Simple Summary
Many species from the Polypedilum generic complex are renowned for their roles in aquatic ecosystems. This genus complex, as one of the most species-rich groups, has been persistently contentious. The current lack of sequence data for the Polypedilum complex limits our comprehension of their species evolution. Here, 14 mitogenomes of the Polypedilum generic complex were sequenced. Combined with three recently published mitochondrial genomes, we analyzed the features of the mitogenomes among genera. Furthermore, the phylogenetic analysis among genera within the Polypedilum complex showed that the Endochironomus + Synendotendipes is the sister group of Phaenopsectra + Sergentia. Our study provides a vital foundation for further study on the evolutionary biology of Chironomidae.
Abstract
Mitochondrial genomics, as a useful marker for phylogenetics and systematics of organisms, are important for molecular biology studies. The phylogenetic relationships of the Polypedilum generic complex remains controversial, due to lack taxonomy and molecular information. In this study, we newly sequenced mitogenomes of 14 species of the Polypedilum generic complex. Coupled with three recently published sequences, we analyzed the nucleotide composition, sequence length, and evolutionary rate of this generic complex. The control region showed the highest AT content. The evolution rate of protein coding genes was as follows: ATP8 > ND6 > ND5 > ND3 > ND2 > ND4L > ND4 > COX1 > ND1 > CYTB > APT6 > COX2 > COX3. We reconstructed the phylogenetic relationships among the genera within the Polypedilum generic complex based on 19 mitochondrial genomes (seventeen ingroups and two outgroups), using Bayesian Inference (BI) and Maximum Likelihood (ML) methods for all databases. Phylogenetic analysis of 19 mitochondrial genomes demonstrated that the Endochironomus + Synendotendipes was sister to Phaenopsectra + Sergentia.
1. Introduction
Mitochondrial genomes are important molecular markers that have usually been used for studies on phylogeny, evolutionary history, speciation and phylogeography in insect groups [1,2,3], benefit by the maternal inheritance, high substitution, and easy availability [4,5]. Mitogenomes are known as the second genetic information system, because of its special genetic characteristics [6]. The mitochondrial genome length of insects ranged from 14,000 to 20,000 bp mostly [1], containing two ribosomal RNAs (rRNAs), thirteen protein-coding genes (PCGs), 22 transfer RNA (tRNAs), and one non-coding control region (CR) [7]. The mitogenomes structure of insects are conserved, with the same genes arrange as the ancestral insect. In addition, the structural features of mitogenomes can provide more information and evidence for morphological classification. The number of complete mitogenomes of insects and Chironomidae have gradually increased, quickening with advances in next-generation sequencing, to resolve the evolutionary history and structure comparison in different taxonomic levels [3,5,7,8,9,10].
Chironomids is one of the most important aquatic insect groups. Almost all Chironomidae species have well-developed resilience and resistance in environmental stressors [3,5,11,12]. Chironomidae is a remarkable cosmopolitan and diverse group, with more than 7500 described species worldwide [11]. Their larvae inhabit varied habitats, including semi-aquatic, terrestrial, and freshwater habitats [12]. Most species sensitively perceive the environmental changes in trophic state temperature, and salinity or acidity, which make them valuable indicator organisms for the aquatic ecosystem [13].
The Polypedilum generic complex belongs to the tribe Chironomini of the subfamily Chironominae, containing Ainurusurika, Endochironomus, Endotribelos, Phaenopsectra, Polypedilum, Sergentia, Stictochironomus, Synendotendipes, Tribelos, and Zhouomyia [14,15]. Species of this group are mainly distributed in neotropical rivers [16]. They can tolerate polluted waters temperately, making them a biological indicator of environments [17]. Their diversity of larval habitat, which includes wood-mining, and aquatic habit therein, also render them an important group for studying evolutionary biology [12,17]. Several taxonomy and molecular studies have examined the phylogenetic relationships of the Polypedilum generic complex [15,18,19], but uncertainties in classification schemes among these groups still persist [19,20,21].
Recently, there were more studies conducted on mitochondrial genomes, which greatly promoted the research progress on systematics evolutionary history in Chironomidae. In addition, the increasing number of available mitogenomes of Chironomidae also assist to explore the Chironomidae mitochondrial structure and evolution pattern [3,5,10], and provide more taxonomic characters. Lin and his colleagues, for example, used mitochondrial genomes to reconstruct the phylogenetic relationships of Prodiamesinae (Diptera: Chironomidae); the results indicated that Prodiamesinae is a subgroup of Orthocladiinae [3]. However, mitogenomes resources of the Polypedilum generic complex are rare. To date, only a few mitogenomes in the Polypedilum generic complex were available, representing only the genera Polypedilum and Stictochironomus [22,23,24,25].
