Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Measured Parameters
- -
- Content of macronutrients on a dry matter basis (%):
- -
- Content of individual fatty acids (FA) on dry matter basis (%):
2.3. Statistical Analysis
3. Results
3.1. Substrate Nutrient Composition
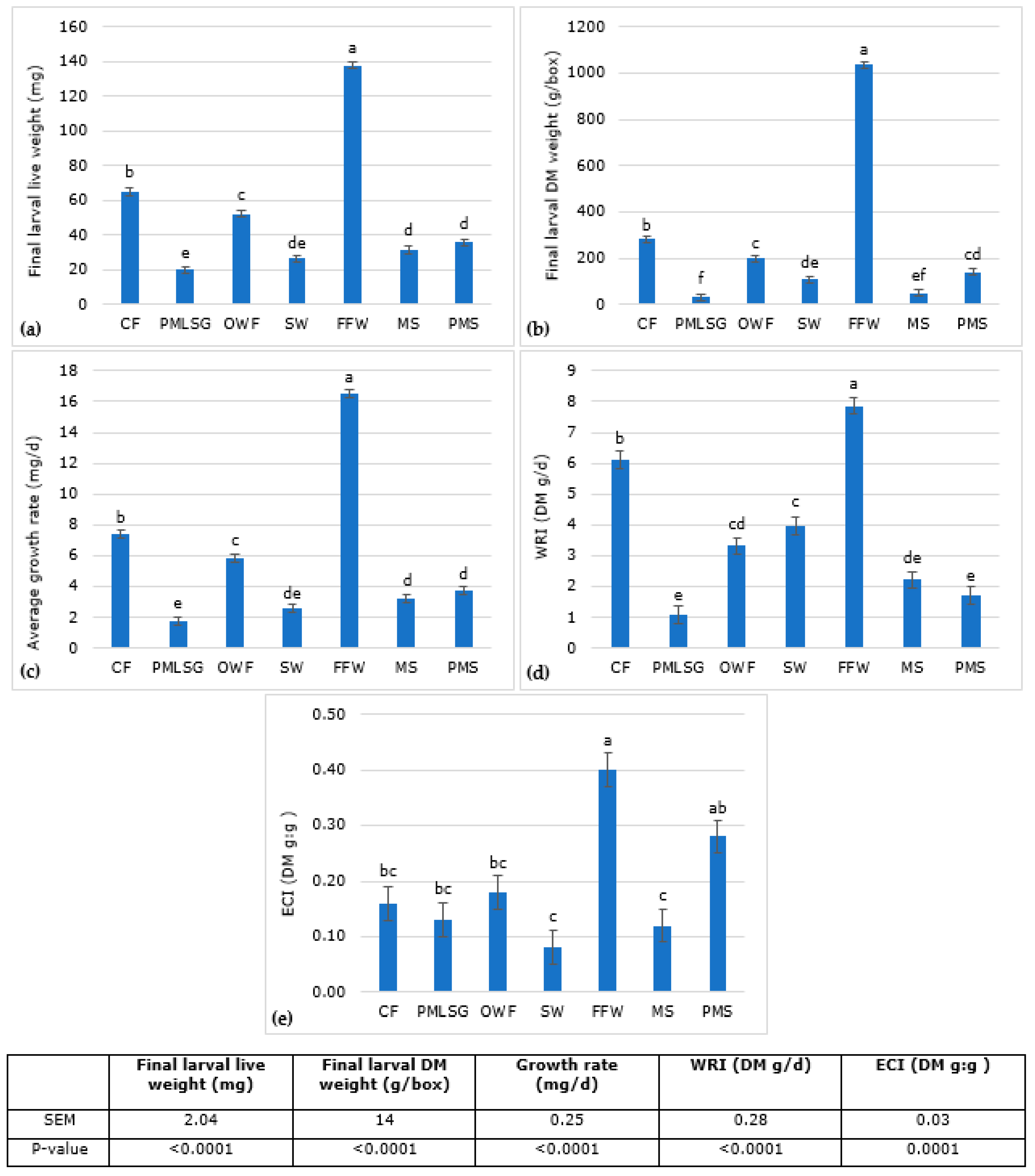
3.2. Substrate Nutrient Composition Larval Growth Performance, Waste Reduction and Efficiency of Conversion
3.3. Larval Chemical Composition and Fatty Acid Profile
3.4. Frass Chemical Composition
3.5. Larval Crude Protein and Fat Masses, Conversions and Losses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forbes, H.; Quested, T.; O’Connor, C. UNEP Food Waste Index Report. 2021. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 21 January 2023).
