The Transcriptomic Response of the Boll Weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae), following Exposure to the Organophosphate Insecticide Malathion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Malathion
2.2. Weevil Samples
2.3. RNA Extraction and Sequencing
2.4. RNA-Seq Analysis
2.5. Acetylcholinesterase SNP Diversity across Populations
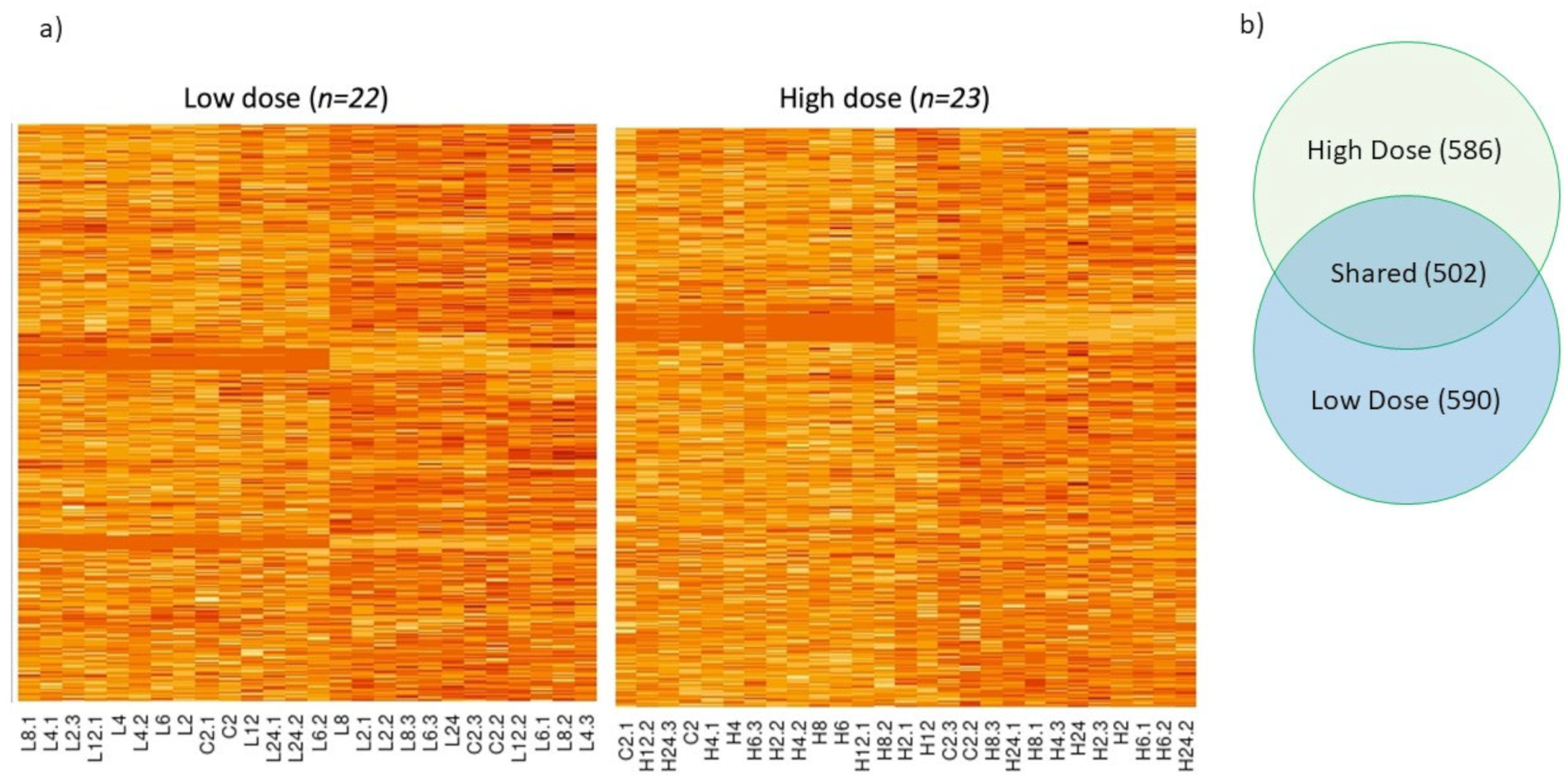
3. Results and Discussion
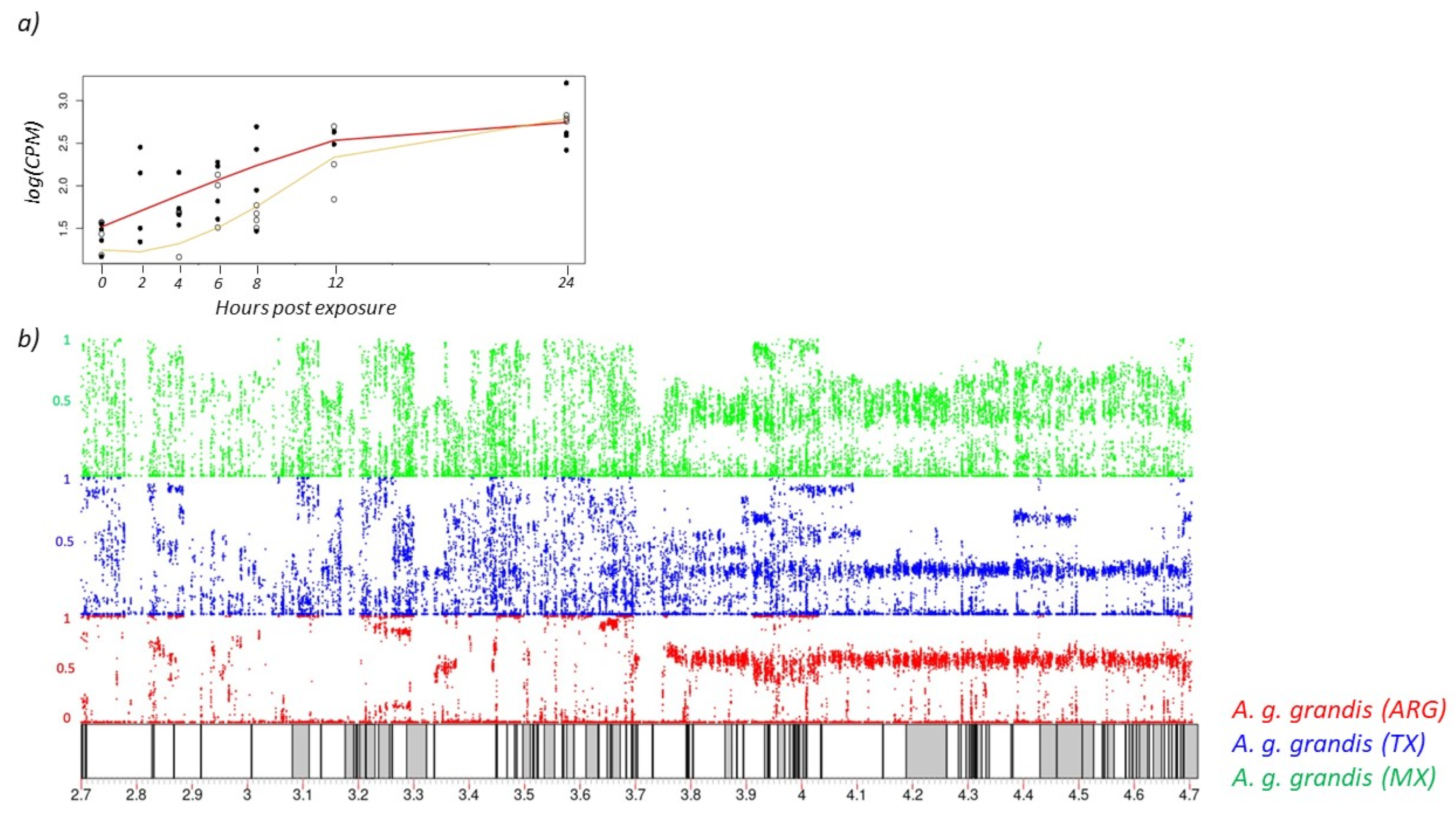
3.1. Acetylcholinesterase Expression Is Similar after Exposure to Low and High Concentrations of Malation
3.2. Population SNP Diversity and Lack of Selection at OP Target Site
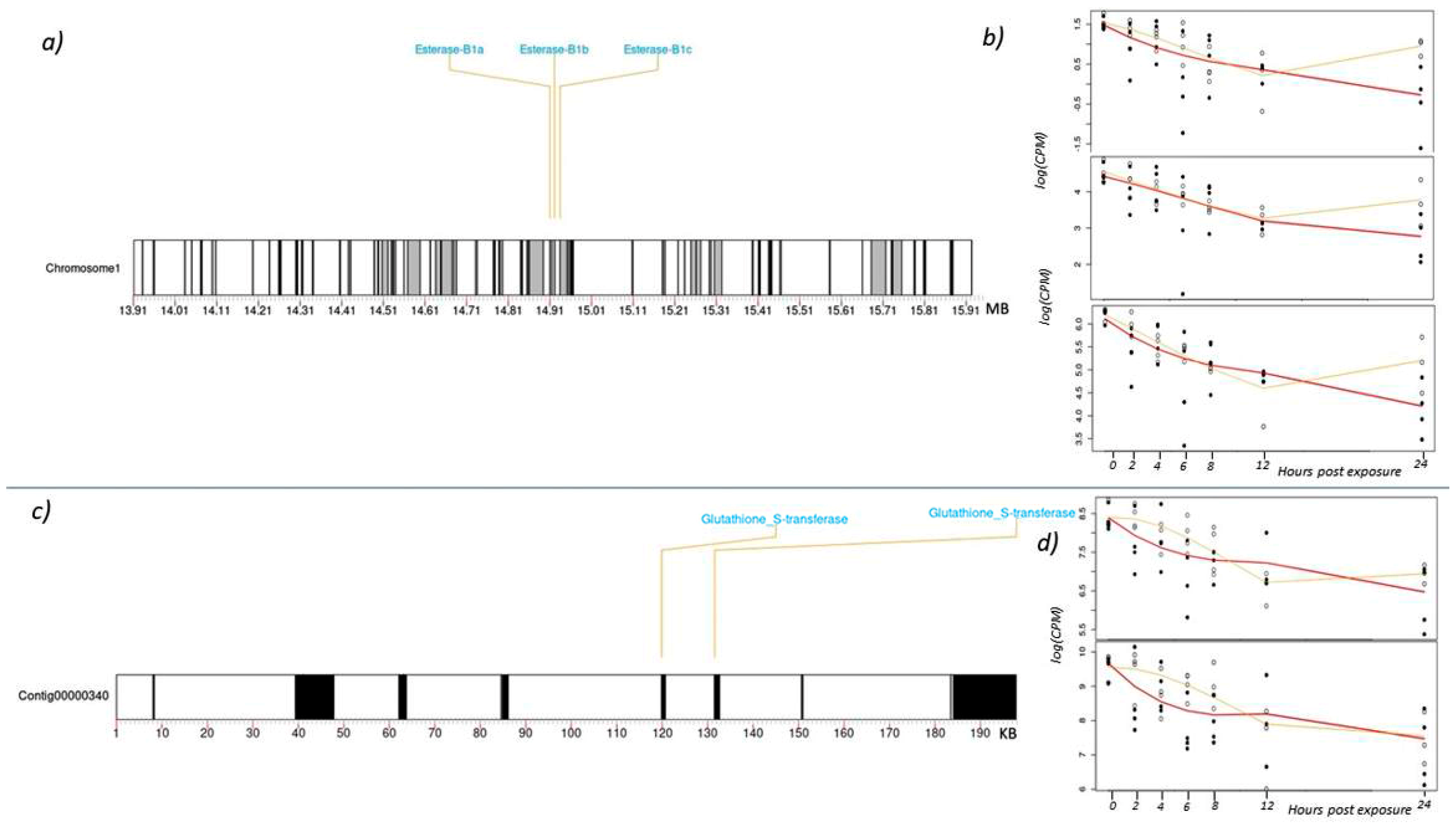
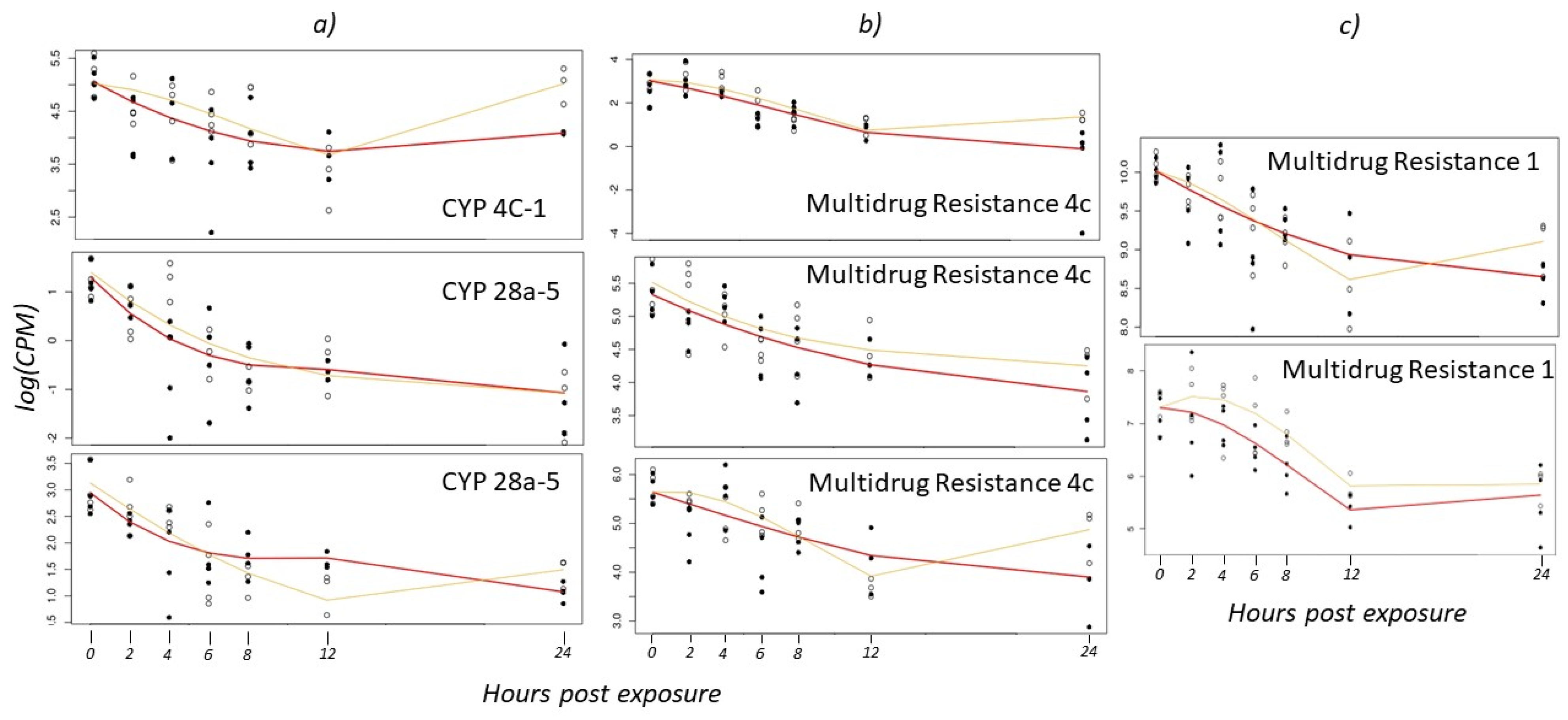
3.3. Expression of Other Detoxification Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burke, R.B.; Clark, W.E.; Cate, J.R.; Fryxell, P.A. Origin and dispersal of the boll weevil. Bull. ESA 1986, 32, 228–238. [Google Scholar] [CrossRef]
- Raszick, T.J.; Dickens, C.M.; Perkin, L.C.; Tessnow, A.E.; Suh, C.P.C.; Ruiz-Arce, R.; Boratynski, T.N.; Falco, M.R.; Johnston, J.S.; Sword, G.A. Population genomics and phylogeography of the boll weevil, Anthonomus grandis Boheman (Coleoptera: Curculionidae), in the United States, northern Mexico, and Argentina. Evol. Appl. 2021, 14, 1778–1793. [Google Scholar] [CrossRef]
- Texas Boll Weevil Eradication Foundation, I. Treatment. Available online: https://www.txbollweevil.org/treatment.html (accessed on 9 February 2023).