Herein, we newly sequenced, assembled, and annotated 14 species of six genera from the Polypedilum generic complex, and analyzed the characters of their mitogenome. Combined with three previously published mitogenomes, we compared the main features, substitution, and evolutionary rates of the mitogenomes among the Polypedilum generic complex. Finally, we reconstructed the phylogenetic relationships of this group based on 19 mitochondrial genomes (17 ingroups and two outgroups), using Maximum Likelihood (ML) and Bayesian Inference (BI) approaches.
2. Materials and Methods
2.1. Taxon Sampling and Sequencing
To reconstruct the phylogenetic relationships of the Polypedilum generic complex, our taxon sampling included most genera in this group. Here, our analysis included 17 taxa of the Polypedilum generic complex. We newly sequenced 14 species, including four Polypedilum species, three Stictochironomus species, three Endochironomus species, two Synendotendipes species, one Phaenopsectra species, and one Sergentia species, which were collected from China, Italy, and Norway by X.X.L. during 2013–2021 (detailed information shown in Table 1). In addition, mitogenomes of three Polypedilum species were retrieved from GenBank for comparative mitogenomic and phylogenetic analyses (Table S1). Based on prior phylogenetic studies of Chironomidae [20], two species of the closely related genus Stenochironomus were selected as outgroups. In total, we sampled 19 species of six members of the Polypedilum generic group, including Endochironomus, Phaenopsectra, Polypedilum, Sergentia, Stictochironomus, and Synendotendipes (detailed information showed in Table 1 and Table S1). All samples were immersed in 85% to 95% ethanol at −20 °C before DNA extraction and morphological examination. Specimen identifications were made by X.L.L. All vouchers were deposited in the College of Fisheries and Life, Shanghai Ocean University, Shanghai, China.

Table 1.
Collection information of newly sequenced species in this study.
Whole genome DNA was extracted with Qiagen DNeasy Blood & Tissue Kit, and the concentration of the DNA was measured with a Qubit® DNA Assay Kit in Qubit® 2.0 Flurometer (ThermoFisher, USA). All whole genomes were sent to company for sequencing (Berry Genomics, Beijing, China). Truseq Nano DNA HT sample preparation Kit (Illumina, USA) was used to generate sequencing libraries. Raw reads were sequenced on the Illumina NovaSeq 6000 platform with 150 bp paired-end reads and were generated with an insert size around 350 bp. Adapters, short, and low-quality reads of raw data were removed using Trimmomatic v0.32 (Jülich, Germany) [26].
2.2. Assembly, Annotation and Composition Analyses
To ensure accuracy, we used two methods for de novo assembly: (1) NOVOPlasty v3.8.3 (Brussel, Belgium) [27] was implemented for mitogenome assembly with COI gene of Polypedilum heberti (GenBank accession: MK505566) as seed sequences and k-mer sizes of 23–39 bp; and (2) IDBA-UD v1.1.3 (Boston, MA, USA) [28] was used to assemble with parameter “--mink 40 --maxk 120”. Geneious 2020.2.1 [29] was used to compare mitogenome sequences which were obtained by the above two methods, and combine them into a single sequence. The secondary structure of tRNAs was implemented in tRNAscan SE 2.0 [30] and MITOS WebServer. The rRNAs and PCGs were annotated manually with the Polypedilum vanderplanki (GenBank accession: KT251040) as a reference using Clustal Omega in Geneious. Clustal W function in MEGA 7 was used to proofread the boundaries of rRNAs and PCGs [31]. Bias of the nucleotide composition and nucleotide composition of each gene was performed via SeqKit v0.16.0 (Chongqing, China) [32].
Rates of non-synonymous substitution rate (Ka)/synonymous substitution rate (Ks) for each PCG were calculated via DnaSP 6.0 [33]. Two formulas were used to calculate AT-skew and GC-skew: AT-skew = (A − T)/ (A + T), GC-skew = (G − C)/ (G + C). An online server CGview (https://cgview.ca/, accessed on 30 November 2022) was used to generate the mitogenome map to show sequence features. Finally, these 14 new mitogenome sequences were deposited in GenBank (for accession numbers, see Table 2).

Table 2.
Nucleotide composition of 19 mitogenomes.