- Eufic. The EU Farm to Fork Strategy: Can We Make the European Food System Healthier and Sustainable? Food Facts for Healthy Choices 2022. 7 March 2022. Available online: https://www.eufic.org/en/food-production/article/the-eu-farm-to-fork-strategy-can-we-make-the-european-food-system-healthier-and-sustainable (accessed on 21 January 2023).
- United Nations—Department of Economic and Social Affairs—Sustainable Development. Do You Know All 17 SDGs? 2015. Available online: https://sdgs.un.org/goals (accessed on 21 January 2023).
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A.; Dicke, M.; van Loon, J. Insects to feed the world. J. Insects Food Feed. 2015, 1, 3–5. [Google Scholar] [CrossRef]
- United Nations—Department of Economic and Social Affairs—Population Division. World Population Prospects. 2022. Available online: https://population.un.org/wpp/ (accessed on 21 January 2023).
- Dunkel, F.; Payne, C. Introduction to edible insects. In Insects as Sustainable Food Ingredients; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- OECD and FAO, OECD-FAO Agricultural Outlook 2021–2030. 2021. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook-2021-2030_19428846-en (accessed on 21 January 2023).
- Van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Pastor, B.; Velásquez, Y.; Gobbi, P.; Rojo, S. Conversion of organic wastes into fly larval biomass: Bottlenecks and challenges. J. Insects Food Feed. 2015, 1, 179–193. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Yuwono, N.W.; Nisa’, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Smets, R.; Verbinnen, B.; Van De Voorde, I.; Aerts, G.; Claes, J.; Van Der Borght, M. Sequential Extraction and Characterisation of Lipids, Proteins, and Chitin from Black Soldier Fly (Hermetia illucens) Larvae, Prepupae, and Pupae. Waste Biomass-Valorization 2020, 11, 6455–6466. [Google Scholar] [CrossRef]
- Addeo, N.F.; Vozzo, S.; Secci, G.; Mastellone, V.; Piccolo, G.; Lombardi, P.; Parisi, G.; Asiry, K.A.; Attia, Y.A.; Bovera, F. Different Combinations of Butchery and Vegetable Wastes on Growth Performance, Chemical-Nutritional Characteristics and Oxidative Status of Black Soldier Fly Growing Larvae. Animals 2021, 11, 3515. [Google Scholar] [CrossRef]
- Spranghers, T.; Ottoboni, M.; Klootwijk, C.; Ovyn, A.; Deboosere, S.; De Meulenaer, B.; Michiels, J.; Eeckhout, M.; De Clercq, P.; De Smet, S. Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J. Sci. Food Agric. 2017, 97, 2594–2600. [Google Scholar] [CrossRef]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- Scieuzo, C.; Franco, A.; Salvia, R.; Triunfo, M.; Addeo, N.F.; Vozzo, S.; Piccolo, G.; Bovera, F.; Ritieni, A.; Di Francia, A.; et al. Enhancement of fruit byproducts through bioconversion by Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023. [Google Scholar] [CrossRef]
- Franco, A.; Scieuzo, C.; Salvia, R.; Mancini, I.; Caniani, D.; Masi, S.; Falabella, P. A mobile black soldier fly farm for on-site disposal of animal dairy manure. Bull. Insectol. 2022, 75, 75–82. [Google Scholar]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource: An overview. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Bockisch, M. Handbuch der Lebensmitteltechnologie: Nahrungsfette Und–Oele; Eugen Ulmer GmBH & Co.: Stuttgart, Germany, 1993; pp. 169–262. [Google Scholar]