- Kanga, L.H.B.; Plapp, F.W.J.; Wall, M.L.; Karner, M.A.; Huffman, R.L.; Fuchs, T.W.; Elzen, G.W.; Martinez-Carrillo, J.L. Monitoring tolerance to insecticides in boll weevil populations (Coleoptera: Curculionidae) from Texas, Arkansas, Oklahoma, Mississippi, and Mexico. J. Econ. Entomol. 1995, 88, 198–204. [Google Scholar]
- Oliveira-Marra, S.O.D.; Guedes, R.N.C.; Bastos, C.S.; Marra, P.H.A.; Vivan, L.M.; Zanine, A.d.M. Insecticide resistance and control failure likelihood among populations of the boll weevil (Anthonomus grandis from Mato Grosso (Brazil). Acta Scientiarum. Agron. 2019, 41, e42714. [Google Scholar] [CrossRef] [Green Version]
- Rolim, G.G.; Coelho, R.R.; Antonino, J.D.; Arruda, L.S.; Rodrigues, A.S.; Barros, E.M.; Torres, J.B. Field-evolved resistance to beta-cyfluthrin in the boll weevil: Detection and characterization. Pest Manag. Sci. 2021, 77, 4400–4410. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, P.V.; Nemec, L.; Sronce, J. Monitoring for resistance to cypermethrin in budworm (H. virescens) and bollworm (H. zea) and to malathion in boll weevil in the Brazos river bottom, Texas. In Proceedings of the Beltwide Cotton Conferences, San Antonio, TX, USA, 4–8 January 2000; pp. 1236–1239. [Google Scholar]
- Campanhola, C.; Plapp, F.W., Jr. Pyrethroid resistance in the tobacco budworm (Lepidoptera: Noctuidae): Insecticide bioassays and field monitoring. J. Econ. Entomol. 1989, 82, 22–28. [Google Scholar] [CrossRef]
- Snodgrass, G.L.; Scott, W.P. Effect of ULV malathion use in boll weevil (Coleoptera: Curculionidae) eradication on resistance in the tarnished plant bug (Heteroptera: Miridae). J. Econ. Entomol. 2003, 96, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Evaluation of Five Organophosphate Insecticides and Herbicides. Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/MonographVolume112-1.pdf (accessed on 2 February 2023).
- International Agency for Research on Cancer. Malathion. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono112-07.pdf (accessed on 2 February 2023).
- Skouras, P.J.; Margaritopoulos, J.T.; Seraphides, N.A.; Ioannides, I.M.; Kakani, E.G.; Mathiopoulos, K.D.; Tsitsipis, J.A. Organophosphate resistance in olive fruit fly, Bactrocera oleae, populations in Greece and Cyprus. Pest Manag. Sci. 2007, 63, 42–48. [Google Scholar] [CrossRef]
- Osta, M.A.; Rizk, Z.J.; Labbé, P.; Weill, M.; Knio, K. Insecticide resistance to organophosphates in Culex pipiens complex from Lebanon. Parasites Vectors 2012, 5, 132. [Google Scholar] [CrossRef] [Green Version]
- Menozzi, P.; Shi, M.; Lougarre, A.; Tang, Z.; Fournier, D. Mutations of acetylcholinesterase which confer insecticide resistance in Drosophila melanogaster populations. BMC Evol. Biol. 2004, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alon, M.; Alon, F.; Morin, S. Organophosphates’ resistance in the B-biotype of Bemisia tabaci (Hemiptera: Aleyrodidae) is associated with a point mutation in an ace1 -type acetylcholinesterase and overexpression of carboxylesterase. Insect Biochem. Mol. Biol. 2008, 38, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwari, D.; Krishna Kumar, N.K.; Manjunatha, H. Multiple Mutations on the Second Acetylcholinesterase Gene Associated with Dimethoate Resistance in the Melon Aphid, Aphis gossypii (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.G.; Cosmidis, N.; Loukas, M.; Tsakas, S.; Hejazi, M.J.; Ayoutanti, A.; Hemingway, J. Altered Acetylcholinesterase Confers Organophosphate Resistance in the Olive Fruit Fly Bactrocera oleae. Pestic. Biochem. Physiol. 2001, 71, 124–132. [Google Scholar] [CrossRef]
- Khajehali, J.; Van Leeuwen, T.; Grispou, M.; Morou, E.; Alout, H.; Weill, M.; Tirry, L.; Vontas, J.; Tsagkarakou, A. Acetylcholinesterase point mutations in European strains of Tetranychus urticae (Acari: Tetranychidae) resistant to organophosphates. Pest Manag. Sci. 2010, 66, 220–228. [Google Scholar] [CrossRef]
