Potential of Insect Life Stages as Functional Ingredients for Improved Nutrition and Health
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Sample Preparation
2.4. Analyses of Fatty Acids
2.5. Analyses of Minerals
2.6. Analyses of Proximate Nutritional Parameters
2.7. Analyses of Vitamins
2.8. Statistical Data Analyses
3. Results and Discussion
3.1. Analyses of Fatty Acids
3.2. Analyses of Minerals
3.3. Analyses of Proximate Nutritional Parameters
3.4. Analyses of Vitamins
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Population Prospects by United Nations (UN). Population Division of the United Nations Department of Economic and Social Affairs; The World Population Prospects by United Nations (UN): New York, NY, USA, 2019. Available online: https://www.un.org/development/desa/en/news/population/world-population-prospects-2019.html (accessed on 1 November 2022).
- Nadeau, L.; Nadeau, I.; Franklin, F.; Dunkel, F. The Potential for Entomophagy to Address Undernutrition. Ecol. Food Nutr. 2015, 54, 200–208. [Google Scholar] [CrossRef] [PubMed]
- FAO; WFP. State of Food Insecurity in the World: Strengthening the Enabling Environment for Food Security and Nutrition; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Gomez-Zavaglia, A.; Mejuto, J.; Simal-Gandara, J. Mitigation of emerging implications of climate change on food production systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- Broucek, J. Production of Methane Emissions from Ruminant Husbandry: A Review. J. Environ. Prot. 2014, 05, 1482–1493. [Google Scholar] [CrossRef]
- Barona, E.; Ramankutty, N.; Hyman, G.; Coomes, O.T. The role of pasture and soybean in deforestation of the Brazilian Amazon. Environ. Res. Lett. 2010, 5. [Google Scholar] [CrossRef]
- Huis, A.V.; Itterbeeck, J.V.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper; FAO: Rome, Italy, 2013; p. 171. [Google Scholar]
- Xu, Q.Y.; Wang, C.A.; Zhao, Z.G.; Luo, L. Effects of replacement of fish meal by soy protein isolate on the growth, digestive enzyme activity and serum biochemical parameters for juvenile Amur sturgeon (Acipenser schrenckii). Asian-Australas. J. Anim. Sci. 2012, 25, 1588–1594. [Google Scholar] [CrossRef] [Green Version]
- Van Huis, A.; Oonincx, D.G. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Numbi Muya, G.M.; Mutiaka, B.K.; Bindelle, J.; Francis, F.; Caparros Megido, R. Human Consumption of Insects in Sub-Saharan Africa: Lepidoptera and Potential Species for Breeding. Insects 2022, 13, 886. [Google Scholar] [CrossRef]
- Lavalette, M. Insects: A New Protein Resource for Human Food. Ph.D. Dissertation, University of Lorraine, Lorraine, France, 2013. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Akinnawo, O.; Ketiku, A.O. Chemical composition and fatty acid profile of edible larva of Cirina forda (Westwood). Afr. J. Biomed. Res. 2000, 3, 93–96. [Google Scholar]
- Bernard, T.; Womeni, H.M. Entomophagy: Insects as food. Insect Physiol. Ecol. 2017, 233–249. [Google Scholar]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein quality of four indigenous edible insect species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Chantawannakul, P. From entomophagy to entomotherapy. Front. Biosci. -Landmark 2020, 25, 179–200. [Google Scholar] [CrossRef]