2.3. Phylogenetic Analyses
A total of 2 rRNAs and 13 PCGs genes of 19 mitogenomes were used for phylogenetic analyses. Nucleotide and protein sequences were aligned via MAFFT v7.450 (Osaka, Japan) [34] with the L-INS-I method. Trimming was performed by Trimal v1.4.1 (Barcelona, Spain) [35] with “-automated1” strategy, and then we concatenated five matrices via FASconCAT-G v1.04 (Santa Cruz, CA, USA) [36] for phylogeny analysis: (1) cds_faa matrix contained all PCGs amino acid reads; (2) cds_fna matrix included all PCGs nucleotide reads; (3) cds_rrna matrix included all PCGs and two rRNA nucleotide reads; (4) cds12_fna matrix contained all PCGs nucleotide reads except the third codon positions; and; (5) cds12_rrna matrix contained PCGs nucleotide reads which removed the third codon positions and two rRNA gene. AliGROOVE v1.06 (Bonn, Germany) [37] was used to calculate the heterogeneity among matrices.
For all matrices, we used ML and BI approaches to infer the phylogenetic relationships of the Polypedilum generic complex. For the ML analysis, we used ModelFinder [38] to select the best-fitting substitution models implemented in IQ-TREE 2 (Canberra, ACT, Australia) [39]. The posterior mean site frequency (PMSF) [40] model was used to minimize long-branch attraction artifacts for matrix cds_faa, with the command ‘−m − mtART + C60 + FO + R’ in IQ-TREE. Phylobayes-MPI (Montréal, QC, Canada) [41] was implemented to generate the BI tree, with the site-heterogeneous mixture model (−m CAT + GTR). We performed two Markov chain Monte Carlo chains (MCMC) with 10,000,000 generations, and stopped when we achieved satisfactory convergence (maxdiff < 0.3). A total of 25% initial trees of each run were discarded as burn-in, and we generated a consensus tree using the remaining trees combined. iTOL, an available online website, was used for tree beautified (https://itol.embl.de/upload.cgi, accessed on 15 December 2022).
3. Results and Discussion
3.1. Mitogenomic Organization
We sequenced about three Gb raw reads for each sample. A total of 14 mitogenomes of Chironomidae were obtained in this study, of which five were complete mitogenomes and nine were linear mitogenomes, and all of them were submitted in GenBank with accession number OP950216–OP950228, OK513041 (Table 2 and Table S1). The whole length of newly obtained sequences ranged from 15,582 (Polypedilum masudai) to 17,810 bp (Sergentia baueri), the variation in which was mainly caused by the unstable size of the CR (Table 2). All newly assembled mitogenomes contained the typeset of one CR and 37 genes which included 13 PCGs, 22 tRNAs, and two rRNAs (Figure 1). Most of the newly assembled mitogenomes were similar to those previously published for Chironomidae in length [3,21,22,23,24]. Sequence features of the represented species are given in Figure 1.
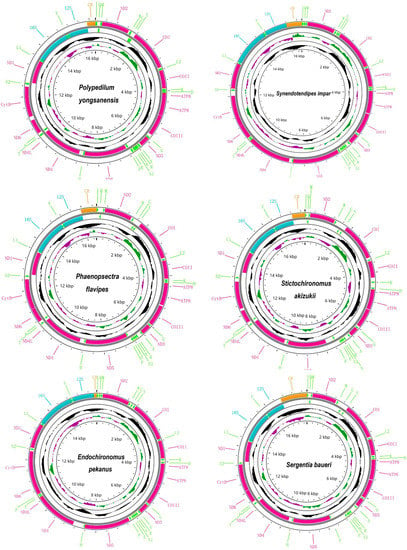
Figure 1.
Mitogenome map showed the mitochondrial genome characteristics of representative species from six genera within the Polypedium generic complex. The arrow indicated the direction of gene transcription. We used normative abbreviations to represent PCGs and rRNAs, and single letter abbreviations were used to represent tRNAs. Red, green, blue, and orange represented PCGs, tRNA, rRNA, and CR, respectively. GC content of complete mitogenome showed in the second circle. GC-skew of complete mitogenome showed in the third circle. The innermost circle showed the length of complete mitogenome.
The nucleotide composition of the newly reported mitogenomes were similar (Table 2), revealing the characteristic AT-biased composition in Chironomidae and other insects [2,3,10,25,42,43,44]. The AT content of the mitochondrial genomes ranged from 75.98% (Stictochironomus akizukii) to 79.94% (Phaenopsectra flavipes; Figure 2; Table 2). The CR showed the highest AT content, ranging from 91.95% (S. akizukii) to 98.18% (Endochironomus albipennis). The AT content in tRNA and PCGs was lower than that in rRNAs (Table 2). All newly assembled mitogenomes had a negative GC-skew, while the AT-skew for most of them was positive, except Endochironomus tendens which showed negative AT-skew. The GC-skew ranged from −32.02 (S. akizukii) to −16.30 (Synendotendipes sp.1), and the AT-skew ranged from −0.002 (Polypedilum masudai) to 0.075 (S. akizukii); the GC content (%) ranged from 20.06 (P. flavipes) to 24.02 (S. akizukii) (detailed information is given in Table 2).