- Matthäus, B.; Piofczyk, T.; Katz, H.; Pudel, F. Renewable Resources from Insects: Exploitation, Properties, and Refining of Fat Obtained by Cold-Pressing from Hermetia illucens (Black Soldier Fly) Larvae. Eur. J. Lipid Sci. Technol. 2019, 121, 1800376. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Sustainable waste management using black soldier fly larva: A review. Int. J. Environ. Sci. Technol. 2021, 19, 12701–12726. [Google Scholar] [CrossRef]
- Fadhillah, N.; Bagastyo, A.Y. Utilization of Hermetia illucens Larvae as A Bioconversion Agent to Reduce Organic Waste. IOP Conf. Ser. Earth Environ. Sci. 2020, 506, 012005. [Google Scholar] [CrossRef]
- Tanga, C.M.; Waweru, J.W.; Tola, Y.H.; Onyoni, A.A.; Khamis, F.M.; Ekesi, S.; Paredes, J.C. Organic Waste Substrates Induce Important Shifts in Gut Microbiota of Black Soldier Fly (Hermetia illucens L.): Coexistence of Conserved, Variable, and Potential Pathogenic Microbes. Front. Microbiol. 2021, 12, 635881. [Google Scholar] [CrossRef]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiab, K.K.M.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C.; Fiaboe, K.K.M.; et al. The nutritive value of black soldier fly larvae reared on common organic waste streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [Green Version]
- Barragan-Fonseca, K.B.; Dicke, M.; Van Loon, J.J.A. Nutritional value of the black soldier fly (Hermetia illucens L.) and its suitability as animal feed—A review. J. Insects Food Feed 2017, 3, 105–120. [Google Scholar] [CrossRef]
- Chia, S.Y.; Tanga, C.M.; Osuga, I.M.; Cheseto, X.; Ekesi, S.; Dicke, M.; van Loon, J.J. Nutritional composition of black soldier fly larvae feeding on agro-industrial by-products. Entomol. Exp. Appl. 2020, 168, 472–481. [Google Scholar] [CrossRef]
- Shumo, M.; Khamis, F.; Tanga, C.M.; Fiaboe, K.K.; Subramanian, S.; Ekesi, S.; Van Huis, A.; Borgemeister, C. Influence of Temperature on Selected Life-History Traits of Black Soldier Fly (Hermetia illucens) Reared on Two Common Urban Organic Waste Streams in Kenya. Animals 2019, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewald, N.; Vidakovic, A.; Langeland, M.; Kiessling, A.; Sampels, S.; Lalander, C. Fatty acid composition of black soldier fly larvae (Hermetia illucens)—Possibilities and limitations for modification through diet. Waste Manag. 2020, 102, 40–47. [Google Scholar] [CrossRef]
- Tschirner, M.; Simon, A. Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J. Insects Food Feed. 2015, 1, 249–259. [Google Scholar] [CrossRef]
- Fuso, A.; Barbi, S.; Macavei, L.; Luparelli, A.; Maistrello, L.; Montorsi, M.; Sforza, S.; Caligiani, A. Effect of the Rearing Substrate on Total Protein and Amino Acid Composition in Black Soldier Fly. Foods 2021, 10, 1773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Chiu, S.L.; Lo, I.M. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef]
