Temperature-Dependent Development of Nitidula rufipes (Linnaeus, 1767) (Coleoptera: Nitidulidae) and Its Significance in Estimating Minimum Postmortem Interval
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Species Identification and Colony Establishment
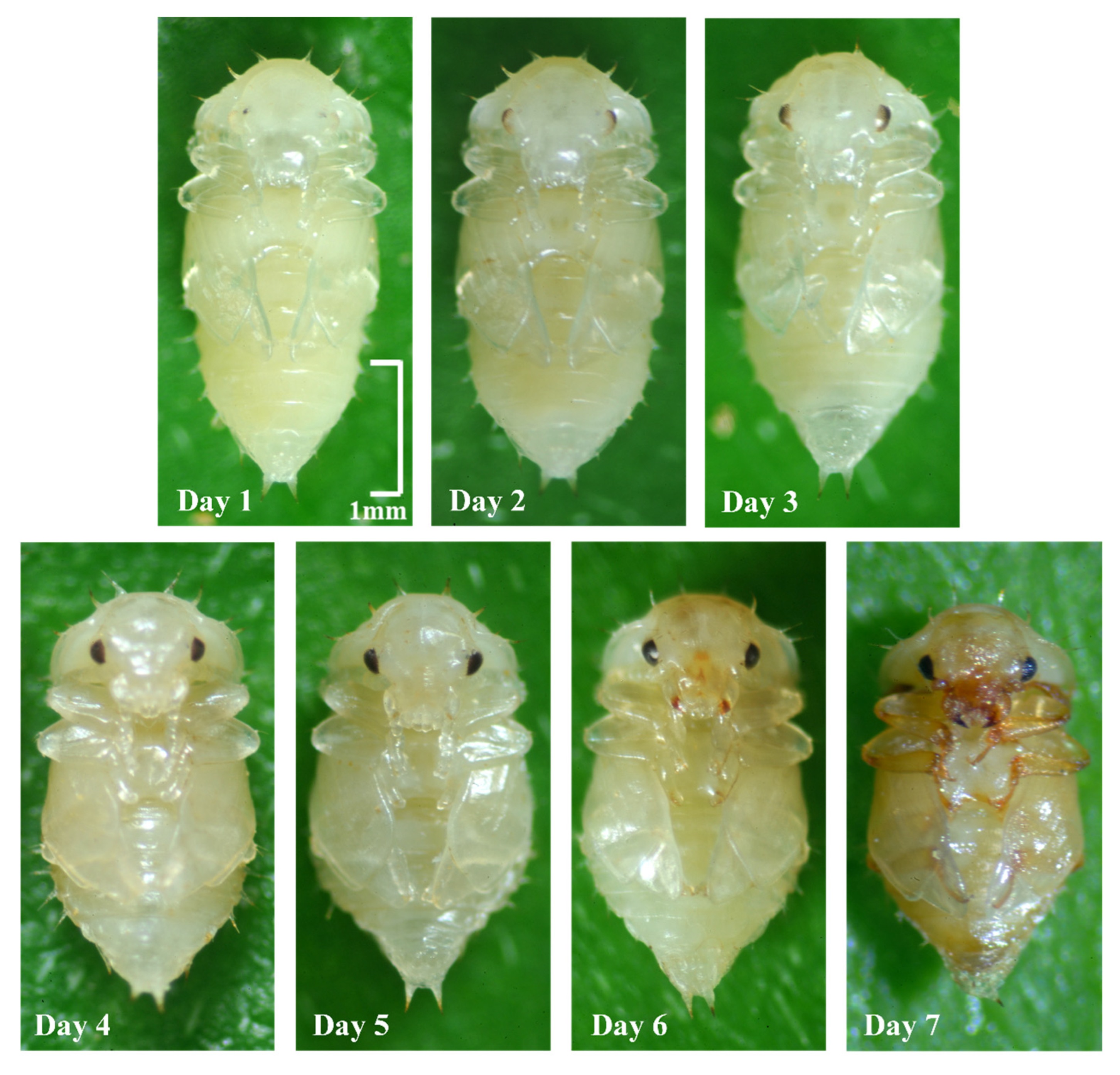
2.2. Observations of Developmental Duration
2.3. Determination of Larval Morphological Indexes
2.4. Data Analysis
3. Results
3.1. Developmental Duration and Isomorphen Diagram
3.2. Thermal Summation Models and Optim SSI Models
3.3. Survival Rates and Larval Body Lengths
3.4. Larval Instar Discrimination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amendt, J.; Krettek, R.; Zehner, R. Forensic entomology. Naturwissenschaften 2004, 91, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Mise, K.M.; Martins, C.; Köb, E.L.; Almeida, L.M. Longer decomposition process and the influence on Coleoptera fauna associated with carcasses. Braz. J. Biol. 2008, 68, 907–908. [Google Scholar] [CrossRef] [PubMed]
- Mądra, A.; Frątczak, K.; Grzywacz, A.; Matuszewski, S. Long-term study of pig carrion entomofauna. Forensic Sci. Int. 2015, 252, 1–10. [Google Scholar] [CrossRef]
- Greenberg, B. Flies as forensic indicators. J. Med. Entomol. 1991, 28, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Amendt, J.; Campobasso, C.P.; Gaudry, E.; Reiter, C.; LeBlanc, H.N.; Hall, M., Jr. Best practice in forensic entomology—Standards and guidelines. Int. J. Legal. Med. 2007, 121, 90–104. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Satpathy, D.K. Use of beetles in forensic entomology. Forensic Sci. Int. 2001, 120, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.; Uribe, A.; Ortiz, A.; Duque, P. A preliminary study of forensic entomology in Medellın, Colombia. Forensic Sci. Int. 2001, 120, 53–59. [Google Scholar] [CrossRef]
- Anderson, G.S.; VanLaerhoven, S.L. Initial studies on insect succession on carrion in southwestern British Columbia. J. Forensic Sci. 1996, 41, 617–625. [Google Scholar] [CrossRef]
- Rodriguez, W.C.; Bass, W.M. Insect activity and its relationship to decay rates of human cadavers in East Tennessee. J. Forensic Sci. 1983, 28, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Midgley, J.M.; Richards, C.S.; Villet, M.H. The Utility of Coleoptera in Forensic Investigations. In Current Concepts in Forensic Entomology; Amendt, J., Goff, M.L., Campobasso, C.P., Grassberger, M., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 57–68. [Google Scholar]
- Wang, Y.; Wang, M.; Hu, G.L.; Xu, W.; Wang, Y.H.; Wang, J.F. Temperature-dependent development of Omosita colon at constant temperature and its implication for PMImin estimation. J. Forensic Leg. Med. 2020, 72, 101946. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, S.; Leschen, R.A.; Lee, S. Evolution of feeding habits of sap beetles (Coleoptera: Nitidulidae) and placement of Calonecrinae. Syst. Entomol. 2020, 45, 911–923. [Google Scholar] [CrossRef]
- Hayashi, N. A contribution to the knowledge of the larvae of Nitidulidae occurring in Japan (Coleoptera: Cucujoidea). Insecta Matsumurana N. S. 1978, 14, 1–97. [Google Scholar]
- Smith, K.G.V. A Manual of Forensic Entomology; The Trustees of the British Museum (Natural History): London, UK, 1986. [Google Scholar]
- Parsons, C.T. A revision of Nearctic Nitidulidae (Coleoptera). Bull. Mus. Comp. Zool. 1943, 92, 121–278. [Google Scholar]
- Reed, H.B. A study of dog carcass communities in Tennessee, with special reference to the insects. Am. Midl. Nat. 1958, 59, 213–245. [Google Scholar] [CrossRef]
- Hinton, H.E. A Monograph of the Beetles Associated with Stored Products; British Museum (Natural History): London, UK, 1945; Volume 1, pp. 1–443. [Google Scholar]
- Dowd, P.F.; Nelsen, T.C. Seasonal variation of sap beetle (Coleoptera: Nitidulidae) populations in central Illinois cornfield—Oak woodland habitat and potential influence of weather patterns. Environ. Entomol. 1994, 23, 1215–1223. [Google Scholar] [CrossRef]
- Zhang, S.F.; Shi, S.F.; Shi, Z.W.; Xue, G.H. Atlas of Beetles Associated with Stored Products; China Agriculture Press: Beijing, China, 2008; p. 134. [Google Scholar]
- Hagstrum, D.W.; Klejdysz, T.; Subramanyam, B.; Nawrot, J. Atlas of Stored-Product Insects and Mites; AACC International: St. Paul, MN, USA, 2013. [Google Scholar]