- Dev, S.; Hassan, K.; Claes, J.; Mozahid, M.; Khatun, H.; Mondal, M. Practices of entomophagy and entomotherapy in Bangladesh. J. Insects Food Feed. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Mokaya, H.; Ndunda, R.; Kegode, T.; Koech, S.; Tanga, C.; Subramanian, S.; Ngoka, B. Silkmoth pupae: Potential and less exploited alternative source of nutrients and natural antioxidants. J. Insects Food Feed. 2022, 1–12. [Google Scholar] [CrossRef]
- Kusia, E.; Borgemeister, C.; Khamis, F.; Copeland, R.; Tanga, C.; Ombura, F.; Subramanian, S. Diversity, Host Plants and Potential Distribution of Edible Saturniid Caterpillars in Kenya. Insects 2021, 12, 600. [Google Scholar] [CrossRef]
- Darge, P.; Terral, G. A new Gonimbrasia from Kenya (Lepidoptera Saturniidae). Alexanor 1992, 17, 468–470. [Google Scholar]
- Glew, R.H.; Jackson, D.; Sena, L.; VanderJagt, D.J.; Pastuszyn, A.; Millson, M. Gonimbrasia belina (Lepidoptera: Saturniidae): A Nutritional Food Source Rich in Protein, Fatty Acids and Minerals. Am. Entomol. 1999, 45, 250–253. [Google Scholar] [CrossRef] [Green Version]
- Siulapwa, N.; Mwambungu, A.; Lungu, E.; Sichilima, W. Nutritional value of four common edible insects in Zambia. Int. J. Sci. Res. 2014, 3, 876–884. [Google Scholar]
- Mnisi, C.M.; Oyeagu, C.E.; Ruzvidzo, O. Mopane Worm (Gonimbrasia belina Westwood) Meal as a Potential Protein Source for Sustainable Quail Production: A Review. Sustainability 2022, 14, 5511. [Google Scholar] [CrossRef]
- Alipanah, M.; Abedian, Z.; Nasiri, A.; Sarjamei, F. Nutritional effects of three mulberry varieties on silkworms in torbat heydarieh. Psyche A J. Entomol. 2020, 29, 2020. [Google Scholar] [CrossRef]
- Narayanamma, V.L.; Padmasri, A. Rearing of Eri Silk Worm and its Economic Viability to Rainfed Castor Farmers. Indian J. Entomol. 2022, 28, 814–818. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiab, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C.; Fiaboe, K.K.M.; et al. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manditsera, F.A.; Luning, P.A.; Fogliano, V.; Lakemond, C.M.M. The contribution of wild harvested edible insects (Eulepida mashona and Henicus whellani) to nutrition security in Zimbabwe. J. Food Compos. Anal. 2019, 75, 17–25. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, Association of Official Analytical Chemist, 19th ed.; AOAC: Washington, DC, USA, 2012. [Google Scholar]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-protein conversion factors for three edible insects: Tenebrio molitor, Alphitobius diaperinus and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Maiyo, N.C.; Khamis, F.M.; Okoth, M.W.; Abong, G.O.; Subramanian, S.; Egonyu, J.P.; Xavier, C.; Ekesi, S.; Omuse, E.R.; Nakimbugwe, D.; et al. Nutritional Quality of Four Novel Porridge Products Blended with Edible Cricket (Scapsipedus icipe) Meal for Food. Foods 2022, 11, 1047. [Google Scholar] [CrossRef]
- Bhatnagar-Panwar, M.; Bhatnagar-Mathur, P.; Bhaaskarla, V.V.; Dumbala, S.R.; Sharma, K.K. Rapid, accurate and routine HPLC method for large-scale screening of pro-vitamin A carotenoids in oilseeds. J. Plant Biochem. Biotechnol. 2015, 24, 84–92. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2018; Available online: https://www.R-project.org (accessed on 10 June 2021).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses R Package Version 1.0. 5.999. 2019. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 10 June 2021).