- Veldkamp, T.; van Rozen, K.; Elissen, H.; van Wikselaar, P.; van der Weide, R. Bioconversion of Digestate, Pig Manure and Vegetal Residue-Based Waste Operated by Black Soldier Fly Larvae, Hermetia illucens L. (Diptera: Stratiomyidae). Animals 2021, 11, 3082. [Google Scholar] [CrossRef]
- Miranda, C.D.; Cammack, J.A.; Tomberlin, J.K. Mass Production of the Black Soldier Fly, Hermetia illucens (L.), (Diptera: Stratiomyidae) Reared on Three Manure Types. Animals 2020, 10, 1243. [Google Scholar] [CrossRef]
- Julita, U.; Suryani, Y.; Kinasih, I.; Yuliawati, A.; Cahyanto, T.; Maryeti, Y.; Permana, A.D.; Fitri, L.L. Growth performance and nutritional composition of black soldier fly, Hermetia illucens (L), (Diptera : Stratiomyidae) reared on horse and sheep manure. IOP Conf. Series Earth Environ. Sci. 2018, 187, 012071. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed Conversion, Survival and Development, and Composition of Four Insect Species on Diets Composed of Food By-Products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Dzepe, D.; Nana, P.; Kuietche, H.M.; Kimpara, J.M.; Magatsing, O.; Tchuinkam, T.; Djouaka, R. Feeding strategies for small-scale rearing black soldier fly larvae (Hermetia illucens) as organic waste recycler. SN Appl. Sci. 2021, 3, 252. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. J. Math. Methods Biosci. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksen, N.T. Dynamic modelling of feed assimilation, growth, lipid accumulation, and CO2 production in black soldier fly larvae. PLoS ONE 2022, 17, e0276605. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, X.; Wang, H.; Yang, Q.; ur Rehman, K.; Li, W.; Cai, M.; Li, Q.; Mazza, L.; Zhang, J.; et al. Dynamic changes of nutrient composition throughout the entire life cycle of black soldier fly. PLoS ONE 2017, 12, e0182601. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of Black Soldier Fly (Diptera: Stratiomyidae) Larvae to Recycle Food Waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Eggink, K.M.; Lund, I.; Pedersen, P.B.; Hansen, B.W.; Dalsgaard, J. Biowaste and by-products as rearing substrates for black soldier fly (Hermetia illucens) larvae: Effects on larval body composition and performance. PLoS ONE 2022, 17, e0275213. [Google Scholar] [CrossRef]
- Gold, M.; Cassar, C.M.; Zurbrügg, C.; Kreuzer, M.; Boulos, S.; Diener, S.; Mathys, A. Biowaste treatment with black soldier fly larvae: Increasing performance through the formulation of biowastes based on protein and carbohydrates. Waste Manag. 2020, 102, 319–329. [Google Scholar] [CrossRef]
- Fischer, H.; Romano, N. Fruit, vegetable, and starch mixtures on the nutritional quality of black soldier fly (Hermetia illucens) larvae and resulting frass. J. Insects Food Feed. 2021, 7, 319–327. [Google Scholar] [CrossRef]
- Danieli, P.P.; Lussiana, C.; Gasco, L.; Amici, A.; Ronchi, B. The Effects of Diet Formulation on the Yield, Proximate Composition, and Fatty Acid Profile of the Black Soldier Fly (Hermetia illucens L.) Prepupae Intended for Animal Feed. Animals 2019, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Minor, M.; Morel, P.C.H.; Najar-Rodriguez, A.J. Bioconversion of Three Organic Wastes by Black Soldier Fly (Diptera: Stratiomyidae) Larvae. Environ. Entomol. 2018, 47, 1609–1617. [Google Scholar] [CrossRef]