- Idrissou, F.O.; Huang, Q.; Yanez, O.; Neumann, P. International beeswax trade facilitates small hive beetle invasions. Sci. Rep. 2019, 9, 10665. [Google Scholar] [CrossRef] [Green Version]
- Jelínek, J.; Audisio, P.; Hájek, J.; Baviera, C.; Moncoutier, B.; Barnouin, T.; Brustel, H.; Genç, H.; Leschen, R. New invasive species of Nitidulidae (Coleoptera) in Europe, with a checklist of sap beetles introduced to Europe and Mediterranean areas. Accad. Peloritana Pericolanti Phys. Math. Nat. Sci. 2016, 94, 1–24. [Google Scholar]
- Neumann, P.; Pettis, J.S.; Schäfer, M.O. Quo vadis Aethina tumida? Biology and control of small hive beetles. Apidologie 2016, 47, 427–466. [Google Scholar] [CrossRef] [Green Version]
- Cline, A.R.; Skelley, P.E.; Audisio, P. Morphology and life history of Brachypeplus glaber LeConte (Coleoptera: Nitidulidae), with a discussion of multiple life stage data for phylogenetic analyses. Zootaxa 2013, 3734, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Dowd, P.F. A labor-saving method for rearing the driedfruit beetle (Coleoptera: Nitidulidae) on pinto bean-based diet. J. Econ. Entomol. 1987, 80, 1351–1353. [Google Scholar] [CrossRef]
- Arbogast, R.T.; Torto, B.; Van Engelsdorp, D.; Teal, P.E. An effective trap and bait combination for monitoring the small hive beetle, Aethina tumida (Coleoptera: Nitidulidae). Fla. Entomol. 2007, 90, 404–406. [Google Scholar] [CrossRef]
- Cline, A.R.; Smith, T.R.; Miller, K.; Moulton, M.; Whiting, M.; Audisio, P. Molecular phylogeny of Nitidulidae: Assessment of subfamilial and tribal classification and formalization of the family Cybocephalidae (Coleoptera: Cucujoidea). Syst. Entomol. 2014, 39, 758–772. [Google Scholar] [CrossRef]
- Adair, T.W.; Kondratieff, B.C. The occurrence of Nitidula flavomaculata (Coleoptera: Nitidulidae) on a human corpse. Entomol. News 1996, 107, 233–236. [Google Scholar]
- De Jong, G.D.; Chadwick, J.W. Additional county records and a correction to the checklist of the Calliphoridae (Diptera) of Colorado, with a new state record for Chrysomya rufifacies. J. Kansas Entomol. Soc. 1997, 70, 47–51. [Google Scholar]
- Tantawi, T.I.; El-Kady, E.M.; Greenberg, B.; El-Ghaffar, H.A. Arthropod succession on exposed rabbit carrion in Alexandria, Egypt. J. Med. Entomol. 1996, 33, 566–580. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Miralbés, M. Artrópodos presentes en carroña de cerdos en la comarca de La Litera (Huesca). Bol. Soc. Entomol. Aragonesa 2001, 28, 133–140. [Google Scholar]
- Schlechter, J. Beetle fauna found on carrion in three woodland sites in Luxembourg (Insecta, Coleoptera). Bull. Soc. Nat. Luxemb 2008, 109, 97–100. [Google Scholar]
- Saiz, F.; Tosti-Croce, E.; Leiva, M. Estudio de los cambios de la mesofauna asociada a la descomposición de cadáveres de conejo en clima mediterráneo. An. Mus. Hist. Nat. Valpso. 1989, 20, 41–74. [Google Scholar]
- Mise, K.M.; Almeida, L.M.D.; Moura, M.O. Levantamento da fauna de Coleoptera que habita a carcaça de Sus scrofa L., em Curitiba, Paraná. Rev. Bras Entomol. 2007, 51, 358–368. [Google Scholar] [CrossRef] [Green Version]
- Battán Horenstein, M.; Linhares, A.X. Seasonal composition and temporal succession of necrophagous and predator beetles on pig carrion in central Argentina. Med. Vet. Entomol. 2011, 25, 395–401. [Google Scholar] [CrossRef]
- Shubeck, P.P.; Downie, N.M.; Wenzel, R.L.; Peck, S.B. Species composition and seasonal abundance of carrion beetles in an oak-beech forest in the Great Swamp National Wildlife Refuge (NJ). Entomol. News 1981, 92, 7–16. [Google Scholar]
- Martinez, E.; Duque, P.; Wolff, M. Succession pattern of carrion-feeding insects in Paramo, Colombia. Forensic Sci. Int. 2007, 166, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Arnaldos, M.; Ubero-Pascal, N.; García, R.; Carles-Tolrá, M.; Presa, J.; García, M. The first report of Telomerina flavipes (Meigen, 1830) (Diptera, Sphaeroceridae) in a forensic case, with redescription of its pupa. Forensic Sci. Int. 2014, 242, e22–e30. [Google Scholar] [CrossRef] [PubMed]
- Maisonhaute, J.E.; Forbes, S.L. Decomposition and insect succession on human cadavers in a humid, continental (Dfb) climate (Quebec, Canada). Int. J. Legal Med. 2022, 137, 493–509. [Google Scholar] [CrossRef] [PubMed]