- Wickham, H.; Chang, W.; Wickham, M.H. Package ‘ggplot2’. In Create Elegant Data Visualisations Using the Grammar of Graphics; Version 2; R Foundation for Statistical Computing: Vienna, Austria, 2016; pp. 1–189. [Google Scholar]
- Son, Y.J.; Choi, S.Y.; Hwang, I.K.; Nho, C.W.; Kim, S.H. Could defatted mealworm (Tenebrio molitor) and mealworm oil be used as food ingredients? Foods 2020, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Kim, T.-K.; Cha, J.-Y.; Yong, H.I.; Kang, M.-C.; Jang, H.W.; Choi, Y.-S. Physicochemical characteristics and aroma patterns of oils prepared from edible insects. LWT 2022. [Google Scholar] [CrossRef]
- Riekkinen, K.; Väkeväinen, K.; Korhonen, J. The Effect of Substrate on the Nutrient Content and Fatty Acid Composition of Edible Insects. Insects 2022, 13, 590. [Google Scholar] [CrossRef] [PubMed]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value and Other Benefits—A Review. Front. Nutr. 2021, 7, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Zou, Y.; Pang, D.; Shi, W.; Mu, L.; Li, E.; Lan, D.; Wang, Y.; Liao, S. Comprehensive Identification of Principal Lipid Classes and Tocochromanols in Silkworm ( Antheraea pernyi and Bombyx mori ) Pupae Oils. Eur. J. Lipid Sci. Technol. 2020, 122, 1900280. [Google Scholar] [CrossRef]
- Barroso, F.G.; Sánchez-Muros, M.J.; Rincón, M.; Rodriguez-Rodriguez, M.; Fabrikov, D.; Morote, E.; Guil-Guerrero, J.L. Production of n-3-rich insects by bioaccumulation of fishery waste. J. Food Compos. Anal. 2019, 82, 103237. [Google Scholar] [CrossRef]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens) – Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.G.A.B.; Laurent, S.; Veenenbos, M.E.; van Loon, J.J. Dietary enrichment of edible insects with omega 3 fatty acids. Insect Sci. 2019, 27, 500–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, K.; Tiuca, I.D. Importance of Fatty Acids in Physiopathology of Human Body. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders—A review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef] [Green Version]
- Sacks, F.M.; Willett, W.W. More on chewing the fat. N. Engl. J. Med. 1991, 325, 1740–1742. [Google Scholar] [CrossRef]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. (Eds.) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Musundire, R.; Zvidzai, C.; Chidewe, C.; Samende, B.; Chemura, A. Habitats and nutritional composition of selected edible insects in Zimbabwe. J. Insects Food Feed. 2016, 2, 189–198. [Google Scholar] [CrossRef]
- da Silva Lucas, A.J.; De Oliveira, L.M.; Da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, M.; Bhattacharyya, B.; Bhagawati, S.; Sarmah, K. Nutritional Composition of Some Commonly Available Aquatic Edible Insects of Assam, India. Insects 2022, 13, 976. [Google Scholar] [CrossRef]