- Ramzy, R.R.; El-Dakar, M.A.; Wang, D.; Ji, H. Conversion Efficiency of Lignin-Rich Olive Pomace to Produce Nutrient-Rich Insect Biomass by Black Soldier Fly Larvae, Hermetia illucens. Waste Biomass-Valorization 2022, 13, 893–903. [Google Scholar] [CrossRef]
- Zheng, L.; Hou, Y.; Li, W.; Yang, S.; Li, Q.; Yu, Z. Biodiesel production from rice straw and restaurant waste employing black soldier fly assisted by microbes. Energy 2012, 47, 225–229. [Google Scholar] [CrossRef]
- Li, X.; Dong, Y.; Sun, Q.; Tan, X.; You, C.; Huang, Y.; Zhou, M. Growth and Fatty Acid Composition of Black Soldier Fly Hermetia illucens (Diptera: Stratiomyidae) Larvae Are Influenced by Dietary Fat Sources and Levels. Animals 2022, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Yun, E.-Y.; Goo, T.-W. Evaluation of Antimicrobial Activity in the Extract of Defatted Hermetia illucens Fed Organic Waste Feed Containing Fermented Effective Microorganisms. Animals 2022, 12, 680. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Romano, N.; Sinha, A. Conversion of Spent Coffee and Donuts by Black Soldier Fly (Hermetia illucens) Larvae into Potential Resources for Animal and Plant Farming. Insects 2021, 12, 332. [Google Scholar] [CrossRef] [PubMed]
- Romano, N.; Fischer, H.; Kumar, V.; Francis, S.A.; Sinha, A.K. Productivity, conversion ability, and biochemical composition of black soldier fly (Hermetia illucens) larvae fed with sweet potato, spent coffee or dough. Int. J. Trop. Insect Sci. 2021, 42, 183–190. [Google Scholar] [CrossRef]
- Bekker, N.S.; Heidelbach, S.; Vestergaard, S.Z.; Nielsen, M.E.; Riisgaard-Jensen, M.; Zeuner, E.J.; Bahrndorff, S.; Eriksen, N.T. Impact of substrate moisture content on growth and metabolic performance of black soldier fly larvae. Waste Manag. 2021, 127, 73–79. [Google Scholar] [CrossRef] [PubMed]
- EL Deen, S.N.; Spranghers, T.; Baldacchino, F.; Deruytter, D. The effects of the particle size of four different feeds on the larval growth of Tenebrio molitor (Coleoptera: Tenebrionidae). Eur. J. Entomol. 2022, 119, 242–249. [Google Scholar] [CrossRef]
- Liland, N.S.; Biancarosa, I.; Araujo, P.; Biemans, D.; Bruckner, C.G.; Waagbø, R.; Torstensen, B.E.; Lock, E.-J. Modulation of nutrient composition of black soldier fly (Hermetia illucens) larvae by feeding seaweed-enriched media. PLoS ONE 2017, 12, e0183188. [Google Scholar] [CrossRef] [Green Version]
- Kebli, H.; Sinaj, S. Agronomic potential of a natural fertiliser based on fly larvae frass. Rech. Agron. Suisse 2017, 88–95. Available online: https://www.agrarforschungschweiz.ch/en/2017/03/agronomic-potential-of-a-natural-fertiliser-based-on-fly-larvae-frass/ (accessed on 21 January 2023).
- Klammsteiner, T.; Turan, V.; Juárez, M.F.-D.; Oberegger, S.; Insam, H. Suitability of Black Soldier Fly Frass as Soil Amendment and Implication for Organic Waste Hygienization. Agronomy 2020, 10, 1578. [Google Scholar] [CrossRef]