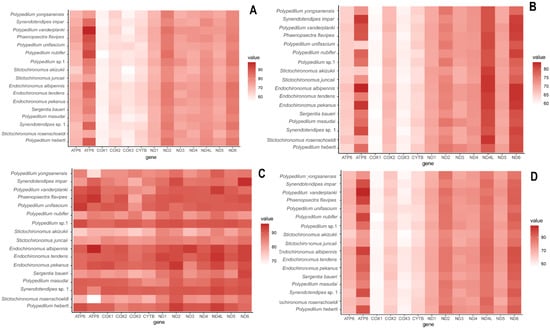
Figure 2.
Difference in AT content of protein coding genes of Polypedilum generic complex mitogenomes. (A) First-codon positions; (B) second-codon positions; (C) third-codon positions; (D) first/second-codon positions.
3.2. Protein-Coding Genes, Codon Usage, and Evolutionary Rates
There was no remarkable difference in the size of tRNA, PCGs, and rRNAs among each species. A total of 13 PCGs of obtained mitogenomes ranged from 11,196 (Polypedilum unifascium) to 11,241 bp (Endochironomus pekanus) in length. Combined and compared with published Chironomidae data, we found that the AT content of the third codon positions was significantly higher than the first and the second positions in PCGs (Figure 2). Most of the 14 mitogenomes exhibited negative GC-skew in PCGs, while E. tendens and E. pekanus were positive; and each of them had negative AT-skew of PCGs, ranging from −0.21 (P. masudai) to −0.17 (E. tendens). The AT content (%) ranged from 71.53 (S. akizukii) to 76.93 (E. albipennis); the GC content (%) ranged from 23.07 (E. albipennis) to 28.47 (S. akizukii) (for detailed information, see Table 2).
All 13 PCGs of newly obtained mitogenomes had the standard start codon ATN, which was most similar to insect mitochondrial. However, several different start codons were found; the start codon of COI gene in 16 species was TTG, in two species was ATG, and in one species was ATA; ATP8 gene in five species was ATA, in nine species was ATT, and in five species was ATC; ND2 was ATT in all species, the ND5 gene in 12 species was GTG, in six species was ATG, in one species was ATC and so on, and detailed statistical information as shown in Figure S1. The codon size ranged from 110 (Endochironomus albipennis) to 1180 bp (Polypedilum heberti) (Table 1).
The Ka/Ks value (ω) was usually used to measure the rate of sequence evolution by natural selection [45,46]. The result of Ka/Ks ratio of all 13 PCGs was less than one, ranging from 0.0958 (COX3) to 0.7251 (ATP8) (Figure 3), which was same as other insects [43,44]. The evolution rate of 13 PCGs was as follows: ATP8 > ND6 > ND5 > ND3 > ND2 > ND4L > ND4 > COX1 > ND1 > CYTB > APT6 > COX2 > COX3. This result indicated that, in most cases, selection eliminated deleterious mutation, and all of them evolved under purifying selection pressure (Figure 3). In PCGs, each gene was under different purifying selection. ATP8, ND6, and ND5 showed higher ω value, indicating that they exhibited relatively relaxed purifying selection pressure. Meanwhile, COX2 and COX3 were under the hardly purifying selection, which were similar to previous research results regarding chironomids [2,3,10,43].
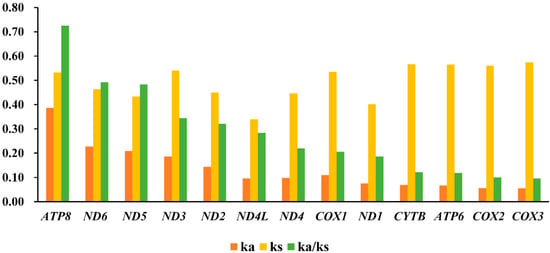
Figure 3.
Evolution rate of 13 PCGs of the Polypedilum generic complex mitogenomes. Ka refers to non-synonymous nucleotide substitutions, Ks refers to synonymous nucleotide substitutions, Ka/Ks refers to the selection pressure of each PCG. The abscissa represented 13 PCGs, and the ordinate represented Ka/Ks values.
An amount of 22 tRNAs ranged from 53 to 77 bp in length, the AT content (%) ranged from 79.17 (S. akizukii) to 82.04 (Polypedilum vanderplanki); except Polypedilum unifascium (−0.007), all others exhibited a positive AT-skew, ranging from 0.016 to 0.063; the GC content (%) ranged from 17.96 (P. vanderplanki) to 20.83 (S. akizukii); the GC-skew ranged from 11.54 (S. akizukii) to 17.36 (Synendotendipes sp.1). rRNA lengths ranged from 2224 in Synendotendipes sp.1 to 2342 bp in S. akizukii; the AT content (%) ranged from 13.81 to 15.68; the GC content (%) ranged from 11.30 to 15.68; the GC-skew of all mitogenomes were obviously positive (28.79 to 39.01); the AT-skew in most mitogenomes were negative (−0.048 to −0.002), while five species were negative (P. heberti, Polypedilum yongsanensis, E. pekanus, S. akizukii, and P. nubifer) for more detailed information, see Table 2).