- Razali, S.M. Physicochemical Characteristics and Microbiological Quality of Silkworm (Bombyx mori) Larval and Pupae Powder: Comparative Study. Sains Malaysiana. 2022, 51, 547–558. [Google Scholar]
- Bhattacharyya, B.; Choudhury, B.; Das, P.; Dutta, S.K.; Bhagawati, S.; Pathak, K. Nutritional Composition of Five Soil-Dwelling Scarab Beetles (Coleoptera: Scarabaeidae) of Assam, India. Coleopt. Bull. 2018, 72, 339–346. [Google Scholar] [CrossRef]
- Montowska, M.; Kowalczewski, P.; Rybicka, I.; Fornal, E. Nutritional value, protein and peptide composition of edible cricket powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jung, C.; Meyer-Rochow, V.B. Nutritional value and chemical composition of larvae, pupae and adults of worker honey bee, Apis mellifera ligustica as a sustainable food source. J. Asia-Pac. Entomol. 2016, 19, 487–495. [Google Scholar] [CrossRef]
- Crenshaw, T.D. Calcium, phosphorus, vitamin D and vitamin K in swine nutrition. In Swine Nutrition; CRC Press: Boca Raton, FL, USA, 2000; pp. 207–232. [Google Scholar]
- Fadipe, L.A.; Babayi, H.; Olayemi, I.K.; Baba, M.B.; Sadiku, J.O. Mineral nutrient profile of grasshopper (Zonocerus variegatus) subjected to different conventional post-processing techniques. Int. J. Appl. Biol. Res. 2018, 9, 152–165. [Google Scholar]
- van Huis, A. Nutrition and health of edible insects. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 228–231. [Google Scholar] [CrossRef]
- Hafeez, A.; Mader, A.; Ruhnke, I.; Röhe, I.; Boroojeni, F.G.; Yousaf, M.; Männer, K.; Zentek, J. Implication of milling methods, thermal treatment and particle size of feed in layers on mineral digestibility and retention of minerals in egg contents. Poult. Sci. 2015, 94, 240–248. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Hurrell, R.F. Nutritional iron deficiency. Lancet 2007, 370, 511–520. [Google Scholar] [CrossRef]
- Muriuki, J.M.; Mentzer, A.J.; Webb, E.L.; Morovat, A.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Crane, R.J.; Berkley, J.A.; Lule, S.A.; et al. Estimating the burden of iron deficiency among African children. BMC Med. 2020, 18, 31. [Google Scholar] [CrossRef] [Green Version]
- Gröber, U.; Schmidt, J.; Kisters, K. Magnesium in Prevention and Therapy. Nutrients 2015, 7, 8199–8226. [Google Scholar] [CrossRef] [Green Version]
- Mokwunye, I.; Igbinadolor, R.; Mokwunye, F.; Asogwa, E.; Ndubuaku, T. Nutrient Composition of Cashew Stem Girdler Analeptes trifasciata (Coleoptera: Cerambycidae) and Its Suitability for Feed and as Food. Afr. Entomol. 2021, 29, 87–95. [Google Scholar] [CrossRef]
- Paiko, Y.B.; Dauda, B.E.; Suleiman, M.A.; Akanya, H.O.; Jacob, J.O. Physicochemical properties and metal ions content of oil extracted from cricket (Brachytrupes membranaceus) in Bosso Local Government Area of Niger State, Nigeria. Asian J. Sci. Technol. 2013, 4, 8–12. [Google Scholar]