- da Silva, N.M.; de Carvalho, R.A.; de Azeredo-Espin, A.M. Acetylcholinesterase cDNA sequencing and identification of mutations associated with organophosphate resistance in Cochliomyia hominivorax (Diptera: Calliphoridae). Vet Parasitol 2011, 177, 190–195. [Google Scholar] [CrossRef]
- Argentine, J.A.; Zhu, K.Y.; Lee, S.H.; Clark, J.M. Biochemical mechanisms of azinphosmethyl resistance in isogenic strains of Colorado potato beetle. Pestic. Biochem. Physiol. 1994, 48, 63–78. [Google Scholar] [CrossRef]
- Kim, H.J.; Yoon, K.S.; Clark, J.M. Functional analysis of mutations in expressed acetylcholinesterase that result in azinphosmethyl and carbofuran resistance in Colorado potato beetle. Pestic. Biochem. Physiol. 2007, 88, 181–190. [Google Scholar] [CrossRef]
- Wang, Y.; Wilson, A.E.; Liu, N. An Overview of Meta-Analysis for the Importance of the Overexpression of Detoxification Genes in Insecticide Resistance. Res. Sq. 2021; preprint. [Google Scholar] [CrossRef]
- Hemingway, J.; Hawkes, N.; Prapanthadara, L.; Jayawardenal, K.G.I.; Ranson, H. The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1998, 353, 1695–1699. [Google Scholar] [CrossRef]
- Oppenoorth, J.F.; van der Pas, L.J.T.; Houx, N.W.H. Glutathione S-transferase and hydrolytic activity in a tetrachlorvinphos-resistant strain of housefly and their influence on resistance. Pestic. Biochem. Physiol. 1979, 11, 176–188. [Google Scholar] [CrossRef]
- Chiang, F.-M.; Sun, C.-N. Glutathione transferase isozymes of diamondback moth larvae and their role in the degradation of some organophosphorus insecticides. Pestic. Biochem. Physiol. 1993, 45, 7–14. [Google Scholar] [CrossRef]
- Huang, H.S.; Hu, N.T.; Yao, Y.E.; Wu, C.Y.; Chiang, S.W.; Sun, C.N. Molecular cloning and heterologous expression of a glutathione S-transferase involved in insecticide resistance from the diamondback moth, Plutella Xylostella. Insect Biochem. Mol. Biol. 1998, 28, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.H.; Clark, A.G.; Syvanen, M. Identification and cloning of a key insecticide-metabolizing glutathione S-transferase (MdGST-6A) from hyper insecticide-resistant strain of the housefly Musca Domestica. Insect Biochem. Mol. Biol. 2001, 31, 1145–1153. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect CYP Genes and P450 Enzymes. In Insect Molecular Biology and Biochemistry; Gilbert, L., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 236–316. Available online: https://www.sciencedirect.com/science/article/pii/B978012384747810008X?via%3Dihub (accessed on 10 November 2022).
- Fragoso, D.B.; Guedes, R.N.C.; Picanço, M.C.; Zambolim, L. Insecticide use and organophosphate resistance in the coffee leaf miner Leucoptera coffeella (Lepidoptera: Lyonetiidae). Bull. Entomol. Res. 2002, 92, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, R.A.; Omoto, C.; Field, L.M.; Williamson, M.S.; Bass, C. Investigating the molecular mechanisms of organophosphate and pyrethroid resistance in the fall armyworm Spodoptera frugiperda. PLoS ONE 2013, 8, e62268. [Google Scholar] [CrossRef] [Green Version]
- Buss, D.S.; McCaffery, A.R.; Callaghan, A. Evidence for p-glycoprotein modification of insecticide toxicity in mosquitoes of the Culex pipiens complex. Med. Vet. Entomol. 2002, 16, 218–222. [Google Scholar] [CrossRef]
- Figueira-Mansur, J.; Ferreira-Pereira, A.; Mansur, J.F.; Franco, T.A.; Alvarenga, E.S.L.; Sorgine, M.H.F.; Neves, B.C.; Melo, A.C.A.; Leal, W.S.; Masuda, H.; et al. Silencing of P-glycoprotein increases mortality in temephos-treated Aedes aegypti larvae. Insect Mol. Biol. 2013, 22, 648–658. [Google Scholar] [CrossRef]
- Chen, Y.; McCarthy, D.; Robinson, M.; Smyth, G. edgeR: Differential Expression Analysis of Digital Gene Expression Data User’s Guide. Bioconductor User’s Guide. 2014. Available online: https://bioconductor.statistik.tu-dortmund.de/packages/3.10/bioc/vignettes/edgeR/inst/doc/edgeRUsersGuide.pdf (accessed on 10 November 2022).