3.3. Phylogenetic Relationships
The heterogeneous divergence analysis indicated that the matrix cds12_rrna and cds_rrna exhibited higher heterogeneity than cds_faa, cds12_fna, and cds_fna (Figure 4). Because of high heterogeneity, third codon positions were rejected to reconstruct the phylogenetic relationship of the Polypedilum generic complex. Furthermore, the genera Endochironomus and Synendotendipes exhibited lower heterogeneity than Polypedilum, Stictochironomus, and Sergentia (Figure 4).
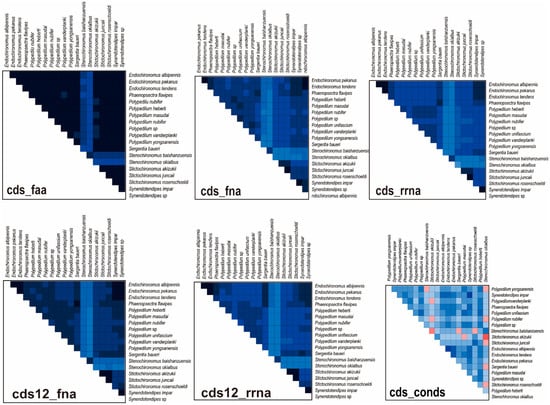
Figure 4.
Heterogeneity analysis for different matrices. Colored squares represented pairwise Aliscore values. Score values ranged from −1 (indicated fully random similarity, dark blue) to +1 (indicated non-random similarity, bright orange, or red).
We used supermatrix cds_faa (3715 sites), cds_fna (10,833 sites), cds_rrna (13,368 sites), cds12_fna (7430 sites), and cds12_rrna (9653 sites) to reconstruct phylogenetic relationships within the Polypedilum generic complex by two different methods. BI and ML approaches based on five matrices yielded five and six trees, respectively (Figure 5 and Figures S2–S9), representing three different topologies. Our result was consistent with phylogenetic tree inferred from previous studies based on four genetic markers [20]. The monophyly of the genera Endochironomus, Polypedilum, Stictochironomus, and Synendotendipes was well supported in all topologies. In BI trees, the phylogenetic analysis of the matrices cds_fna, cds_faa, and cds12_rrna resulted in ((Endochironomus + Synendotendipes) + (Phaenopsectra + Sergentia) + (Polypedilum + Stictochironomus)) (Figure 5A, Figures S7 and S9), while the matrices cds_rrna and cds12_fna yielded (Stictochironomus + ((Endochironomus + Synendotendipes) + (Sergentia + Phaenopsectra)) + Polypedilum) (Figure 5B and Figure S8). All ML trees recovered ((((Endochironomus + Synendotendipes) + (Phaenopsectra + Sergentia)) + Stictochironomus) + Polypedilum) (Figure 5 and Figures S2–S6).
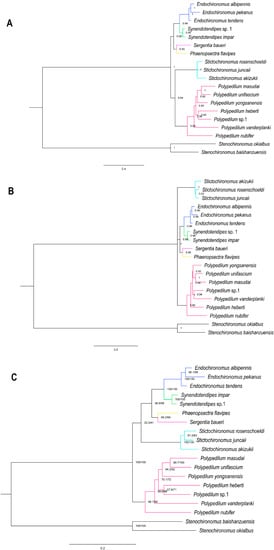
Figure 5.
Phylogenetic trees of the Polypedilum generic complex: (A) BI tree based on analysis cds_fna in Phylobayes. (B) BI tree based on analysis of cds_rrna in Phylobayes. (C) ML tree based on the anaysis cds_faa with PMSF model in IQTREE. Support values on nodes indicate Bayesian posterior probabilities in topology A and B, while they represent SH-aLRT/UFBoot2 in topology C.
Due to the lack of Phaenopsectra and Synendotendipes species, the relationships of the Polypedilum generic complex were unclear in a recent dated molecular phylogeny [20]. Our analyses included a wider range of samples, recovering a new insight for the phylogenetic relationships within the Polypedilum generic complex. According to our data, Endochironomus + Synendotendipes is sister to Phaenopsectra + Sergentia (Figure 5). Although the BI and ML topologies differed, the phylogenetic relationships of the genera Synendotendipes, Endochironomus, Phaenopsectra, and Sergentia were stably supported in all trees.