- Ries, A.C.R.; Costa-Silva, V.; Santos, C.F.; Blochtein, B.; Thyssen, P.J. Factors affecting the composition and succession of beetles in exposed pig carcasses in Southern Brazil. J. Med. Entomol. 2021, 58, 104–113. [Google Scholar] [CrossRef]
- Al-Khalifa, M.S.; Mashaly, A.; Alqhtani, A. Impacts of antemortem ingestion of alcoholic beverages on insect successional patterns. Saudi J. Biol. Sci. 2021, 28, 685–692. [Google Scholar] [CrossRef]
- Castro, M.; Centeno, N.; González-Vainer, P. An initial study of insect succession on pig carcasses in open pastures in the northwest of Uruguay. Forensic Sci. Int. 2019, 302, 109837. [Google Scholar] [CrossRef]
- Zanetti, N.I.; Camina, R.; Visciarelli, E.C.; Centeno, N.D. Active search on carcasses versus pitfall traps: A comparison of sampling methods. Neotrop. Entomol. 2016, 45, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, N.I.; Visciarelli, E.C.; Centeno, N.D. Trophic roles of scavenger beetles in relation to decomposition stages and seasons. Rev. Bras. Entomol. 2015, 59, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Rysavy, N.M.; Goff, M.L. Preliminary observations of arthropods associated with buried carrion on Oahu. J. Forensic Sci. 2015, 60, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Caballero, U.; LeónCortés, J.L. Beetle succession and diversity between clothed sun-exposed and shaded pig carrion in a tropical dry forest landscape in Southern Mexico. Forensic Sci. Int. 2014, 245, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, R.C.; Almeida, L.M.; Moura, M.O. Coleoptera associated with buried carrion: Potential forensic importance and seasonal composition. J. Med. Entomol. 2014, 51, 1057–1066. [Google Scholar] [CrossRef]
- Mariani, R.; García-Mancuso, R.; Varela, G.L.; Inda, A.M. Entomofauna of a buried body: Study of the exhumation of a human cadaver in Buenos Aires, Argentina. Forensic Sci. Int. 2014, 237, 19–26. [Google Scholar] [CrossRef]
- Archer, M. Comparative analysis of insect succession data from Victoria (Australia) using summary statistics versus preceding mean ambient temperature models. J. Forensic Sci. 2014, 59, 404–412. [Google Scholar] [CrossRef]
- Azwandi, A.; Nina Keterina, H.N.; Owen, L.C.; Nurizzati, M.D.; Omar, B. Adult carrion arthropod community in a tropical rainforest of Malaysia: Analysis on three common forensic entomology animal models. Trop. Biomed. 2014, 30, 481–494. [Google Scholar]
- Castro, C.P.E.; García, M.D.; Silva, P.D.M.; Silva, I.F.E.; Serrano, A. Coleoptera of forensic interest: A study of seasonal community composition and succession in Lisbon, Portugal. Forensic Sci. Int. 2013, 232, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tumer, A.R.; Karacaoglu, E.; Namli, A.; Keten, A.; Farasat, S.; Akcan, R.; Sert, O.; Odabasi, A.B. Effects of different types of soil on decomposition: An experimental study. Legal Med. 2013, 15, 149–156. [Google Scholar] [CrossRef]
- Benbow, M.E.; Lewis, A.J.; Tomberlin, J.K.; Pechal, J.L. Seasonal necrophagous insect community assembly during vertebrate carrion decomposition. J. Med. Entomol. 2013, 50, 440–450. [Google Scholar] [CrossRef]
- Horenstein, M.B.; Rosso, B.; Garcia, M.D. Seasonal structure and dynamics of sarcosaprophagous fauna on pig carrion in a rural area of Cordoba (Argentina): Their importance in forensic science. Forensic Sci. Int. 2012, 217, 146–156. [Google Scholar] [CrossRef]
- Hobischak, N.R.; VanLaerhoven, S.L.; Anderson, G.S. Successional patterns of diversity in insect fauna on carrion in sun and shade in the boreal forest region of Canada, near Edmonton, Alberta. Can. Entomol. 2006, 138, 376–383. [Google Scholar] [CrossRef]
- Schroeder, H.; Klotzbach, H.; Puschel, K. Insects’ colonization of human corpses in warm and cold season. Leg. Med. 2003, 5, S372–S374. [Google Scholar] [CrossRef]
- Davis, J.B.; Goff, M.L. Decomposition patterns in terrestrial and intertidal habitats on Oahu Island and Coconut Island, Hawaii. J. Forensic Sci. 2000, 45, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.N.; Goff, M.L. Arthropod succession on exposed carrion in three contrasting tropical habitats on Hawaii Island, Hawaii. J. Med. Entomol. 1997, 34, 328–339. [Google Scholar] [CrossRef]
