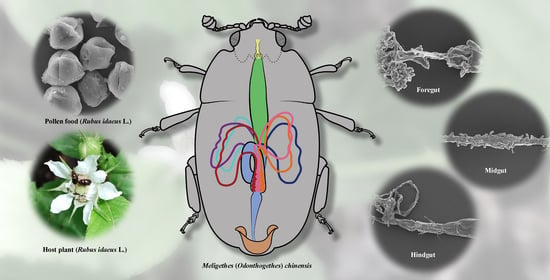
Morphological Study of the Alimentary Canal and Malpighian Tubules in the Adult of the Pollen Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. Light Microscopy (LM) Research
2.3. Fluorescence Microscopy (FM) Research
2.4. Scanning Electron Microscope (SEM) Research
2.5. Data Analysis
| An: anus | Hg: hindgut | Ph: pharynx |
| Cm: circular muscle | Il: ileum | Pl: pylorus region |
| Co: colon | Lbr: labrum | Pv: proventriculus |
| Cry: cryptonephridial system | Lm: longitudinal muscle | Rc: rectum |
| Cv: cardiac valve | Mb: muscle bundle | Se: setae |
| Ep: epithelium | Mg: midgut | Tl: tracheole |
| Es: esophagus | Ml: muscle layer | Tm: transverse muscle |
| Fg: foregut | Mp: mouthpart | Tr: trachea |
| Gc: gastric cecum | Mt (1, 2, 3, 4, 5, 6): Malpighian tubule (1, 2, 3, 4, 5, 6) | |
3. Results
3.1. General Morphology of Alimentary Canal and Malpighian Tubules
3.2. Foregut
3.3. Midgut
3.4. Hindgut
3.5. Malpighian Tubules
4. Discussion
4.1. Structural Comparison and Functional Speculation of the Foregut
4.1.1. The Relationship between Crop Structure and Function and Beetle Feeding Habits
4.1.2. The Relationship between the Structure of the Proventriculus and the Texture of Food
4.2. Structural Comparison, Functional Hypotheses, and Application in the Classification of the Midgut
4.2.1. Study on the Structure and Function of Midgut “Saccate Protrusions”
4.2.2. The Application of Midgut “Saccate Protrusions” in Classification
4.3. Structural Comparison and Functional Hypotheses of the Hindgut
4.4. Structural Comparison and Functional Hypotheses of the Malpighian Tubules and Cryptonephridial System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D. The co-radiations of pollinating insects and angiosperms in the Cretaceous. Ann. Mo. Bot. Gard. 1999, 86, 373–406. [Google Scholar] [CrossRef]
- Smith, S.A.; Beaulieu, J.M.; Donoghue, M.J. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc. Natl. Acad. Sci. USA 2010, 107, 5897–5902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.A.; Nicolson, S.W. Pollen digestion by flower-feeding Scarabaeidae: Protea beetles (Cetoniini) and monkey beetles (Hopliini). J. Insect Physiol. 2001, 47, 725–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tihelka, E.; Li, L.Q.; Fu, Y.Z.; Su, Y.T.; Huang, D.Y.; Cai, C.Y. Angiosperm pollinivory in a Cretaceous beetle. Nat. Plants 2021, 7, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Dafni, A.; Bernhardt, P.; Shmida, A.; Ivri, Y.; Greenbaum, S.; O’Toole, C.; Losito, L. Red bowl-shaped flowers: Convergence for beetle pollination in the Mediterranean region. Isr. J. Bot. 1990, 39, 81–92. [Google Scholar] [CrossRef]
- Proctor, M.; Yeo, P.; Lack, A. The Natural History of Pollination; Harper Collins: London, UK, 1996; pp. 79–779. [Google Scholar]
- Goldblatt, P.; Manning, J.C. Hopliine beetles (Scarabaeidae: Rutelinae: Hopliini), specialized pollinators of the Southern African flora. Curtis’s Bot. Mag. 2011, 28, 238–259. [Google Scholar] [CrossRef]
- Harmon, J.P.; Ganguli, A.C.; Solga, M.J. An overview of pollination in rangelands: Who, why, and how. Rangelands 2011, 33, 4–8. [Google Scholar] [CrossRef] [Green Version]