Different topologies between BI and ML trees indicated that the phylogenetic relationships based on mitogenomes among this group were still erratic, i.e., the systematic position of Stictochironomus, and the trees which were inferred by the heterogeneity model (CAT + GTR) were also not well supported. Therefore, we need to await further taxonomic and phylogenomic studies with more taxon sampling and availably molecular markers, such as ultra-conserved elements and single-copy orthologous genes, which have been successfully used in other insect groups [47,48,49] to explore the evolutionary history of the Polypedilum generic complex.
4. Conclusions
Fourteen mitogenomes of six genera within the Polypedilum generic complex were obtained, including six complete mitogenomes and eight linear mitogenomes. All newly sequenced mitogenomes had similar structural characters and nucleotide compositions to previously published Chironomidae data. In adding Phaenopsectra and Synendotendipes, we could also reconstruct the phylogenetic relationships among the genera within the Polypedilum generic complex. Our results showed that Endochironomus + Synendotendipes are sister to Phaenopsectra + Sergentia, which is a new systematic finding for Chironomidae.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects14030238/s1, Table S1. Sample resources used in this study. Figure S1. Start codons of protein-coding genes among Polypedilum generic complex mitogenomes. Figure S2. Maximum likelihood phylogenetic tree of Polypedilum generic complex based on the analysis cds_faa with Partition model in IQTREE. Support values on nodes indicate SH-aLRT/UFBoot2, respectively. Figure S3. Maximum likelihood phylogenetic tree of Polypedilum generic complex based on the analysis cds_fna with a Partitioned model in IQTREE. Support values on nodes indicate SH-aLRT/UFBoot2, respectively. Figure S4. Maximum likelihood phylogenetic tree of Polypedilum generic complex based on the analysis cds_rrna with a Partitioned model in IQTREE. Support values on nodes indicate SH-aLRT/UFBoot2, respectively. Figure S5. Maximum likelihood phylogenetic tree of Polypedilum generic complex based on the analysis cds12_fna with a Partitioned model in IQTREE. Support values on nodes indicate SH-aLRT/UFBoot2, respectively. Figure S6. Maximum likelihood phylogenetic tree of Polypedilum generic complex based on the analysis cds12_rrna with a Partitioned model in IQTREE. Support values on nodes indicate SH-aLRT/UFBoot2, respectively. Figure S7. Bayesian inference phylogenetic tree of Polypedilum generic complex based on the analysis cds_fna with a GTR + CAT model in phylobayes. Support values on nodes indicate Bayesian posterior probabilities. Figure S8. Bayesian inference phylogenetic tree of Polypedilum generic complex based on the analysis cds12_fna with a GTR + CAT model in phylobayes. Support values on nodes indicate Bayesian posterior probabilities. Figure S9. Bayesian inference phylogenetic tree of Polypedilum generic complex based on the analysis cds12_rrna with a GTR + CAT model in phylobayes. Support values on nodes indicate Bayesian posterior probabilities.
Author Contributions
X.-L.L. conceived and supervised this study; X.-L.L., F.-X.H. and Z.A. collected species; X.-L.L. identified species; D.Z., F.-X.H. and X.-B.L. performed phylogenetic analyses; D.Z. and F.-X.H. wrote the paper; X.-L.L., X.-B.L. and Z.A. reviewed and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (31900344), Project of Biodiversity Survey and Assessment in Guiyang (GZZC-2021-018), and Youth Innovation Team Development Project of Shandong Universities (2019KJE018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The following information was supplied regarding the availability of DNA sequences: The new mitogenomes are deposited in GenBank of NCBI and the accession numbers are OP950216–OP950228, OK513041.