- Bonacci, T.; Mendicino, F.; Bonelli, D.; Carlomagno, F.; Curia, G.; Scapoli, C.; Pezzi, M. Investigations on arthropods associated with decay stages of buried animals in Italy. Insects 2021, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Sharanowski, B.J.; Walker, E.G.; Anderson, G.S. Insect succession and decomposition patterns on shaded and sunlit carrion in Saskatchewan in three different seasons. Forensic Sci. Int. 2008, 179, 219–240. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, Z.G.; Yu, L.C.; Chen, Q.S.; Huang, A.H.; Liao, M.Q.; Xie, Y.T.; Chen, Y.C. Succession and development of insects on pig carcasses and their significances in estimating PMI in the Pearl River Delta region. Forensic Sci. Int. 2008, 179, 11–18. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.M.; Corrêa, R.C.; Grossi, P.C. Coleoptera species of forensic importance from Brazil: An updated list. Rev. Bras. Entomol. 2015, 59, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Byrd, J.H.; Tomberlin, J.K. Forensic Entomology: The Utility of Arthropods in Legal Investigations; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Torres, H.H.; Martínez, O.G.; García, I.S.; Uribe, L.A.A.; Peña, S.R.S. Primer registro de Omosita colon (Linnaeus) (Coleoptera: Nitidulidae: Nitidulinae) recolectada en cadáveres de cabrito (Capra aegagrus hircus Linnaeus) en Buenavista, Saltillo Coahuila, México. Insecta Mundi 2018, 0626, 1–5. [Google Scholar]
- Cao, Y.K.; Huang, M. A SEM study of the antenna and mouthparts of Omosita colon (Linnaeus) (Coleoptera: Nitidulidae). Microsc. Res. Tech. 2016, 79, 1152–1164. [Google Scholar] [CrossRef]
- Perris, É. Larves de coléoptères. Ann. Soc. Linn. Lyon 1877, 23, 1–430. [Google Scholar]
- Ortloff, A.; Zanetti, N.; Centeno, N.; Silva, R.; Bustamante, F.; Olave, A. Ultramorphological characteristics of mature larvae of Nitidula carnaria (Schaller 1783) (Coleoptera: Nitidulidae), a beetle species of forensic importance. Forensic Sci. Int. 2014, 239, e1–e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Aranda, L.M.; Martín-Vega, D.; Baz, A.; Cifrián, B. Larval identification key to necrophagous Coleoptera of medico-legal importance in the western Palaearctic. Int. J. Legal Med. 2018, 132, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.A.; Clitheroe, C.L.; Villet, M.H.; Midgley, J.M. The first record of Omosita nearctica Kirejtshuk (Coleoptera, Nitidulidae) in South Africa, with the first description of its mature larva. Afr. Invertebr. 2021, 62, 257–271. [Google Scholar] [CrossRef]
- Zanetti, N.I.; Visciarelli, E.C.; Centeno, N.D. Preliminary data on larval morphology and life cycle of Nitidula carnaria (Coleoptera: Nitidulidae), a species of forensic interest. Rev. Soc. Entomol. Argent. 2014, 72, 195–198. [Google Scholar]
- Hu, G.W.; Kang, C.T.; Zhu, R.; Guo, Y.; Li, L.L.; Wang, Y.H.; Zhang, Y.N.; Wang, Y.; Wang, J.F. A preliminary study of body decomposition and arthropod succession in an arid area in Northwest China during summer. J. Med. Entomol. 2023, 60, 306–315. [Google Scholar] [CrossRef]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Molec. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Ikemoto, T.; Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ. Entomol. 2000, 29, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Shi, P.J.; Ikemoto, T.; Egami, C.; Sun, Y.C.; Ge, F. A modified program for estimating the parameters of the SSI model. Environ. Entomol. 2011, 40, 462–469. [Google Scholar] [CrossRef]
- Lyu, Z.; Wan, L.H.; Yang, Y.Q.; Tang, R.; Xu, L.Z. A checklist of beetles (Insecta, Coleoptera) on pig carcasses in the suburban area of southwestern China: A preliminary study and its forensic relevance. J. Forensic Leg. Med. 2016, 41, 42–48. [Google Scholar] [CrossRef]
- Anton, E.; Niederegger, S.; Beutel, R.G. Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med. Vet. Entomol. 2011, 25, 353–364. [Google Scholar] [CrossRef]
- Kočárek, P. Decomposition and Coleoptera succession on exposed carrion of small mammal in Opava, the Czech Republic. Eur. J. Soil Biol. 2003, 39, 31–45. [Google Scholar] [CrossRef]