- Labandeira, C.C. Insect mouthparts: Ascertaining the paleobiology of insect feeding strategies. Annu. Rev. Ecol. Syst. 1997, 28, 153–193. [Google Scholar] [CrossRef] [Green Version]
- Labandeira, C.C. The pollination of mid Mesozoic seed plants and the early history of long-proboscid insects. Ann. Mo. Bot. Gard. 2010, 97, 469–513. [Google Scholar] [CrossRef]
- Nel, P.; Bertrand, S.; Nel, A. Diversification of insects since the Devonian: A new approach based on morphological disparity of mouthparts. Sci. Rep. 2018, 8, 3516. [Google Scholar] [CrossRef] [Green Version]
- Kirejtshuk, A.G. On formation of phyllophagy (phyllophaglzation) among beetles (Coleoptera). Proc. Zool.Inst. Leningr. 1989, 202, 147–182. [Google Scholar]
- Kirejtshuk, A.G. On the evolution of anthophilous Nitidulidae (Coleoptera) in tropical and subtropical regions. Bonn. Zool. Beitr. 1997, 47, 111–134. [Google Scholar]
- Kirejtshuk, A.G. Sistema, evolutsiya obraza zhizni i filogeniya otryada zhukov (Coleoptera). I. (System, evolution of mode of life and phylogeny of the order Coleoptera. I). Entomol. Obozr. 1994, 73, 266–288. [Google Scholar]
- Kirejtshuk, A.G. Historical fate of groups, application in systematics with references to fam. Nitidulidae and order Coleoptera. In Proceedings of the Fifth International Congress of Systematic and Evolutionary Biology, Budapest, Hungary, 17–14 August 1996; p. 258, Abstract ICSEBV. [Google Scholar]
- Kirejtshuk, A.G. System, evolution of mode of life and phylogeny of the order Coleoptera. Entomol. Obozr. 1996, 75, 39–62. [Google Scholar]
- Kirejtshuk, A.G. A current generic classification of sap beetles (Coleoptera, Nitidulidae). Zoosystematica Ross. 2008, 17, 107–122. [Google Scholar] [CrossRef]
- Audisio, P. Coleoptera, Nitidulidae-Kateretidae; Calderini Edizioni: Bologna, Italy, 1993; pp. xvi + 971. [Google Scholar]
- Audisio, P.; Cline, A.R.; De Biase, A.; Antonini, G.; Mancini, E.; Trizzino, M.; Costantini, L.; Strika, S.; Lamanna, F.; Cerretti, P. Preliminary re-examination of genus-level taxonomy of the pollen beetle subfamily Meligethinae (Coleoptera: Nitidulidae). Acta Entomol. Musei Natl. Pragae 2009, 49, 341–504. [Google Scholar]
- Audisio, P.; Sabatelli, S.; Jelínek, J. Revision of the pollen beetle genus Meligethes (Coleoptera: Nitidulidae). Fragm. Entomol. 2015, 46, 19–112. [Google Scholar] [CrossRef] [Green Version]
- Jelínek, J.; Carlton, C.E.; Cline, A.R.; Leschen, R.A.B. Nitidulidae Latreille. In Handbook of Zoology. Arthropoda: Insecta. Coleoptera, 2 Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia Partim); Beutel, R.G., Leschen, R.A.B., Lawrence, J.F., Eds.; Walter De Gruyter: Berlin, Germany, 2010; pp. 390–407. [Google Scholar]
- Liu, M.; Yang, X.; Huang, M.; Jelínek, J.; Audisio, P. Four new species of Meligethes Stephens from China and additional data on other species of the genus (Coleoptera: Nitidulidae: Meligethinae). Zootaxa 2016, 4121, 101–116. [Google Scholar] [CrossRef]