Acknowledgments
We sincerely thank Hai-Jun Yu and Yu Peng for collecting the materials. We thank Michael Orr for language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef]
- Ge, X.Y.; Peng, L.; Vogler, A.P.; Morse, J.C.; Yang, L.; Sun, C.; Wang, B.X. Massive gene rearrangements of mitochondrial genomes and implications for the phylogeny of Trichoptera (Insecta). Syst. Entomol. 2022. online. [Google Scholar] [CrossRef]
- Lin, X.L.; Zhao, Y.M.; Yan, L.P.; Liu, W.B.; Bu, W.J.; Wang, X.H.; Zheng, C.G. Mitogenomes provide new insights into the evolutionary history of Prodiamesinae (Diptera: Chironomidae). Zool. Scr. 2022, 51, 119–132. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Liu, Z.; Yan, L.P.; Duan, X.; Bu, W.J.; Wang, X.H.; Zheng, C.G. Mitogenomes provide new insights of evolutionary history of Boreheptagyiini and Diamesini (Diptera: Chironomidae: Diamesinae). Ecol. Evol. 2022, 12, e8957. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Lee, S.Y.; Bang, I.C.; Nam, Y.K. Complete mitogenome sequence of an endangered freshwater fish, Iksookimia choii (Teleostei; Cypriniformes; Cobitidae). Mitochondrial DNA 2008, 19, 438–445. [Google Scholar] [CrossRef]
- Brown, W.M. The mitochondrial genome of animals. In Molecular Evolutionary Genetics; Plenum Press: New York, NY, USA, 1985; pp. 95–130. [Google Scholar]
- Kang, Z.; Li, X.; Yang, D. The complete mitochondrial genome of Dixella sp. (Diptera: Nematocera, Dixidae). Mitochondr. DNA Part A Resour. 2016, 27, 1528–1529. [Google Scholar] [CrossRef]
- Li, X.Y.; Yan, L.Y.; Pape, T.; Gao, Y.Y.; Zhang, D. Evolutionary insights into bot flies (Insecta: Diptera: Oestridae) from comparative analysis of the mitochondrial genomes. Int. J. Biol. Macromol. 2020, 149, 371–380. [Google Scholar] [CrossRef]
- Tang, L.; Yan, L.; Gao, Y.; Zhang, D. First report of mitochondrial genome from the subfamily Bengaliinae (Diptera: Calliphoridae). Mitochondr. DNA Part B Resour. 2019, 4, 1560–1561. [Google Scholar] [CrossRef]
- Lencioni, V.; Cranston, P.S.; Makarchenko, E. Recent advances in the study of Chironomidae: An overview. J. Limnol. 2018, 77, 1–6. [Google Scholar] [CrossRef]
- Armitage, P.D.; Pinder, L.; Cranston, P. The Chironomidae: Biology and ecology of non-biting midges. J. N. Am. Benthol. Soc. 1995, 14, 611–738. [Google Scholar] [CrossRef]
- Giłka, W.; Zakrzewska, M.; Lukashevich, E.D.; Vorontsov, D.D.; Soszyńska-Maj, A.; Skibińska, K.; Cranston, P.S. Wanted, tracked down and identified: Mesozoic non-biting midges of the subfamily Chironominae (Chironomidae, Diptera). Zool. J. Linn. Soc. 2021, 194, 874–892. [Google Scholar] [CrossRef]
- Sasa, M.; Kikuchi, M. Chironomidae (Diptera) of Japan; University of Tokyo Press: Tokyo, Japan, 1995; 333p. [Google Scholar]
- Han, W.; Wei, J.; Lin, X.; Tang, H. The afro-oriental genus Yaeprimus Sasa et Suzuki (Diptera: Chironomidae: Chironomini): Phylogeny, new species and expanded diagnoses. Diversity 2020, 12, 31. [Google Scholar] [CrossRef]
- Villamarín, C.; Villamarín-Cortez, S.; Salcido, D.M.; Herrera-Madrid, M.; Ríos-Touma, B. Drivers of diversity and altitudinal distribution of Chironomids (Diptera: Chironomidae) in the Ecuadorian. Andes. Rev. Biol. Trop. 2021, 69, 113–126. [Google Scholar] [CrossRef]
- Cranston, P.S. A new genus and species of Chironominae (Diptera: Chironomidae) with wood-mining larvae. Aust. J. Entomol. 2006, 45, 227–234. [Google Scholar] [CrossRef]
- Han, W.; Tang, H. Phylogeny of marine Ainuyusurika tuberculata (Tokunaga) (Diptera: Chironomidae: Chironominae), with description of the immature stages. Zootaxa 2019, 4695, 131–147. [Google Scholar] [CrossRef]
- Tang, H.; Cheng, Q.; Han, W.; Cranston, P.S. Integrative taxonomy: Molecular phylogenetics of Polypedilum (Cerobregma) and revisited morphology of Yaethauma and Collartomyia (Diptera: Chironomidae) reveals synonymy and supports new classification. Zool. J. Linn. Soc. 2021, 194, 102–119. [Google Scholar] [CrossRef]
- Cranston, P.S.; Hardy, N.B.; Morse, G.E. A dated molecular phylogeny for the Chironomidae (Diptera). Syst. Entomol. 2012, 37, 172–188. [Google Scholar] [CrossRef]
- Zheng, C.G.; Liu, Z.; Zhao, Y.M.; Wang, Y.; Bu, W.J.; Wang, X.H.; Lin, X.L. First report on mitochondrial gene rearrangement in non-biting midges, revealing a synapomorphy in Stenochironomus Kieffer (Diptera: Chironomidae). Insects 2022, 13, 115. [Google Scholar] [CrossRef]