- Matuszewski, S.; Frątczak, K.; Konwerski, S.; Bajerlein, D.; Szpila, K.; Jarmusz, M.; Szafałowicz, M.; Grzywacz, A.; Mądra, A. Effect of body mass and clothing on carrion entomofauna. Int. J. Legal Med. 2016, 130, 221–232. [Google Scholar] [CrossRef] [Green Version]
- De Jong, G.D.; Hoback, W.W. Effect of investigator disturbance in experimental forensic entomology: Succession and community composition. Med. Vet. Entomol. 2006, 20, 248–258. [Google Scholar] [CrossRef] [Green Version]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci. Int. 2010, 195, 42–51. [Google Scholar] [CrossRef]
- Watson, E.J.; Carlton, C.E. Insect succession and decomposition of wildlife carcasses during fall and winter in Louisiana. J. Med. Entomol. 2005, 42, 193–203. [Google Scholar] [CrossRef]
- Matuszewski, S.; Szafałowicz, M.; Jarmusz, M. Insects colonising carcasses in open and forest habitats of Central Europe: Search for indicators of corpse relocation. Forensic Sci. Int. 2013, 231, 234–239. [Google Scholar] [CrossRef]
- Bourel, B.; Martin-Bouyer, L.; Hedouin, V.; Cailliez, J.C.; Derout, D.; Gosset, D. Necrophilous insect succession on rabbit carrion in sand dune habitats in northern France. J. Med. Entomol. 1999, 36, 420–425. [Google Scholar] [CrossRef]
- Williams, R.N.; Blackmer, J.L.; Richmond, D.S.; Ellis, M.S. Nitidulidae (Coleoptera) diversity in three natural preserves in Portage County, Ohio. Ohio J. Sci. 1992, 92, 82–87. [Google Scholar]
- Saloña, M.I.; Moraza, M.L.; Carles Tolrá, M.; Iraola, V.; Bahillo, P.; Yélamos, T.; Outerelo, R.; Alcaraz, R. Searching the soil: Forensic importance of edaphic fauna after the removal of a corpse. J. Forensic Sci. 2010, 55, 1652–1655. [Google Scholar] [CrossRef]
- Watson, E.J.; Carlton, C.E. Spring succession of necrophilous insects on wildlife carcasses in Louisiana. J. Med. Entomol. 2003, 40, 338–347. [Google Scholar] [CrossRef]
- Li, L.L.; Guo, Y.; Zhou, Y.X.; Yang, Y.; Kang, C.T.; Hu, G.W.; Wang, Y.H.; Zhang, Y.N.; Wang, Y.; Wang, J.F. Succession patterns of sarcosaprophagous insects on pig carcasses in different months in Yangtze River Delta, China. Forensic Sci. Int. 2022, 342, 111518. [Google Scholar] [CrossRef]
- Pastula, E.C.; Merritt, R.W. Insect arrival pattern and succession on buried carrion in Michigan. J. Med. Entomol. 2013, 50, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Park, S.H.; Lee, J.H.; Woo, D.; Ji, B.H.; Moon, T.Y. Insect diversity and succession patterns on pig cadavers in Changwon, South Korea. Entomol. Res. 2022, 52, 241–250. [Google Scholar] [CrossRef]
- Probst, C.; Gethmann, J.; Amendt, J.; Lutz, L.; Teifke, J.P.; Conraths, F.J. Estimating the postmortem interval of wild boar carcasses. Vet. Sci. 2020, 7, 6. [Google Scholar] [CrossRef] [Green Version]
- Lutz, L.; Amendt, J.; Moreau, G. Carcass concealment alters assemblages and reproduction of forensically important beetles. Forensic Sci. Int. 2018, 291, 124–132. [Google Scholar] [CrossRef]
- Perez, A.E.; Haskell, N.H.; Wells, J.D. Evaluating the utility of hexapod species for calculating a confidence interval about a succession based postmortem interval estimate. Forensic Sci. Int. 2014, 241, 91–95. [Google Scholar] [CrossRef]
- Michaud, J.P.; Majka, C.G.; Privé, J.P.; Moreau, G. Natural and anthropogenic changes in the insect fauna associated with carcasses in the North American Maritime lowlands. Forensic Sci. Int. 2010, 202, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Jarmusz, M.; Grzywacz, A.; Bajerlein, D. A comparative study of the entomofauna (Coleoptera, Diptera) associated with hanging and ground pig carcasses in a forest habitat of Poland—ScienceDirect. Forensic Sci. Int. 2020, 309, 110212. [Google Scholar] [CrossRef]
- Baz, A.; Botías, C.; Martín-Vega, D.; Cifrián, B.; Díaz-Aranda, L.M. Preliminary data on carrion insects in urban (indoor and outdoor) and periurban environments in central Spain. Forensic Sci. Int. 2015, 248, 41–47. [Google Scholar] [CrossRef]
- Díaz-Aranda, L.M.; Martín-Vega, D.; Gómez-Gómez, A.; Cifrián, B.; Baz, A. Annual variation in decomposition and insect succession at a periurban area of central Iberian Peninsula. J. Forensic Leg. Med. 2018, 56, 21–31. [Google Scholar] [CrossRef]
- Zanetti, N.I.; Visciarelli, E.C.; Centeno, N.D. Associational patterns of scavenger beetles to decomposition stages. J. Forensic Sci. 2015, 60, 919–927. [Google Scholar] [CrossRef]