- Liu, M.; Huang, M.; Cline, A.R.; Sabatelli, S.; Audisio, P. New species of Meligethes Stephens from China and additional data on members of the M. chinensis species-complex (Coleoptera: Nitidulidae, Meligethinae). Fragm. Entomol. 2017, 49, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Huang, M.; Cline, A.R.; Audisio, P. New and poorly known Meligethes Stephens from China, with bionomical data on some species (Coleoptera: Nitidulidae: Meligethinae). Zootaxa 2018, 4392, 546–566. [Google Scholar] [CrossRef]
- Liu, M.; Huang, M.; Cline, A.R.; Cardoli, P.; Audisio, P.; Sabatelli, S. Re-examination of the genus-level taxonomy of the pollen beetle subfamily Meligethinae—Part 1. Sagittogethes Audisio & Cline 2009 and allied genera; with description of a new genus (Coleoptera: Nitidulidae). Fragm. Entomol. 2020, 52, 119–135. [Google Scholar]
- Liu, M.; Huang, M.; Cline, A.R.; Mancini, E.; Scaramuzzi, A.; Paradisi, S.; Audisio, P.; Badano, D.; Sabatelli, S. Rosaceae, Brassicaceae and pollen beetles: Exploring relationships and evolution in an anthophilous beetle lineage (Nitidulidae, Meligethes-complex of genera) using an integrative approach. Front. Zool. 2021, 18, 9. [Google Scholar] [CrossRef]
- Sabatelli, S.; Liu, M.; Badano, D.; Mancini, E.; Trizzino, M.; Cline, A.R.; Endrestøl, A.; Huang, M.; Audisio, P. Molecular phylogeny and host-plant use (Lamiaceae) of the Thymogethes pollen beetles (Coleoptera). Zool. Scr. 2019, 49, 28–46. [Google Scholar] [CrossRef]
- Crome, W. Zur Morphologie und Anatomie der Larve von Oryctes nasicornis L. (Col. Dynastidae). Dtsch. Entomol. Z. 1957, 4, 254–257. [Google Scholar] [CrossRef]
- Wang, Y.Z. Insect Physiology; China Agriculture Press: Beijing, China, 2004; pp. 39–183. [Google Scholar]
- Slansky, F., Jr. Insect nutrition: An adaptation’s perspective. Fla. Entomol. 1982, 65, 45–71. [Google Scholar] [CrossRef]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw-Hill: New York, NY, USA, 1935; pp. 280–311. [Google Scholar]
- Aslam, N.A. An assessment of some internal characters in the higher classification of the Curculionidae S. L. (Coleoptera). Trans. R. Entomol. Soc. Lond. 1961, 113, 417–480. [Google Scholar] [CrossRef]
- Crowson, R.A. The Biology of Coleoptera; Academic press: London, UK, 1981; pp. 69–85. [Google Scholar]
- Terra, R.W. Evolution of digestive systems of insects. Annu. Rev. Entomol. 1990, 35, 181–200. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Candan, S. Characterization of the alimentary canal and malpighian tubules of Chrysolina herbacea (Duftschmid, 1825) (Coleoptera: Chrysomelidae): Anatomical and histological approaches. Microsc. Res. Tech. 2020, 84, 1135–1144. [Google Scholar] [CrossRef]
- Arnett, R.H. The tarnished beetles: A Study of Underpopulation. J. Wash. Acad. Sci. 1962, 52, 9–15. [Google Scholar]
- Nobuchi, A. A Comparative Morphological Study of the Proventriculus in the Adult of the Superfamily Scolytoidea (Coleoptera). Doctoral Dissertation, Kyushu University, Fukuoka, Japan, 1969. [Google Scholar]
- Jaspar-Versali, M.F.; Goffinet, G.; Jeuniaux, C. The digestive system of adult carabid beetles: An ultrastructural and histoenzymological study. Acta Bot. Acad. Sci. Hung. 1987, 22, 375–382. [Google Scholar]