- Arasakumar, E.; Manimegalai, S.; Mohankumar, S.; Parthiban, K.T.; Priyadharshini, P. Analysis of proximate composition and oil yield from spent pupae of mulberry silkworm, Bombyx mori L. & eri silkworm, Samia ricini D. Indian J. 2021, 16, 555–559. [Google Scholar]
- Cerda, H.; Martinez, R.; Briceno, N.; Pizzoferrato, L.; Manzi, P.; Tommaseo Ponzetta, M.; Marin, O.; Paoletti, M.G. Palm worm: (Rhynchophorus palmarum) traditional food in Amazonas, Venezuela–nutritional composition, small scale production and tourist palatability. Ecol. Food Nutr. 2001, 40, 13–32. [Google Scholar] [CrossRef]
- Finke, M.D. Complete Nutrient Content of Four Species of Feeder Insects. Zoo Biol. 2012, 32, 27–36. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of three species of wild caught insects, pallid-winged grasshopper, rhinoceros beetles and white-lined sphinx moth. J. Insects Food Feed. 2015, 1, 281–292. [Google Scholar] [CrossRef]
- Vanqa, N.; Mshayisa, V.V.; Basitere, M. Proximate, Physicochemical, Techno-Functional and Antioxidant Properties of Three Edible Insect (Gonimbrasia belina, Hermetia illucens and Macrotermes subhylanus) Flours. Foods 2022, 11, 976. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.; van Loon, J.J.; van Loon, L.J. Consideration of insects as a source of dietary protein for human consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, J.M.; Kim, B.; Nam, J.-O.; Hahn, D.; Choi, M.B. Nutritional Value of the Larvae of the Alien Invasive Wasp Vespa velutina nigrithorax and Amino Acid Composition of the Larval Saliva. Foods 2020, 9, 885. [Google Scholar] [CrossRef]
- Oonincx, D.; Finke, M. Nutritional value of insects and ways to manipulate their composition. J. Insects Food Feed. 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Deori, M.; Devi, D.; Devi, R. Nutrient composition and antioxidant activities of muga and eri silkworm pupae. Int. J. Sci. Nat. 2014, 5, 636–640. [Google Scholar]
- Longvah, T.; Mangthya, K.; Ramulu, P. Nutrient composition and protein quality evaluation of eri silkworm (Samia ricinii) prepupae and pupae. Food Chem. 2011, 128, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.; Gangopadhyay, D. Effect of maturation stage and sex on proximate, fatty acid and mineral composition of eri silkworm (Samia ricini) from India. J. Food Compos. Anal. 2021, 100, 103898. [Google Scholar] [CrossRef]
- Narzari, S.; Sarmah, J. Proximate composition of wild edible insects consumed by the Bodo tribe of Assam, India. Int. J. Bioassays. 2015, 4, 4050–4054. [Google Scholar]
- Bhulaidok, S.; Sihamala, O.; Shen, L.; Li, D. Nutritional and fatty acid profiles of sun-dried edible black ants (Polyrhachis vicina Roger). Maejo Int. J. Sci. Technol. 2010, 4, 101–112. [Google Scholar]