- Özdemir, S.; Sert, O. Determination of Coleoptera fauna on carcasses in Ankara province, Turkey. Forensic Sci. Int. 2009, 183, 24–32. [Google Scholar] [CrossRef]
- Bajerlein, D.; Taberski, D.; Matuszewski, S. Estimation of postmortem interval (PMI) based on empty puparia of Phormia regina (Meigen) (Diptera: Calliphoridae) and third larval stage of Necrodes littoralis (L.) (Coleoptera: Silphidae)–Advantages of using different PMI indicators. J. Forensic Leg. Med. 2018, 59, 59. [Google Scholar] [CrossRef]
- Martín-Vega, D.; Baz, A.; Cifrián, B.; Gómez-Gómez, A.; Díaz-Aranda, L.M. Long-term insect successional patterns on pig carcasses in central Spain. Int. J. Legal. Med. 2019, 133, 1581–1592. [Google Scholar] [CrossRef]
- Matuszewski, S.; Mądra-Bielewicz, A. Post-mortem interval estimation based on insect evidence in a quasi-indoor habitat. Sci. Justice 2019, 59, 109–115. [Google Scholar] [CrossRef]
- Mashaly, A.; Al Khalifa, M.; Al Qahtni, A.; Alshehri, A. Analysis of insects colonised on human corpses during autopsy in Riyadh, Saudi Arabia. Entomol. Res. 2020, 50, 351–360. [Google Scholar] [CrossRef]
- Pérez-Marcos, M.; Arnaldos-Sanabria, M.I.; García, M.D.; Presa, J.J. Pérez-Marcos, M.; Arnaldos-Sanabria, M.I.; García, M.D.; Presa, J.J. Examining the sarcosaprophagous fauna in a natural mountain environment (Sierra Espuña, Murcia, Spain). Ann. Soc. Entomol. Fr. 2016, 52, 264–280. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Keshavarzi, D.; Fereidooni, M.; Soltani, A. First survey of forensically important insects from human corpses in Shiraz, Iran. J. Forensic Leg. Med. 2018, 54, 62–68. [Google Scholar] [CrossRef]
- Bonacci, T.; Mendicino, F.; Carlomagno, F.; Bonelli, D.; Marchetti, M.G.; Vicenzi, A.; Scapoli, C.; Pezzi, M. Necrodes littoralis (Coleoptera: Silphidae) visiting and breeding on a carcass in Italy. Trop. Biomed. 2022, 39, 203–208. [Google Scholar]
- Oliva, A. Insects of forensic significance in Argentina. Forensic Sci. Int. 2001, 120, 145–154. [Google Scholar] [CrossRef]
- Jung, J.B.; Yoon, M.H. A study on the arthropod succession in exposed pig carrion. J. Life Sci. 2008, 18, 1400–1409. [Google Scholar] [CrossRef]
- Frątczak, K.; Matuszewski, S. Instar determination in forensically useful beetles Necrodes littoralis (Silphidae) and Creophilus maxillosus (Staphylinidae). Forensic Sci. Int. 2014, 241, 20–26. [Google Scholar] [CrossRef]
- Karabey, T.; Sert, O. The analysis of pupal development period in Lucilia sericata (Diptera: Calliphoridae) forensically important insect. Int. J. Legal Med. 2018, 132, 1185–1196. [Google Scholar] [CrossRef]
- Davies, K.; Harvey, M.L. Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J. Forensic Sci. 2013, 58, 79–84. [Google Scholar] [CrossRef]
- Novák, M.; Frątczak-Łagiewska, K.; Mądra-Bielewicz, A.; Matuszewski, S. Eye-background contrast as a quantitative marker for pupal age in a forensically important carrion beetle Necrodes littoralis L. (Silphidae). Sci. Rep. 2021, 10, 14494. [Google Scholar] [CrossRef]
- Matuszewski, S.; Bajerlein, D.; Konwerski, S.; Szpila, K. An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci. Int. 2008, 180, 61–69. [Google Scholar] [CrossRef]
- Payne, J.A.; King, E.W. Coleoptera associated with pig carrion. Entomol. Mon. Mag. 1970, 105, 1265–1267. [Google Scholar]
- Másmela, L.A.O. Entomofauna sucesional en el cadáver de un cánido en condiciones de campo en la Universidad del Valle (Cali-Colombia). Cuad. Med. Forense 2001, 23, 5–14. [Google Scholar]
- Piñero, F.S. Analysis of spatial and seasonal variability of carrion beetle (Coleoptera) assemblages in two arid zones of Spain. Environ. Entomol. 1997, 26, 805–814. [Google Scholar] [CrossRef]
- James, D.G.; Vogele, B. Development and survivorship of Carpophilus hemipterus (L.), Carpophilus mutilatus Erichson and Carpophilus humeralis (F.) (Coleoptera: Nitidulidae) over a range of constant temperatures. Aust. J. Entomol. 2000, 39, 180–184. [Google Scholar] [CrossRef]
- Nielsen, P.S.; Axelsen, J. Developmental time and mortality of the immature stages of the pollen beetle (Meligethes aeneus F.) under natural conditions. J. Appl. Entomol. 1988, 105, 198–204. [Google Scholar] [CrossRef]