- Johnson, K.S.; Rabosky, D. Phylogenetic distribution of cysteine proteinases in beetles: Evidence for an evolutionary shift to an alkaline digestive strategy in Cerambycidae. Comp. Biochem. Physiol. Part B 2000, 126, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Dow, J.A.T. Insect midgut function. Adv. Insect Physiol. 1986, 19, 187–328. [Google Scholar] [CrossRef]
- Thomas, J.B. A comparative study of gastric caeca in adult and larval stages of bark beetles (Coleoptera: Scolytidae). Proc. Entomol. Soc. Ont. 1967, 97, 71–90. [Google Scholar]
- Calder, A.A. The alimentary canal and nervous system of Curculionoidea (Coleoptera): Gross morphology and systematic significance. J. Nat. Hist. 1989, 23, 1205–1265. [Google Scholar] [CrossRef]
- Candan, S.; Özyurt Koçakoğlu, N.; Serttaş, A. Histoanatomy of Malpighian tubules and the digestive tract of adult of biocontrol agent Calosoma sycophanta L. (Coleoptera: Carabidae). Int. J. Trop. Insect Sci. 2020, 41, 1373–1386. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Candan, S.; Erbey, M. Structure of the mouthparts and alimentary canal of Eusomus ovulum Germar, 1824 (Coleoptera: Curculionidae). Rev. Bras. Entomol. 2020, 64, e20200004. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Çağlar, Ü.; Candan, S. Anatomy and histology of digestive tract in Melanophila (Trachypteris) picta decastigma (Fabricius, 1787) (Coleoptera: Buprestidae). Eur. J. Biol. 2021, 80, 1–8. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Candan, S.; Güllü, M. Anatomical and histological descriptions of digestive canal and excretory system of Mylabris cernyi Pan & Bologna, 2014 (Coleoptera: Meloidae). Orient. Insects 2022, 56, 362–378. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Candan, S.; Güllü, M. Anatomy and histology of digestive tract in the red poplar leaf beetle Chrysomela populi Linnaeus, 1758 (Coleoptera: Chrysomelidae). Int. J. Trop. Insect Sci. 2022, 42, 927–939. [Google Scholar] [CrossRef]
- Toni, A.S.B.; Fialho, V.S.; Cossolin, J.F.S.; Serrão, J.E. Larval and adult digestive tract of the carrion beetle Oxelytrum discicolle (Brullé, 1840) (Coleoptera: Silphidae). Arthropod Struct. Dev. 2022, 71, 101213. [Google Scholar] [CrossRef]
- Bess, H.A. The alimentary canal of Calosoma sycophanta Linnaeus. Ohio J. Sci. 1935, 35, 54–61. [Google Scholar]
- Ekis, G.; Gupta, A.P. Digestive system of Cleridae (Coleoptera). Int. J. Insect Morphol. Embryol. 1971, 1, 51–86. [Google Scholar] [CrossRef]
- Gillott, C. Entomology, 3rd ed.; Springer Science and Business Media: Dordrecht, The Netherlands, 2005; pp. 487–510. [Google Scholar]
- Stammer, H.J. Bau und bedeutung der malpighischen gefässe der Copeopteren. Z. Für Morphol. Ökologie Tiere 1934, 29, 196–217. [Google Scholar] [CrossRef]
- Poll, M. Contribution à l’étude des tubes de Malpighi des Coléoptères: Leur utilité en phylogenèse. Recl. L’institut Zool. Torley-Rousseau 1932, 4, 47–80. [Google Scholar]
- Saini, S.R. Histology and physiology of the cryptonephridial system of insects. Trans. R. Entomol. Soc. Lond. 2009, 116, 347–392. [Google Scholar] [CrossRef]
- Yang, Q.F.; Li, Q.; Zhi, Y.R. Anatomical study of the alimentary canal of Xylosandrus germanus. Chin. Bull. Entomol. 2009, 46, 623–626. [Google Scholar]