- Alexa, A.; Rahnenfuhrer, J. TopGo: Enrichment Analysis for Gene Ontology, Version 2.40.0. R Package. 2020. Available online: https://bioconductor.org/packages/release/bioc/html/topGO.html (accessed on 10 November 2022).
- Cohen, Z.P.; Perkin, L.C.; Sim, S.B.; Stahlke, A.R.; Geib, S.M.; Childers, A.K.; Smith, T.P.L.; Suh, C. Insight into weevil biology from a reference quality genome of the boll weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae). G3 Genes Genomes Genet. 2022, G3, jkac309. [Google Scholar] [CrossRef]
- Gel, B.; Serra, E. karyoploteR: An R/Bioconductor package to plot customizable genomes displaying arbitrary data. Bioinformatics 2017, 33, 3088–3090. [Google Scholar] [CrossRef] [Green Version]
- Cook, D.E.; Andersen, E.C. VCF-kit: Assorted utilities for the variant call format. Bioinformatics 2017, 33, 1581–1582. [Google Scholar] [CrossRef] [Green Version]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Greenberg, D.S. Acetylcholinesterase involvement in apoptosis. Front. Mol. Neurosci. 2012, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.B.; Sawicki, R.M. Characterization of the resistance mechanisms to diazinon, parathion and diazoxon in the organophosphorus-resistant SKA strain of house flies (Musca domestica L.). Pestic. Biochem. Physiol. 1971, 1, 275–285. [Google Scholar] [CrossRef]
- Enayati, A.A.; Ranson, H.; Hemingway, J. Insect glutathione transferases and insecticide resistance. Insect Mol. Biol. 2005, 14, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.H.; Syvanen, M. A complex glutathione transferase gene family in the housefly Musca domestica. Mol. Gen. Genet. MGG 1997, 256, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Pavlidi, N.; Tseliou, V.; Riga, M.; Nauen, R.; Van Leeuwen, T.; Labrou, N.E.; Vontas, J. Functional characterization of glutathione S-transferases associated with insecticide resistance in Tetranychus urticae. Pestic. Biochem. Physiol. 2015, 121, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Reidy, G.F.; Rose, H.A.; Visetson, S.; Murray, M. Increased glutathione S-transferase activity and glutathione content in an insecticide-resistant strain of Tribolium castaneum (Herbst). Pestic. Biochem. Physiol. 1990, 36, 269–276. [Google Scholar] [CrossRef]
- Daborn, P.J.; Yen, J.L.; Bogwitz, M.R.; Goff, G.L.; Feil, E.; Jeffers, S.; Tijet, N.; Perry, T.; Heckel, D.; Batterham, P.; et al. A single P450 allele associated with insecticide resistance in Drosophila. Science 2002, 297, 2253–2256. [Google Scholar] [CrossRef]
- Gahan, L.J.; Gould, F.; Heckel, D.G. Identification of a gene associated with Bt resistance in Heliothis virescens. Science 2001, 293, 857–860. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Daborn, P.J.; Le Goff, G. The genetics and genomics of insecticide resistance. Trends Genet. 2004, 20, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Salvador, R.; Niz, J.M.; Nakaya, P.A.; Pedarros, A.; Hopp, H.E. Midgut Genes Knockdown by Oral dsRNA Administration Produces a Lethal Effect on Cotton Boll Weevil. Neotrop. Entomol. 2021, 50, 121–128. [Google Scholar] [CrossRef]