- Mussen, E.C.; Chiang, H.C. Development of the picnic beetle, Glischrochilus quadrisignatus (Say), at various temperatures. Environ. Entomol. 1974, 3, 1032–1034. [Google Scholar] [CrossRef]
- Midgley, J.M.; Villet, M.H. Development of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) at constant temperatures. Int. J. Legal Med. 2009, 123, 285–292. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.B.; Wang, J.F.; Li, L.L.; Wang, M.; Yang, L.J.; Tao, L.Y.; Chu, J.; Hou, Y.D. Development of the forensically important beetle Creophilus maxillosus (Coleoptera: Staphylinidae) at constant temperatures. J. Med. Entomol. 2017, 54, 281–289. [Google Scholar]
- Wang, Y.H.; Li, L.L.; Hu, G.W.; Kang, C.T.; Guo, Y.; Zhang, Y.N.; Wang, Y.; Wang, J.F. Development of Necrobia ruficollis (Fabricius) (Coleoptera: Cleridae) under Different Constant Temperatures. Insects 2022, 13, 319. [Google Scholar] [CrossRef]
- Midgley, J.M.; Villet, M.H. Effect of the killing method on post-mortem change in length of larvae of Thanatophilus micans (Fabricius 1794) (Coleoptera: Silphidae) stored in 70% ethanol. Int. J. Legal Med. 2009, 123, 103–108. [Google Scholar] [CrossRef]
- Frątczak-Łagiewska, K.; Matuszewski, S. The quality of developmental reference data in forensic entomology: Detrimental effects of multiple, in vivo measurements in Creophilus maxillosus L. (Coleoptera: Staphylinidae). Forensic Sci. Int. 2019, 298, 316–322. [Google Scholar] [CrossRef]
- McCalla, K.A.; Keçeci, M.; Milosavljević, I.; Ratkowsky, D.A.; Hoddle, M.S. The influence of temperature variation on life history parameters and thermal performance curves of Tamarixia radiata (Hymenoptera: Eulophidae), a parasitoid of the Asian citrus psyllid (Hemiptera: Liviidae). J. Econ. Entomol. 2019, 112, 1560–1574. [Google Scholar] [CrossRef]
- Milosavljević, I.; McCalla, K.A.; Ratkowsky, D.A.; Hoddle, M.S. Effects of constant and fluctuating temperatures on development rates and longevity of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae). J. Econ. Entomol. 2019, 112, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Hagstrum, D.W.; Milliken, G.A. Modeling differences in insect developmental times between constant and fluctuating temperatures. Ann. Entomol. Soc. Am. 1991, 84, 369–379. [Google Scholar] [CrossRef]










| Developmental Events | Temperature (°C) | |||||||
|---|---|---|---|---|---|---|---|---|
| 16 °C | 19 °C | 22 °C | 25 °C | 28 °C | 31 °C | 34 °C | ||
| Hatching | min | 10.14 ± 0.38 a | 8.44 ± 0.42 b | 6.14 ± 0.17 c | 4.79 ± 0.21 d | 3.71 ± 0.15 e | 3.10 ± 0.26 f | 2.82 ± 0.07 f |
| mean | 11.21 ± 0.89 a | 8.80 ± 0.40 b | 6.91 ± 0.44 c | 5.47 ± 0.28 d | 4.19 ± 0.26 e | 3.65 ± 0.36 f | 3.32 ± 0.25 g | |
| max | 12.14 ± 1.06 a | 9.65 ± 0.20 b | 7.97 ± 0.48 c | 6.13 ± 0.05 d | 4.64 ± 0.17 e | 4.31 ± 0.12 e | 3.83 ± 0.30 e | |
| 1st ecdysis | min | 18.47 ± 0.51 a | 14.44 ± 0.42 b | 10.47 ± 0.75 c | 8.46 ± 0.77 d | 6.71 ± 0.15 e | 5.77 ± 0.79 ef | 5.15 ± 0.59 f |
| mean | 20.30 ± 0.92 a | 16.00 ± 0.81 b | 11.90 ± 0.71 c | 9.53 ± 0.68 d | 7.30 ± 0.53 e | 6.03 ± 0.61 f | 5.87 ± 0.51 f | |
| max | 22.47 ± 0.85 a | 16.99 ± 0.55 b | 12.64 ± 0.17 c | 10.79 ± 0.54 d | 7.64 ± 0.17 e | 6.64 ± 0.64 ef | 6.17 ± 0.85 f | |
| 2nd ecdysis | min | 26.14 ± 1.00 a | 21.11 ± 0.67 b | 15.14 ± 0.17 c | 11.46 ± 0.41 d | 8.71 ± 0.15 e | 7.77 ± 0.79 f | 6.82 ± 0.07 g |
| mean | 28.03 ± 1.03 a | 23.57 ± 1.17 b | 16.33 ± 0.80 c | 11.77 ± 0.77 d | 9.63 ± 0.67 e | 8.43 ± 0.82 f | 8.13 ± 0.57 f | |
| max | 30.47 ± 0.48 a | 25.32 ± 0.38 b | 16.97 ± 0.48 c | 13.13 ± 0.05 d | 9.97 ± 0.48 e | 9.31 ± 0.12 f | 8.83 ± 0.30 f | |
| Wandering | min | 33.14 ± 1.58 a | 25.11 ± 0.98 b | 19.47 ± 2.17 c | 14.12 ± 0.77 d | 11.04 ± 0.73 e | 9.43 ± 0.82 ef | 8.82 ± 0.07 f |
| mean | 38.00 ± 3.91 a | 29.80 ± 3.33 b | 22.37 ± 2.64 c | 16.15 ± 1.41 d | 12.71 ± 1.57 e | 11.42 ± 1.91 f | 11.61 ± 1.96 f | |
| max | 44.14 ± 7.47 a | 38.32 ± 4.56 a | 27.64 ± 4.58 b | 19.46 ± 1.20 c | 18.97 ± 2.69 c | 17.64 ± 1.56 c | 17.17 ± 0.36 c | |
| Pupation | min | 48.47 ± 2.50 a | 36.44 ± 1.42 b | 26.14 ± 1.79 c | 20.79 ± 1.21 d | 16.38 ± 0.63 e | 14.77 ± 0.79 ef | 13.50 ± 0.85 f |
| mean | 52.40 ± 2.70 a | 39.88 ± 2.31 b | 29.36 ± 2.94 c | 23.07 ± 1.54 d | 18.15 ± 1.47 e | 15.38 ± 0.95 f | 14.94 ± 1.28 f | |