- Yang, Y.H.; Shi, M.W.; Yuan, L. Function and microstructure observation of Malpighian tubules in Tenebrio Molitor. J. Henan Inst. Sci. Technol. Nat. Sci. Ed. 2011, 39, 30–32. [Google Scholar]
- Song, Y.Q.; Dong, J.F.; Sun, H.Z.; Liu, S.T. Anatomical structure of the digestive tract of Holotrichia oblita Fald (Coleoptera: Melolonthidae). J. Hunan Agric. Univ. Nat. Sci. 2012, 38, 511–514. [Google Scholar] [CrossRef]
- Candan, S.; Özyurt Koçakoğlu, N.; Güllü, M.; Çağlar, Ü. Anatomical and histological studies of the alimentary canal of adult maize leaf weevil, Tanymecus dilaticollis Gyllenhal, 1834 (Coleoptera: Curculionidae). Microsc. Res. Tech. 2020, 83, 1153–1162. [Google Scholar] [CrossRef]
- Zhang, H.Y. Co-evolution of entomophilous plants and pollination insects (II)–the adaptation of features of entomophilous flowers to insect pollination. J. Sichuan For. Sci. Technol. 2005, 26, 22–27. [Google Scholar]
- Bao, T.; Wang, B.; Li, J.; Dilcher, D. Pollination of Cretaceous flowers. Proc. Natl. Acad. Sci. USA 2019, 116, 24707–24711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peris, D.; Labandeira, C.C.; Barrón, E.; Delclòs, X.; Rust, J.; Wang, B. Generalist Pollen-Feeding Beetles during the Mid-Cretaceous. iScience 2020, 23, 100913. [Google Scholar] [CrossRef] [Green Version]
- Latty, T.; Trueblood, J.S. How do insects choose flowers? A review of multi-attribute flower choice and decoy effects in flower-visiting insects. J. Anim. Ecol. 2020, 89, 2750–2762. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Jácome, A.; Fernández-Tlapa, F.; Munguía-Rosas, A.M. Visiting and feeding behavior of sap beetles (Carpophilus lugubris) in the flowers of a chiropterophilic columnar cactus (Pilosocereus leucocephalus). J. Arid Environ. 2021, 189, 104482. [Google Scholar] [CrossRef]
- Kirejtshuk, A.G. Novye vidy zhukov-blestyanok podsem. Meligethinae (Coleoptera, Nitidulidae) iz Aziatskoi chasti SSSR i sopredel’nykh territorii [New species of subfamily Meligethinae (Coleoptera, Nitidulidae) from the Asian part of the USSR and adjacent territories]. Tr. Zool. Inst. Akad. Nauk SSSR 1979, 88, 50–68. [Google Scholar]
- Li, Q.; Chen, L.; Liu, M.; Wang, W.; Sabatelli, S.; Di Giulio, A.; Audisio, P. Scanning electron microscope study of antennae and mouthparts in the pollen-beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae). Insects 2021, 12, 659. [Google Scholar] [CrossRef]
- Borges, I.; Nóia, M.; Camarinho, R.; Rodrigues, A.S.; Soares, A.O. Characterization of the alimentary canal of the aphidophagous ladybird, Adalia bipunctata (Coleoptera: Coccinellidae): Anatomical and histological approaches. Entomol. Sci. 2015, 18, 66–73. [Google Scholar] [CrossRef]
- Zheng, X.L.; Zhang, Y.J.; Wang, Y.L.; Dong, Z.S.; Hu, D.X.; Lu, W. Observation on digestive system in Dactylispa setifera Chapuis (Coleoptera: Hispidae). J. Sichuan For. Sci. Technol. 2016, 47, 223–226. [Google Scholar]
- Liu, Y.; Long, W.C.; Liu, A.X.; Xiao, X.M.; Luo, C.B.; Liao, H.; Yang, Y.J. On anatomical morphology and electron microscope scanning structure of alimentary canal of Cyrtotrachelus buqueti (Coleoptera: Curculionidae). J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2019, 44, 30–36. [Google Scholar]