- Brattsten, L.B. Inducibility of metabolic insecticide defenses in boll weevils and tobacco budworm caterpillars. Pestic. Biochem. Physiol. 1987, 27, 13–23. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Porretta, D.; Gargani, M.; Bellini, R.; Medici, A.; Punelli, F.; Urbanelli, S. Defence mechanisms against insecticides temephos and diflubenzuron in the mosquito Aedes caspius: The P-glycoprotein efflux pumps. Med. Vet. Entomol. 2008, 22, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Bariami, V.; Jones, C.M.; Vontas, J.; Ranson, H. Gene amplification, ABC transporters and cytochrome P450s: Unraveling the molecular basis of pyrethroid resistance in the dengue vector, Aedes Aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matowo, J.; Jones, C.M.; Kabula, B.; Ranson, H.; Steen, K.; Mosha, F.; Rowland, M.; Weetman, D. Genetic basis of pyrethroid resistance in a population of Anopheles arabiensis, the primary malaria vector in Lower Moshi, north-eastern Tanzania. Parasites Vectors 2014, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Yan, Z.; Si, F.; Zhou, Y.; Fu, W.; Chen, B. ATP-Binding Cassette (ABC) Transporter Genes Involved in Pyrethroid Resistance in the Malaria Vector Anopheles sinensis: Genome-Wide Identification, Characteristics, Phylogenetics, and Expression Profile. Int. J. Mol. Sci. 2019, 20, 1409. [Google Scholar] [CrossRef] [Green Version]
- Mamidala, P.; Wijeratne, A.J.; Wijeratne, S.; Kornacker, K.; Sudhamalla, B.; Rivera-Vega, L.J.; Hoelmer, A.; Meulia, T.; Jones, S.C.; Mittapalli, O. RNA-Seq and molecular docking reveal multi-level pesticide resistance in the bed bug. BMC Genom. 2012, 13, 6. [Google Scholar] [CrossRef] [Green Version]
- Gaddelapati, S.C.; Kalsi, M.; Roy, A.; Palli, S.R. Cap ‘n’ collar C regulates genes responsible for imidacloprid resistance in the Colorado potato beetle, Leptinotarsa decemlineata. Insect. Biochem. Mol. Biol. 2018, 99, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rösner, J.; Merzendorfer, H. Identification of two ABCC transporters involved in malathion detoxification in the red flour beetle, Tribolium castaneum. Insect Sci. 2022, 29, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Labbé, R.; Caveney, S.; Donly, C. Expression of multidrug resistance proteins is localized principally to the Malpighian tubules in larvae of the cabbage looper moth, Trichoplusia ni. J. Exp. Biol. 2011, 214, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.Z.; Xu, J.P.; Wang, X.Y.; Ma, Y.; Yu, D.; Fei, D.Q.; Zhang, S.Z.; Wang, W.L. Identification of Four ATP-Binding Cassette Transporter Genes in Cnaphalocrocis medinalis and Their Expression in Response to Insecticide Treatment. J. Insect. Sci. 2017, 17, 44. [Google Scholar] [CrossRef] [Green Version]
- Onstad, D.W. Insect Resistance Management: Biology, Economics, and Prediction; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perkin, L.C.; Cohen, Z.P.; Carlson, J.W.; Suh, C.P.-C. The Transcriptomic Response of the Boll Weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae), following Exposure to the Organophosphate Insecticide Malathion. Insects 2023, 14, 197. https://doi.org/10.3390/insects14020197
Perkin LC, Cohen ZP, Carlson JW, Suh CP-C. The Transcriptomic Response of the Boll Weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae), following Exposure to the Organophosphate Insecticide Malathion. Insects. 2023; 14(2):197. https://doi.org/10.3390/insects14020197
Chicago/Turabian StylePerkin, Lindsey C., Zachary P. Cohen, Jason W. Carlson, and Charles P.-C. Suh. 2023. "The Transcriptomic Response of the Boll Weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae), following Exposure to the Organophosphate Insecticide Malathion" Insects 14, no. 2: 197. https://doi.org/10.3390/insects14020197
APA StylePerkin, L. C., Cohen, Z. P., Carlson, J. W., & Suh, C. P.-C. (2023). The Transcriptomic Response of the Boll Weevil, Anthonomus grandis grandis Boheman (Coleoptera: Curculionidae), following Exposure to the Organophosphate Insecticide Malathion. Insects, 14(2), 197. https://doi.org/10.3390/insects14020197




