Vermicompost Amendments Disrupt Feeding Behavior of Diaphorina citri Kuwayama and Boost Activities of Salicylic Acid and Jasmonic Acid Pathway-Related Enzymes in Citrus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Vermicompost and Soil
2.2. Plants and Insects
2.3. Feeding Behavior of D. citri
2.4. Activity of Key Enzymes in the SA and JA Defense Pathway
2.5. Statistical Analysis
3. Results
3.1. Feeding Behavior of D. citri
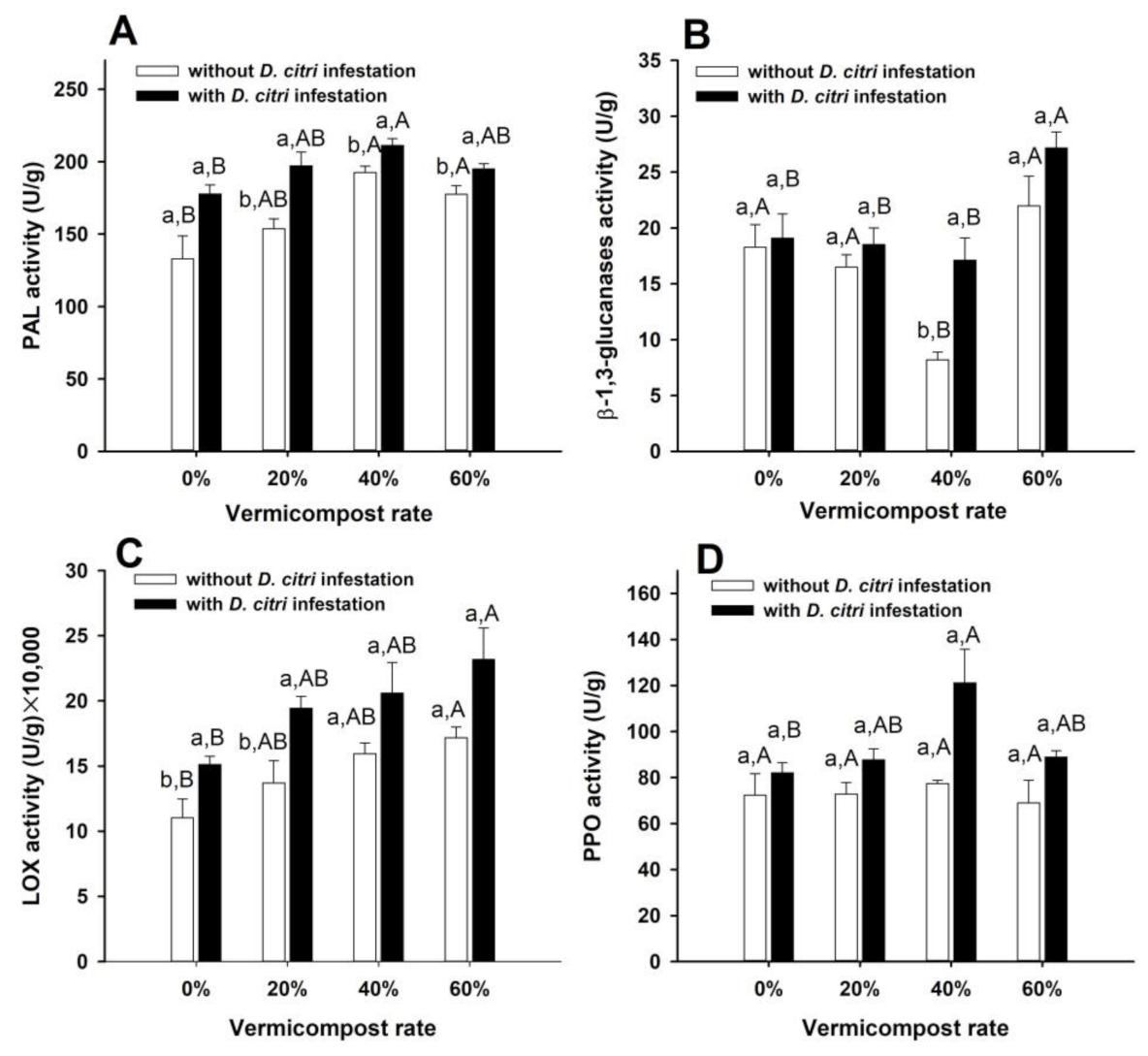
3.2. Activities of SA and JA Pathway-Related Enzymes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, U.; Sajid, N.; Khalid, A.; Riaz, L.; Rabbani, M.M.; Syed, J.H.; Malik, R.N. A review on vermicomposting of organic wastes. Environ. Prog. Sustain. Energy 2015, 34, 1050–1062. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Ali, M.; Baba, Z.A.; Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron. Sustain. Dev. 2021, 41, 7. [Google Scholar] [CrossRef]
- Blouin, M.; Barrere, J.; Meyer, N.; Lartigue, S.; Barot, S.; Mathieu, J. Vermicompost Significantly Affects Plant Growth. A Meta-Analysis. Agron. Sustain. Dev. 2019, 39, 34. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric. Ecosyst. Environ. 2021, 315, 107425. [Google Scholar]
- Arancon, N.Q.; Edwards, C.A.; Yardim, E.N.; Oliver, T.J.; Byrne, R.J.; Keeney, G. Suppression of two-spotted spider mite (Tetranychus urticae), mealy bug (Pseudococcus sp.) and aphid (Myzus persicae) populations and damage by vermicomposts. Crop Prot. 2007, 26, 29–39. [Google Scholar]
- Razmjou, J.; Mohammadi, M.; Hassanpour, M. Effect of vermicompost and cucumber cultivar on population growth attributes of the melon aphid (Hemiptera: Aphididae). J. Econ. Entomol. 2011, 104, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Sedaghatbaf, R.; Samih, M.A.; Zohdi, H.; Zarabi, M. Vermicomposts of different origins protect tomato plants against the sweetpotato whitefly. J. Econ. Entomol. 2018, 111, 146–153. [Google Scholar] [CrossRef]
- Mardani-Talaee, M.; Nouri-Ganblani, G.; Razmjou, J.; Hassanpour, M.; Naseri, B.; Asgharzadeh, A. Effects of chemical, organic and bio-fertilizers on some secondary metabolites in the leaves of bell pepper (Capsicum annuum) and their impact on life table parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2016, 109, 1231–1240. [Google Scholar] [CrossRef]
- Ravi, M.; Dhandapani, N.; Sathiah, N.; Murugan, M. Influence of organic manures and fertilizers on the incidence of sucking pests of sunflower, Helianthus annuus L. Ann. Plant Prot. Sci. 2006, 14, 41–44. [Google Scholar]
- Fong, C.-J.; Chuang, Y.-Y.; Lai, H.-Y. Effect of sulfur-enriched vermicompost on the growth of Brassica chinensis L. and the Spodoptera litura Fabricius larvae feeding. Agriculture 2022, 12, 494. [Google Scholar] [CrossRef]