- Medel, V.; Molina, B.; Seguel, J.; Rebolledo, R.; Quiroz, A. Morphology and histology of the digestive system of raspberry weevil Aegorhinus superciliosus (Coleoptera: Curculionidae). Rev. Colomb. Entomol. 2013, 39, 260–266. [Google Scholar] [CrossRef]
- Chen, Y.Y. The Comparative Study of Alimentary Canal Morphology and Cellulase in Eucryptorrhynchus scrobiculatus and E. brandti. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, 2016. [Google Scholar]
- Candan, S.; Özyurt Koçakoğlu, N.; Erbey, M. Morphology and histology of the alimentary canal of Epiphaneus malachiticus Boheman, 1842 (Coleoptera: Curculionidae). Entomol. Rev. 2019, 99, 326–336. [Google Scholar] [CrossRef]
- Ingerson-Mahar, J.M. Relating Diet and Morphology of the Head, Mandibles and Proventriculus in Adult Carabid Beetles. Doctoral Dissertation, Graduate School-New Brunswick, New Brunswick, NJ, USA, 2014. [Google Scholar]
- McAllister, J.C.; Steelman, C.D.; Carlton, C.E. Histomorphology of the larval and adult digestive systems of Alphitobius diaperinus (Coleoptera: Tenebrionidae). J. Kans. Entomol. Soc. 1995, 68, 195–205. [Google Scholar]
- Schedl, K.E. Morphology of the bark beetles of the genus Gnathotrichus Eichh. Smithson. Misc. Collect. 1931, 82, 1–88. [Google Scholar]
- Gebhardt, A. Beiträge zur Anatomie des Darmkanals der Buprestiden (Col.). Sb. Entomol. Oddelení Národniho Mus. V Praze 1931, 9, 99–130. [Google Scholar]
- Ameen, M.; Shafiq, S.A. Proepithelial regeneration in the midgut of beetles. Nature 1959, 183, 913–914. [Google Scholar] [CrossRef]
- Kasap, H.; Crowson, R.A. A comparative study of the internal anatomy and abdominal structures of Curculionoidea. Hacet. Bull. Nat. Sci. Eng. Ank. 1977, 6, 35–86. [Google Scholar]
- Chapman, R.F. Alimentary canal, digestion and absorption. In The Insects Structure and Function, 4th ed.; Simpson, S.J., Douglas, A.E., Eds.; Cambridge University Press: Cambridge CB2 8RU, UK, 1998; Volume 3, pp. 38–68. [Google Scholar] [CrossRef]
- Özyurt Koçakoğlu, N.; Candan, S.; Çağlar, Ü. Histomorphology of the adult digestive tract of Capnodis tenebrionis (L. 1758) (Coleoptera: Buprestidae). Microsc. Microanal. 2020, 26, 1245–1254. [Google Scholar] [CrossRef]
- Ali, H.A. An Introduction to the Taxonomy of Iraqi Carabidae Col., with an Examination of the Taxonomic Value of Internal Characters. Doctoral Dissertation, Imperial College of Science and Technology, London, UK, 1964. [Google Scholar]
- Davidson, R.H. The alimentary canal of Criocerus asparagi Linn. Ohio J. Sci. 1931, 31, 396–405. [Google Scholar]
- Miller, W.C. The alimentary canal of Meracantha contracta Beauv. (Tenebrionidae). Ohio J. Sci. 1931, 31, 143–156. [Google Scholar]
- Sinha, R.N. The alimentary canal of the adult of Tribolium castaneum Herbst (Coleoptera, Tenebrionidae). J. Kans. Entomol. Soc. 1958, 31, 118–125. [Google Scholar]
- Sarwade, A.B.; Bhawane, G.P. Anatomical and histological structure of digestive tract of adult Platynotus belli. F (Coleoptera: Tenebrionidae). Biol. Forum-Int. J. 2013, 5, 47–55. [Google Scholar]