- Paoletti, M.G.; Norberto, L.; Damini, R.; Musumeci, S. Human Gastric Juice Contains Chitinase That Can Degrade Chitin. Ann. Nutr. Metab. 2007, 51, 244–251. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Boudrant, J.; Meyer, D.; Manno, N.; DeMarchis, M.; Paoletti, M.G. Current views on fungal chitin/chitosan, human chitinases, food preservation, glucans, pectins and inulin: A tribute to Henri Braconnot, precursor of the carbohydrate polymers science, on the chitin bicentennial. Carbohydr. Polym. 2012, 87, 995–1012. [Google Scholar] [CrossRef]
- Yoo, J.S.; Cho, K.H.; Hong, J.S.; Jang, H.S.; Chung, Y.H.; Kwon, G.T.; Shin, D.G.; Kim, Y.Y. Nutrient ileal digestibility evaluation of dried mealworm (Tenebrio molitor) larvae compared to three animal protein by-products in growing pigs. Asian-Australas. J. Anim. Sci. 2019, 32, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). Micronutrient Deficiencies Nutrition. 2014. Available online: https://www.who.int/nutrition/topics/ida/en/ (accessed on 1 November 2022).
- Weber, G.M.; Windisch, W. Producing sufficient animal-source protein for the growing world population. In Sustainable Nutrition in a Changing World; Springer: Cham, Switzerland, 2017; pp. 321–334. [Google Scholar]
- Bramley, P.M.; Elmadfa, I.; Kafatos, A.; Kelly, F.J.; Manios, Y.; Roxborough, H.E.; Wagner, K.H. Review vitamin E. J. Sci. Food Agric. 2000, 80, 913–938. [Google Scholar] [CrossRef]
- Chen, W.; Liu, D.; Zhou, L.; Li, Q.; Wu, D. Antioxidant activity of vitamin E enhanced by cyclodextrin inclusion complex. Br. Food J. 2021, 123, 3988–3998. [Google Scholar] [CrossRef]
- Zhang, G.R.; Xu, C.; You, C.H.; Wang, S.Q.; Xie, D.Z.; Hasan, A.M.; Li, Y.Y. Effects of dietary vitamin E on growth performance, antioxidant capacity and lipid metabolism of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2021, 27, 2205–2217. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, S.; Duan, H.; Wang, J.; Yan, W. Silkworm Pupae: A Functional Food with Health Benefits for Humans. Foods 2022, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Cermeño, M.; Bascón, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Hu, D.; Liu, Q.; Cui, H.; Wang, H.; Han, D.; Xu, H. Effects of amino acids from selenium-rich silkworm pupas on human hepatoma cells. Life Sci. 2005, 77, 2098–2110. [Google Scholar] [CrossRef]
- Weixin, L.; Lixia, M.; Leiyan, W.; Yuxiao, Z.; Haifeng, Z.; Sentai, L. Effects of silkworm pupa protein hydrolysates on mitochondrial substructure and metabolism in gastric cancer cells. J. Asia-Pac. Entomol. 2019, 22, 387–392. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, X.; Liu, X.; Zhu, W.; Mei, Y.; Li, W.; Wang, J. Structured lipids enriched with unsaturated fatty acids produced by enzymatic acidolysis of silkworm pupae oil using oleic acid. Eur. J. Lipid Sci. Technol. 2015, 117, 879–889. [Google Scholar] [CrossRef]