- Lopes, I.G.; Lalander, C.; Vidotti, R.M.; Vinnerås, B. Using Hermetia illucens larvae to process biowaste from aquaculture production. J. Clean. Prod. 2020, 251, 119753. [Google Scholar] [CrossRef]
- Chiam, Z.; Lee, J.T.E.; Tan, J.K.N.; Song, S.; Arora, S.; Tong, Y.W.; Tan, H.T.W. Evaluating the potential of okara-derived black soldier fly larval frass as a soil amendment. J. Environ. Manag. 2021, 286, 112163. [Google Scholar] [CrossRef] [PubMed]
- Tanga, C.; Beesigamukama, D.; Kassie, M.; Egonyu, P.; Ghemoh, C.J.; Nkoba, K.; Subramanian, S.; Anyega, A.; Ekesi, S. Performance of black soldier fly frass fertiliser on maize (Zea mays L.) growth, yield, nutritional quality, and economic returns. J. Insects Food Feed. 2022, 8, 185–196. [Google Scholar] [CrossRef]
- Gärttling, D.; Kirchner, S.M.; Schulz, H. Assessment of the N- and P-Fertilization Effect of Black Soldier Fly (Diptera: Stratiomyidae) By-Products on Maize. J. Insect Sci. 2020, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.O.; Lind, A.-M.; Sommer, S.G. Nitrogen and organic matter losses during storage of cattle and pig manure. J. Agric. Sci. 1998, 130, 69–79. [Google Scholar] [CrossRef]


| Substrate | Source | Quantity of Wet Substrate (kg) | Number of BSFL |
|---|---|---|---|
| Chicken feed (CF; control diet) | Agruniek Rijnvallei Voer BV (Wageningen, The Netherlands) | 8.0 | 14,800 |
| Pig manure slurry mixed with roadside silage grass (PMLSG) | Van Beek SPF Varkens B.V. (Lelystad, The Netherlands) | 5.0 | 9250 |
| Organic wet fraction (OWF) | Attero Holding N.V. (Arnhem, The Netherlands) | 10.0 | 18,500 |
| Secondary sludge from slaughter waste (SW) | Esbro (Doetinchem, The Netherlands) | 10.0 | 18,500 |
| Fast food waste (FFW) | McDonald’s restaurants (The Netherlands) | 10.0 | 18,500 |
| Mushroom stems (MS) | CNC Grondstoffen BV (Milsbeek, The Netherlands) | 5.0 | 9250 |
| Pig manure solid (PMS) | Van Beek SPF Varkens B.V. (Lelystad, The Netherlands) | 9.0 | 16,650 |
| Parameter | CF | PMLSG | OWF | SW | FFW | MS | PMS | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 39.80 a | 31.40 c | 35.77 ac | 34.33 bc | 38.33 ab | 32.97 c | 35.73 ac | 0.98 | 0.00075 |
| Crude ash | 5.19 c | 5.45 c | 37.79 a | 3.49 c | 3.13 c | 3.44 c | 15.40 b | 0.80 | <0.0001 |
| Crude protein | 19.77 b | 8.22 d | 6.87 d | 26.60 a | 18.09 b | 6.18 d | 15.86 c | 0.42 | <0.0001 |
| Total fat | 5.28 bc | - | 4.39 c | 27.38 a | 27.74 a | - | 5.98 b | 0.22 | <0.0001 |
| Crude fiber | 6.20 c | 55.54 a | 29.30 b | 26.50 b | 1.13 d | 58.62 a | 26.02 b | 0.81 | <0.0001 |
| Starch | 42.88 | - | - | - | 45.92 | - | - | 0.66 | 0.08336 |
| N-free substances | 63.57 a | 30.80 c | 21.66 d | 16.02 d | 49.91 b | 31.77 c | 36.74 c | 1.61 | <0.0001 |
| Calcium | 0.70 b | 0.74 b | 3.12 a | 0.89 b | 0.13 c | 0.71 b | 3.13 a | 0.08 | <0.0001 |
| Phosphorus | 0.64 b | 0.31 cd | 0.19 e | 0.40 c | 0.24 de | 0.16 e | 1.50 a | 0.02 | <0.0001 |
| pH | 5.26 e | 7.69 b | 5.79 c | 5.67 cd | 4.79 f | 5.38 de | 8.35 a | 0.08 | <0.0001 |
| Parameter | CF | PMLSG | OWF | SW | FFW | MS | PMS | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Capric acid C 10:0 | 0.13 b | - | 0.01 b | 0.08 b | 0.32 a | - | 0.04 b | 0.03 | 0.0002 |