| max | 55.14 ± 1.02 a | 45.32 ± 2.36 b | 34.64 ± 3.53 c | 26.13 ± 1.05 d | 20.97 ± 0.48 e | 17.98 ± 1.22 e | 17.50 ± 1.82 e | |
| Eclosion | min | 65.14 ± 5.01 a | 45.78 ± 1.85 b | 35.8 ± 2.36 c | 26.46 ± 1.34 d | 20.71 ± 1.15 de | 18.77 ± 0.79 de | 17.17 ± 0.85 e |
| mean | 71.05 ± 4.37 a | 52.94 ± 4.12 b | 40.09 ± 3.43 c | 30.07 ± 2.13 d | 24.20 ± 2.04 e | 20.95 ± 2.31 f | 20.78 ± 2.40 f | |
| max | 80.47 ± 4.29 a | 59.65 ± 5.15 b | 48.3 ± 5.13 c | 35.46 ± 1.48 d | 30.3 ± 1.94 de | 30.31 ± 1.01 de | 26.5 ± 0.84 e | |
| Developmental Stage | K ± SE (Degree Days) | TL ± SE (°C) | R2 |
|---|---|---|---|
| Hatching | 83.12 ± 4.73 | 9.11 ± 0.70 a | 0.97 |
| 1st ecdysis | 139.39 ± 8.74 | 9.67 ± 0.72 b | 0.97 |
| 2nd ecdysis | 181.50 ± 14.96 | 10.26 ± 0.89 c | 0.96 |
| Wandering | 255.15 ± 20.08 | 9.78 ± 0.90 b | 0.95 |
| Pupation | 347.21 ± 17.41 | 9.74 ± 0.57 b | 0.98 |
| Eclosion | 471.40 ± 25.46 | 9.65 ± 0.62 b | 0.98 |
| Parameter (Unit) | Hatching | 1st Ecdysis | 2nd Ecdysis | Wandering | Pupation | Emergence |
|---|---|---|---|---|---|---|
| TΦ (°C) | 20.38 | 20.23 | 21.06 | 21.30 | 24.05 | 24.15 |
| ρΦ (day−1) | 0.13 | 0.07 | 0.06 | 0.04 | 0.04 | 0.03 |
| ∆HA (cal/mol) | 1.35 × 104 | 1.45 × 104 | 1.56 × 104 | 1.49 × 104 | 1.42 × 104 | 1.40 × 104 |
| ∆HL (cal/mol) | −1.53 × 105 | −1.19 × 105 | −1.44 × 105 | −1.31 × 105 | −3.87 × 104 | −5.72 × 104 |
| ∆HH (cal/mol) | 8.67 × 104 | 1.07 × 105 | 8.19 × 104 | 8.56 × 104 | 6.92 × 104 | 7.88 × 104 |
| TL (°C) | 11.03 | 7.24 | 12.68 | 12.19 | 6.20 | 10.12 |
| TH (°C) | 37.19 | 35.96 | 35.76 | 35.46 | 36.64 | 36.00 |
| χ2 | 2.73 × 10−4 | 7.72 × 10−5 | 9.62 × 10−4 | 3.67 × 10−4 | 2.51 × 10−5 | 4.06 × 10−5 |
| R2 | 0.999 | 0.999 | 0.992 | 0.994 | 0.999 | 0.999 |
| Temperature (°C) | Equation | df | R2 |
|---|---|---|---|
| 16 | L = −2.146E-4T3 + 0.011T2 + 0.109T + 1.107 | 793 | 0.929 |
| 19 | L = −4.651E-4T3 + 0.018T2 + 0.108T + 1.175 | 799 | 0.926 |
| 22 | L = −0.002T3 + 0.036T2 + 0.235T + 0.927 | 502 | 0.931 |
| 25 | L = −0.014T3 + 0.245T2 − 0.455T + 1.584 | 311 | 0.897 |
| 28 | L = −0.015T3 + 0.204T2 + 0.140T + 0.900 | 276 | 0.952 |
| 31 | L = −0.023T3 + 0.278T2 + 0.067T + 0.938 | 300 | 0.955 |
| 34 | L = −0.013T3 + 0.117T2 + 0.773T + 0.211 | 265 | 0.922 |
| Morphological Indexes | Instar | Mean ± SD | Range | Sample Size |
|---|---|---|---|---|
| The widths of head capsules | 1st | 0.21 ± 0.02 | 0.15–0.28 | 1055 |
| 2nd | 0.37 ± 0.04 | 0.24–0.49 | 905 | |
| 3rd | 0.55 ± 0.05 | 0.37–0.73 | 1387 | |
| The distance of urogomphi | 1st | 0.11 ± 0.01 | 0.07–0.16 | 1055 |
| 2nd | 0.20 ± 0.02 | 0.14–0.28 | 905 | |
| 3rd | 0.30 ± 0.03 | 0.20–0.39 | 1387 |
| Instar | Sample Size | Size Prediction of Classification Precision | Precision Rate | ||
|---|---|---|---|---|---|
| 1st | 2nd | 3rd | |||
| 1st | 802 | 802 | 0 | 0 | 100.00% |
| 2nd | 838 | 0 | 823 | 15 | 98.21% |
| 3rd | 1030 | 0 | 2 | 1028 | 99.81% |
| Total | 2660 | 802 | 825 | 1043 | 99.74% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Li, L.; Guo, Y.; Kang, C.; Wang, Y.; Zhang, Y.; Zhang, Z.; Wang, J.; Wang, Y. Temperature-Dependent Development of Nitidula rufipes (Linnaeus, 1767) (Coleoptera: Nitidulidae) and Its Significance in Estimating Minimum Postmortem Interval. Insects 2023, 14, 299. https://doi.org/10.3390/insects14030299
Hu G, Li L, Guo Y, Kang C, Wang Y, Zhang Y, Zhang Z, Wang J, Wang Y. Temperature-Dependent Development of Nitidula rufipes (Linnaeus, 1767) (Coleoptera: Nitidulidae) and Its Significance in Estimating Minimum Postmortem Interval. Insects. 2023; 14(3):299. https://doi.org/10.3390/insects14030299
Chicago/Turabian StyleHu, Gengwang, Liangliang Li, Yi Guo, Chengtao Kang, Yinghui Wang, Yanan Zhang, Zhixiang Zhang, Jiangfeng Wang, and Yu Wang. 2023. "Temperature-Dependent Development of Nitidula rufipes (Linnaeus, 1767) (Coleoptera: Nitidulidae) and Its Significance in Estimating Minimum Postmortem Interval" Insects 14, no. 3: 299. https://doi.org/10.3390/insects14030299
APA StyleHu, G., Li, L., Guo, Y., Kang, C., Wang, Y., Zhang, Y., Zhang, Z., Wang, J., & Wang, Y. (2023). Temperature-Dependent Development of Nitidula rufipes (Linnaeus, 1767) (Coleoptera: Nitidulidae) and Its Significance in Estimating Minimum Postmortem Interval. Insects, 14(3), 299. https://doi.org/10.3390/insects14030299