- Yin, Y.P. Comparative anatomy of the alimentary canal of longicorn beetles and their significance in taxonomy (Coleoptera: Cerambycidae). Entomotaxonomia 1987, 9, 313–320. [Google Scholar]
- Yin, X.M. Study on adult and larval alimentary canals of Philus antenatus (Gyllenhal). Entomol. Knowl. 1996, 33, 216–218. [Google Scholar]
- Potts, S.F. The alimentary canal of the Mexican bean beetle. Ohio J. Sci. 1927, 27, 127–137. [Google Scholar]
- Aldigail, S.A.; Alsaggaff, A.I.; Al-Azab, A.M. Anatomical and histological study on the digestive canal of Epilachna chrysomelina (Coleoptera: Coccinellidae). Biosci. Biotechnol. Res. Asia 2013, 10, 183–192. [Google Scholar] [CrossRef]
- Lyal, C.H.; Favreau, E.A. The rectal valve in Curculionoidea (Insecta: Coleoptera). Zootaxa 2015, 3926, 451–479. [Google Scholar] [CrossRef] [Green Version]
- Gullan, P.J.; Cranston, P.S. The Insects. An Outline of Entomology, 5th ed.; Blackwell Publishing Ltd.: Malden, MA, USA; Oxford, UK; Victoria, TX, USA, 2014; p. 88. [Google Scholar]
- Singh, O.L.; Prasad, B. Histomorphology of the alimentary tract of adult, Odoiporus longicollis (Oliv.) (Coleoptera: Curculionidae). J. Entomol. Zool. Stud. 2013, 1, 109–115. [Google Scholar]










| Organ | Organ Width (Mean ± SE) (mm) | Student’s t-Test (t, p) or Mann–Whitney Test (Z, p) | |
|---|---|---|---|
| Male | Female | ||
| Esophagus | 0.074 ± 0.005 a | 0.075 ± 0.006 a | t = −0.132, p = 0.897 |
| Proventriculus | 0.152 ± 0.013 a | 0.174 ± 0.009 a | Z = −1.440, p = 0.150 |
| Midgut | 0.372 ± 0.029 a | 0.422 ± 0.028 a | Z = −1.437, p = 0.151 |
| Ileum | 0.164 ± 0.012 a | 0.139 ± 0.009 a | Z = −1.551, p = 0.121 |
| Colon | 0.231 ± 0.015 a | 0.227 ± 0.019 a | t = 0.179, p = 0.860 |
| Rectum | 0.170 ± 0.010 a | 0.151 ± 0.008 a | t = 1.449, p = 0.165 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Liu, M.; Di Giulio, A.; Chen, X.; Sabatelli, S.; Wang, W.; Audisio, P. Morphological Study of the Alimentary Canal and Malpighian Tubules in the Adult of the Pollen Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae). Insects 2023, 14, 298. https://doi.org/10.3390/insects14030298
Chen L, Liu M, Di Giulio A, Chen X, Sabatelli S, Wang W, Audisio P. Morphological Study of the Alimentary Canal and Malpighian Tubules in the Adult of the Pollen Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae). Insects. 2023; 14(3):298. https://doi.org/10.3390/insects14030298
Chicago/Turabian StyleChen, Longyan, Meike Liu, Andrea Di Giulio, Xinxin Chen, Simone Sabatelli, Wenkai Wang, and Paolo Audisio. 2023. "Morphological Study of the Alimentary Canal and Malpighian Tubules in the Adult of the Pollen Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae)" Insects 14, no. 3: 298. https://doi.org/10.3390/insects14030298
APA StyleChen, L., Liu, M., Di Giulio, A., Chen, X., Sabatelli, S., Wang, W., & Audisio, P. (2023). Morphological Study of the Alimentary Canal and Malpighian Tubules in the Adult of the Pollen Beetle Meligethes (Odonthogethes) chinensis (Coleoptera: Nitidulidae: Meligethinae). Insects, 14(3), 298. https://doi.org/10.3390/insects14030298