| Lauric acid C 12:0 | 5.40 b | - | 0.42 c | 2.05 bc | 13.44 a | - | 1.23 c | 0.83 | <0.0001 |
| Myristic acid C 14:0 | 1.36 ab | - | 0.22 c | 0.72 bc | 2.46 a | - | 0.45 bc | 0.23 | 0.0009 |
| Palmitic acid C 16:0 | 2.17 | - | 1.64 | 4.57 | 4.94 | - | 3.02 | 1.23 | 0.3222 |
| Sum saturated fatty acids | 9.72 b | - | 2.65 b | 8.32 b | 22.49 a | - | 5.45 b | 2.42 | 0.0034 |
| Sum monounsaturated fatty acids | 3.16 ab | - | 2.40 b | 6.42 ab | 11.04 a | - | 4.38 ab | 1.73 | 0.0459 |
| Sum polyunsaturated fatty acids | 2.99 | - | 1.28 | 3.04 | 5.41 | - | 1.97 | 0.85 | 0.0674 |
| Sum trans fatty acids | 0.12 | - | 0.05 | 0.22 | 0.26 | - | 0.15 | 0.05 | 0.1028 |
| Omega-3 fatty acids | 0.25 | - | 0.14 | 0.28 | 0.42 | - | 0.15 | 0.08 | 0.1711 |
| Omega-6 fatty acids | 2.66 ab | - | 1.12 b | 2.67 ab | 4.86 a | - | 1.77 ab | 0.75 | 0.0609 |
| Parameter | CF | PMLSG | OWF | SW | FFW | MS | PMS | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Dry matter | 71.90 a | 53.87 c | 45.64 d | 59.33 bc | 78.00 a | 63.03 b | 55.87 bc | 1.49 | <0.0001 |
| Crude ash | 7.80 c | 5.64 cd | 39.82 a | 4.55 cd | 6.28 cd | 3.75 d | 16.82 b | 0.66 | <0.0001 |
| Crude protein | 23.57 b | 6.57 d | 6.96 d | 31.44 a | 22.73 b | 5.07 d | 14.98 c | 0.43 | <0.0001 |
| Total fat | 2.86 c | - | 1.24 c | 9.79 b | 26.45 a | - | - | 0.66 | <0.0001 |
| Crude fiber | 26.92 d | 61.07 b | 35.75 c | 35.14 c | 10.98 e | 67.59 a | 32.56 c | 0.90 | <0.0001 |
| N-free substances | 38.85 a | 26.73 b | 16.23 d | 19.07 cd | 33.56 a | 23.58 bc | 35.04 a | 1.27 | <0.0001 |
| Calcium | 0.81 bc | 0.65 bc | 2.30 ab | 1.06 bc | 0.07 c | 0.55 bc | 3.30 a | 0.38 | 0.0007 |
| Phosphorus | 0.96 b | 0.30 d | 0.18 e | 0.49 c | 0.34 d | 0.17 e | 1.71 a | 0.02 | <0.0001 |
| pH | 8.01 abc | 8.72 ab | 7.34 cd | 7.67 bd | 6.81 d | 7.96 abc | 8.87 a | 0.22 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naser El Deen, S.; van Rozen, K.; Elissen, H.; van Wikselaar, P.; Fodor, I.; van der Weide, R.; Hoek-van den Hil, E.F.; Rezaei Far, A.; Veldkamp, T. Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae). Insects 2023, 14, 204. https://doi.org/10.3390/insects14020204
Naser El Deen S, van Rozen K, Elissen H, van Wikselaar P, Fodor I, van der Weide R, Hoek-van den Hil EF, Rezaei Far A, Veldkamp T. Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae). Insects. 2023; 14(2):204. https://doi.org/10.3390/insects14020204
Chicago/Turabian StyleNaser El Deen, Somaya, Klaas van Rozen, Hellen Elissen, Piet van Wikselaar, Istvan Fodor, Rommie van der Weide, Elise Federica Hoek-van den Hil, Arya Rezaei Far, and Teun Veldkamp. 2023. "Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae)" Insects 14, no. 2: 204. https://doi.org/10.3390/insects14020204
APA StyleNaser El Deen, S., van Rozen, K., Elissen, H., van Wikselaar, P., Fodor, I., van der Weide, R., Hoek-van den Hil, E. F., Rezaei Far, A., & Veldkamp, T. (2023). Bioconversion of Different Waste Streams of Animal and Vegetal Origin and Manure by Black Soldier Fly Larvae Hermetia illucens L. (Diptera: Stratiomyidae). Insects, 14(2), 204. https://doi.org/10.3390/insects14020204