- Wu, X.; He, K.; Velickovic, T.C.; Liu, Z. Nutritional, functional and allergenic properties of silkworm pupae. Food Sci. Nutr. 2021, 9, 4655–4665. [Google Scholar] [CrossRef]
- Jeong, K.Y.; Lee, J.S.; Yuk, J.E.; Song, H.; Lee, H.J.; Kim, K.J.; Kim, B.J.; Lim, K.-J.; Park, K.H.; Lee, J.-H.; et al. Allergenic characterization of Bomb m 4, a 30-kDa Bombyx mori lipoprotein 6 from silkworm pupa. Clin. Exp. Allergy 2022, 52, 888–897. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Li, K.; Li, X.; Yang, A.; Tong, P.; Chen, H. Allergenicity assessment on thermally processed peanut influenced by extraction and assessment methods. Food Chem. 2019, 281, 130–139. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed. Available online: http://ipiff.org/insects-eu-legislation (accessed on 8 January 2023).
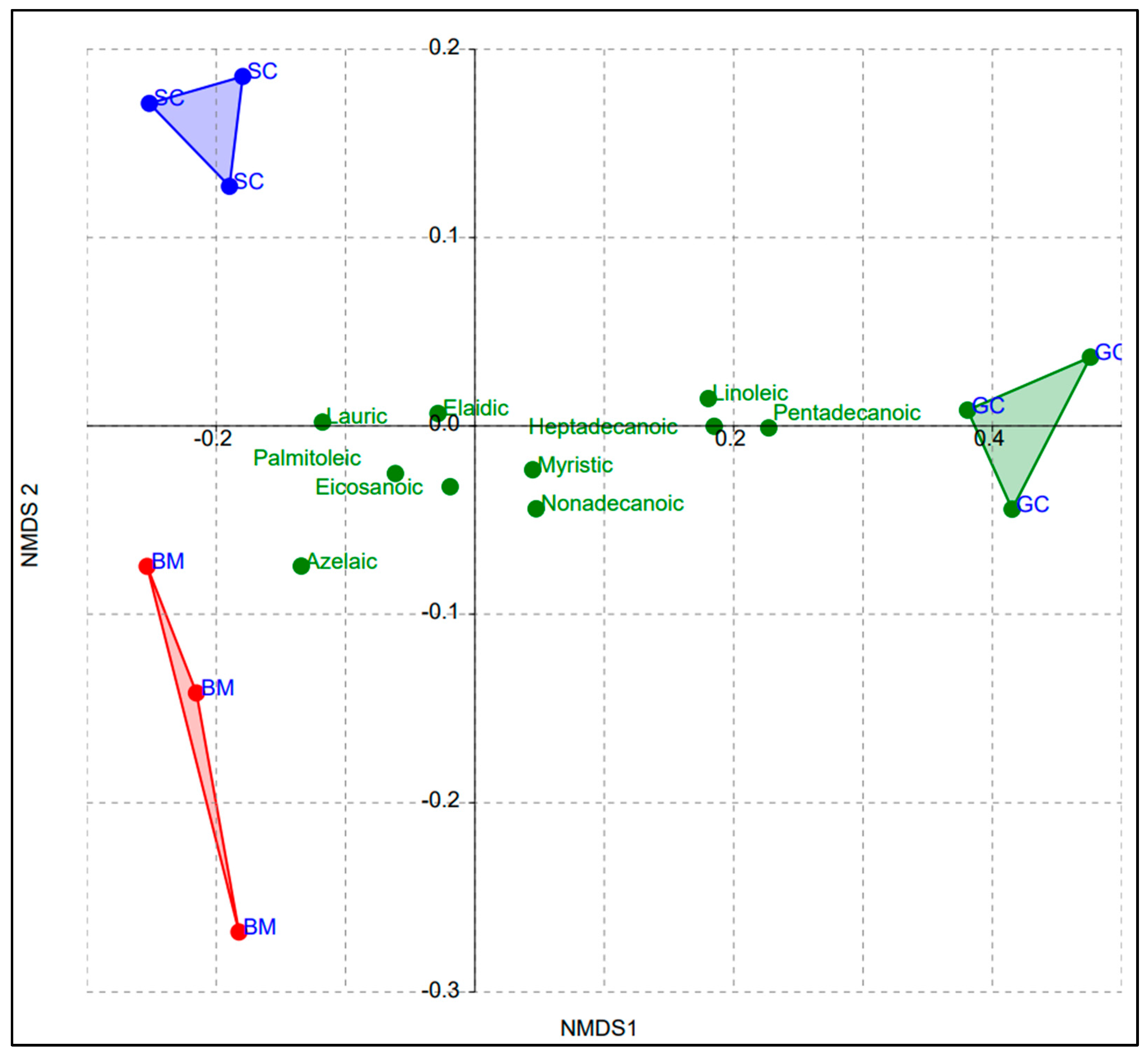




| Free Fatty Acids | Insects | |||||
|---|---|---|---|---|---|---|
| B. mori | S. cynthia ricini | G. cocaulti | F-Value | df | p-Value | |
| Lauric | 0.23 ± 0.07 b | 0.23 ± 0.06 b | 0.08 ± 0.04 a | 7.146 | 2 | 0.0259 |
| Azelaic | 2.82 ± 0.41 b | 0.96 ± 0.07 a | 0.53 ± 0.20 a | 62.97 | 2 | <0.0001 |
| Myristic | 1.80 ± 0.48 ab | 1.24 ± 0.10 a | 2.04 ± 0.02 b | 6.334 | 2 | 0.0332 |
| Pentadecanoic | 0.33 ± 0.09 a | 0.35 ± 0.02 a | 1.52 ± 0.03 b | 391.2 | 2 | <0.0001 |
| Palmitoleic | 9.47 ± 1.14 b | 6.57 ± 0.64 a | 4.97 ± 0.51 a | 23.78 | 2 | 0.0014 |
| Heptadecanoic | 1.89 ± 0.11 a | 1.85 ± 0.12 a | 6.18 ± 0.40 b | 298.9 | 2 | <0.0001 |
| Linoleic | 4.52 ± 0.35 a | 6.99 ± 1.16 a | 18.18 ± 1.69 b | 109.7 | 2 | <0.0001 |
| Elaidic | 68.88 ± 3.10 b | 76.08 ± 1.42 c | 59.03 ± 1.92 a | 43.08 | 2 | 0.0003 |
| Nonadecanoic | 1.69 ± 0.65 a | 0.81 ± 0.07 b | 1.69 ± 0.07 a | 5.362 | 2 | 0.0462 |
| Eicosanoic | 8.36 ± 1.15 b | 4.92 ± 0.32 a | 5.78 ± 0.36 a | 18.32 | 2 | 0.0028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanga, C.M.; Mokaya, H.O.; Kasiera, W.; Subramanian, S. Potential of Insect Life Stages as Functional Ingredients for Improved Nutrition and Health. Insects 2023, 14, 136. https://doi.org/10.3390/insects14020136
Tanga CM, Mokaya HO, Kasiera W, Subramanian S. Potential of Insect Life Stages as Functional Ingredients for Improved Nutrition and Health. Insects. 2023; 14(2):136. https://doi.org/10.3390/insects14020136
Chicago/Turabian StyleTanga, Chrysantus M., Hosea O. Mokaya, Wendie Kasiera, and Sevgan Subramanian. 2023. "Potential of Insect Life Stages as Functional Ingredients for Improved Nutrition and Health" Insects 14, no. 2: 136. https://doi.org/10.3390/insects14020136





