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Elith, J.; Peterson, A.T.; Graham, C.H.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Fan, S.; Chen, C.; Zhao, Q.; Wei, J.; Zhang, H. Identifying potentially climatic suitability areas for Arma custos (Hemiptera: Pentatomidae) in China under climate change. Insects 2020, 11, 674. [Google Scholar] [CrossRef]
- Maclean, I.M.D.; Wilson, R.J. Recent ecological responses to climate change support predictions of high extinction risk. Proc. Natl. Acad. Sci. USA 2011, 108, 12337–12342. [Google Scholar] [CrossRef] [Green Version]
- Roth, T.; Plattner, M.; Amrhein, V. Plants, birds and butterflies: Short-term responses of species communities to climate warming vary by taxon and with altitude. PLoS ONE 2014, 9, e82490. [Google Scholar] [CrossRef]
- Stoeckli, S.; Felber, R.; Haye, T. Current distribution and voltinism of the brown marmorated stink bug, Halyomorpha halys, in Switzerland and its response to climate change using a high-resolution CLIMEX model. Int. J. Biometeorol. 2020, 64, 2019–2032. [Google Scholar] [CrossRef]
- Tôrres, N.M.; De Marco, P.; Santos, T.; Silveira, L.; de Almeida Jácomo, A.T.; Diniz-Filho, J.A.F. Can species distribution modelling provide estimates of population densities? A case study with jaguars in the Neotropics. Divers. Distrib. 2012, 18, 615–627. [Google Scholar] [CrossRef]
- Oliver, T.H.; Gillings, S.; Girardello, M.; Rapacciuolo, G.; Brereton, T.M.; Siriwardena, G.M.; Roy, D.B.; Pywell, R.; Fuller, R.J. Population density but not stability can be predicted from species distribution models. J. Appl. Ecol. 2012, 49, 581–590. [Google Scholar] [CrossRef] [Green Version]
- Skidmore, I.H.; Hansen, A.K. The evolutionary development of plant-feeding insects and their nutritional endosymbionts. Insect Sci. 2017, 24, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guan, F.; Zhang, X.; Li, C.; Zhou, Y. Response of moso bamboo growth and soil nutrient content to strip cutting. Forests 2022, 13, 1293. [Google Scholar] [CrossRef]
- Zhai, W.L.; Wang, Y.; Luan, J.W.; Liu, S.R. Effects of nitrogen addition on clonal integration between mother and daughter ramets of moso bamboo: A 13C-CO2 pulse labeling study. J. Plant Ecol. 2022, 15, 756–770. [Google Scholar] [CrossRef]


| Abbreviation | Environmental Variables | Pearson > 0.9 | Operation |
|---|---|---|---|
| Bio1 | Annual mean temp (°C) | Retain | |
| Bio2 | Mean diurnal range (°C) | Retain | |
| Bio3 | Isothermality | Retain | |
| Bio4 | Temperature seasonality | Retain | |
| Bio5 | Maximum temp of warmest month (°C) | Bio10 | Eliminate |
| Bio6 | Minimum temp of coldest month (°C) | Bio11 | Eliminate |
| Bio7 | Temperature annual range (°C) | Retain | |
| Bio8 | Mean temp of wettest quarter (°C) | Retain | |
| Bio9 | Mean temp of driest quarter (°C) | Retain | |
| Bio10 | Mean temp of warmest quarter (°C) | Bio5 | Retain |
| Bio11 | Mean temp of coldest quarter (°C) | Bio6 | Retain |
| Bio12 | Annual precipitation (mm) | Bio13 | Retain |
| Bio13 | Precipitation of wettest month (mm) | Bio16, Bio12 | Eliminate |
| Bio14 | Precipitation of driest month (mm) | Retain | |
| Bio15 | Precipitation seasonality (mm) | Retain | |
| Bio16 | Precipitation of wettest quarter (mm) | Bio13 | Retain |
| Bio17 | Precipitation of driest quarter (mm) | Retain | |
| Bio18 | Precipitation of warmest quarter (mm) | Retain | |
| Bio19 | Precipitation of coldest quarter (mm) | Retain |
| Variable | Percent Contribution | Permutation Importance |
|---|---|---|
| Bio18 | 42 | 1.5 |
| Bio4 | 23 | 0 |
| Bio14 | 17.6 | 0.7 |
| Bio15 | 8.1 | 0 |
| Bio1 | 3.9 | 0.6 |
| Bio19 | 3.3 | 1.2 |
| Bio2 | 1 | 0.4 |
| Bio3 | 0.5 | 57.2 |
| Bio10 | 0.4 | 1.6 |
| Bio11 | 0.2 | 36 |
| Bio17 | 0 | 0.4 |
| Bio8 | 0 | 0.1 |
| Bio9 | 0 | 0.4 |
| Bio16 | 0 | 0 |
| Bio12 | 0 | 0 |
| Bio7 | 0 | 0 |
| 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 1 | ||||||||
| 02 | 0.923 ** | 1 | |||||||
| 0.000 | |||||||||
| 03 | 0.488 | 0.432 | 1 | ||||||
| 0.182 | 0.245 | ||||||||
| 04 | 0.406 | 0.389 | 0.923 ** | 1 | |||||
| 0.278 | 0.301 | 0.000 | |||||||
| 05 | 0.679 * | 0.784 * | 0.483 | 0.529 | 1 | ||||
| 0.044 | 0.012 | 0.188 | 0.143 | ||||||
| 06 | 0.060 | 0.118 | 0.026 | −0.075 | −0.060 | 1 | |||
| 0.877 | 0.763 | 0.946 | 0.848 | 0.878 | |||||
| 07 | 0.698 * | 0.792 * | 0.579 | 0.605 | 0.976 ** | −0.005 | 1 | ||
| 0.037 | 0.011 | 0.103 | 0.084 | 0.000 | 0.990 | ||||
| 08 | 0.488 | 0.452 | 0.323 | 0.345 | 0.782 * | −0.544 | 0.739 * | 1 | |
| 0.182 | 0.222 | 0.396 | 0.363 | 0.013 | 0.130 | 0.023 | |||
| 09 | 0.273 | 0.376 | 0.226 | 0.215 | 0.481 | 0.708 * | 0.515 | 0.089 | 1 |
| 0.477 | 0.318 | 0.559 | 0.578 | 0.190 | 0.033 | 0.156 | 0.819 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Duan, Q.; Shen, X.; Zhang, S. Climate Change Influences the Population Density and Suitable Area of Hippotiscus dorsalis (Hemiptera: Pentatomidae) in China. Insects 2023, 14, 135. https://doi.org/10.3390/insects14020135
Zhao M, Duan Q, Shen X, Zhang S. Climate Change Influences the Population Density and Suitable Area of Hippotiscus dorsalis (Hemiptera: Pentatomidae) in China. Insects. 2023; 14(2):135. https://doi.org/10.3390/insects14020135
Chicago/Turabian StyleZhao, Mingzhen, Qian Duan, Xiayang Shen, and Shaoyong Zhang. 2023. "Climate Change Influences the Population Density and Suitable Area of Hippotiscus dorsalis (Hemiptera: Pentatomidae) in China" Insects 14, no. 2: 135. https://doi.org/10.3390/insects14020135