- Park, K.; Jo, H.; Choi, B.; Kwak, I.S. Complete mitochondrial genome of Stictochironomus akizukii (Tokunaga) (Chironomidae, Diptera) assembled from next-generation sequencing data. Mitochondr. DNA Part B Resour. 2020, 5, 2310–2311. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Xu, Z.G.; Wang, J.X.; Fang, X.L.; Fu, Y. Complete mitochondrial genome of a eurytopic midge, Polypedilum nubifer (Diptera: Chironomidae). Mitochondr. DNA Part B Resour. 2022, 7, 1936–1938. [Google Scholar] [CrossRef] [PubMed]
- Deviatiiarov, R.; Kikawada, T.; Gusev, O. The complete mitochondrial genome of an anhydrobiotic midge Polypedilum vanderplanki (Chironomidae, Diptera). Mitochondr. DNA Part A Resour. 2017, 2, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Song, C.; Zhu, X.D.; Xu, B.Y.; Qi, X. The complete mitochondrial genome of a non-biting midge Polypedilum unifascium (Tokunaga, 1938) (Diptera: Chironomidae). Mitochondr. DNA Part B Resour. 2021, 6, 2212–2213. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dierckxsens, N.; Mardulyn, P.; Smits, G.J. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Leung, H.C.; Yiu, S.M.; Chin, F.Y.L. DBA-UD: A de novo assembler for single-cell and metagenomic sequencing data with highly uneven depth. Bioinformatics 2012, 28, 1420–1428. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Shen, W.; Le, S.; Li, Y.; Hu, F.Q. SeqKit: A cross-platform and ultrafast toolkit for FASTA/Q file manipulation. PLoS ONE 2016, 11, e0163962. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Kück, P.; Longo, G.C. FASconCAT-G: Extensive functions for multiple sequence alignment preparations concerning phylogenetic studies. Front. Zool. 2014, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Kück, P.; Meid, S.A.; Groß, C.; Wägele, J.W.; Misof, B. AliGROOVE–visualization of heterogeneous sequence divergence within multiple sequence alignments and detection of inflated branch support. BMC Bioinform. 2014, 15, 294. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef]
- Wang, H.C.; Minh, B.Q.; Susko, E.; Roger, A.J. Modeling site heterogeneity with posterior mean site frequency profiles accelerates accurate phylogenomic estimation. Syst. Biol. 2017, 67, 216–235. [Google Scholar] [CrossRef]
- Lartillot, N.; Rodrigue, N.; Stubbs, D.; Richer, J. PhyloBayes MPI: Phylogenetic reconstruction with infinite mixtures of profiles in a parallel environment. Syst. Biol. 2013, 62, 611–615. [Google Scholar] [CrossRef]
- Wei, S.J.; Shi, M.; Chen, X.X.; Sharkey, M.J.; van Achterberg, C.; Ye, G.Y.; He, J.H. New views on strand asymmetry in insect mitochondrial genomes. PLoS ONE 2010, 5, e12708. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhao, Y.M.; Guo, B.X.; Li, C.H.; Sun, B.J.; Lin, X.L. Comparative analysis of mitogenomes of Chironomus (Diptera: Chironomidae). Insects 2022, 13, 1164. [Google Scholar] [CrossRef]
- Ge, X.Y.; Zang, H.M.; Ye, X.Y.; Peng, L.; Wang, B.X.; Lian, G.; Sun, C.H. Comparative mitogenomic analyses of hydropsychidae revealing the novel rearrangement of protein-coding gene and tRNA (Trichoptera: Annulipalpia). Insects 2022, 13, 759. [Google Scholar] [CrossRef] [PubMed]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Gemet. 2002, 18, 486. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Bielawski, J.P. Statistical methods for detecting molecular adaptation. Trends Ecol. Evol. 2000, 15, 496–503. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, Z.Q.; Luo, A.R.; Orr, M.C.; Ferrari, R.R.; Jin, J.F.; Wu, Q.T.; Zhu, C.D. Testing the systematic status of Homalictus and Rostrohalictus with weakened cross-vein groups within Halictini (Hymenoptera: Halictidae) using low-coverage whole-genome sequencing. Insect Sci. 2022, 29, 1819–1833. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, Y.H.; Zhu, C.D.; Zhou, X.; Orr, M.C.; Scheu, S.; Luan, Y.X. Phylogenomics from low-coverage whole-genome sequencing. Methods Ecol. Evol. 2019, 10, 507–517. [Google Scholar] [CrossRef]
- Branstetter, M.G.; Müller, A.; Griswold, T.L.; Orr, M.C.; Zhu, C.D. Ultraconserved element phylogenomics and biogeography of the agriculturally important mason bee subgenus Osmia (Osmia). Syst. Entomol. 2021, 46, 453–472. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).