- Morkunas, I.; Mai, V.C.; Gabrys, B. Phytohormonal signaling in plant responses to aphid feeding. Acta Physiol. Plant. 2011, 33, 2057–2073. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Sun, J.; Shi, W.; Harwood, J.D.; Monticelli, L.S.; Tan, X.; Chen, J. Effects of field simulated warming on feeding behavior of Sitobion avenae (Fabricius) and host defense systems. Entomol. Gen. 2021, 41, 567–578. [Google Scholar] [CrossRef]
- Gao, J.; Tao, T.; Arthurs, S.P.; Ye, F.; An, X.; Hussain, M.; Mao, R. Plant jasmonic acid mediated contrasting effects of two citrus aphid species on Diaphorina citri Kuwayama. Pest Manag. Sci. 2022, 79, 811–820. [Google Scholar] [CrossRef]
- Islam, W.; Tayyab, M.; Khalil, F.; Hua, Z.; Huang, Z.; Chen, H.Y. Silicon-mediated plant defense against pathogens and insect pests. Pestic. Biochem. Physiol. 2020, 168, 104641. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.H.-O.; Chung, Y.R. Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Song, Y.; Long, J.; Wang, R.; Baerson, S.R.; Pan, Z.; Zhu-Salzman, K.; Xie, J.; Cai, K.; Luo, S. Priming of jasmonate-mediated antiherbivore defense responses in rice by silicon. Proc. Natl. Acad. Sci. USA 2013, 110, E3631–E3639. [Google Scholar] [CrossRef] [PubMed]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and Management of Asian Citrus Psyllid, Vector of the Huanglongbing Pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Backus, E.A.; Guedes, R.N.C.; Reif, K.E. AC–DC electropenetrography: Fundamentals, controversies, and perspectives for arthropod pest management. Pest Manag. Sci. 2021, 77, 1132–1149. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, H.; Ge, F. Plant–aphid interactions under elevated CO2: Some cues from aphid feeding behavior. Front. Plant Sci. 2016, 7, 502. [Google Scholar] [CrossRef]
- Maluta, N.K.P.; Lopes, J.R.S.; Fiallo-Olivé, E.; Navas-Castillo, J.; Lourenção, A.L. Foliar application of systemic insecticides disrupts feeding behavior of the whitefly Bemisia tabaci MEAM1 and the transmission of tomato chlorosis virus in potato plants. J. Pest Sci. 2021, 94, 1265–1276. [Google Scholar] [CrossRef]
- Shi, Q.; George, J.; Krystel, J.; Zhang, S.; Lapointe, S.L.; Stelinski, L.L.; Stover, E. Hexaacetyl-chitohexaose, a chitin-derived oligosaccharide, transiently activates citrus defenses and alters the feeding behavior of Asian citrus psyllid. Hortic. Res. 2019, 6, 76. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Shivankar, V.; Sandnya, D.; Dhengre, V. Effect of time of breaking water stress, planting density, organic manuring and certain new insecticides on incidence of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Indian J. Agric. Sci. 2013, 83, 1022–1026. [Google Scholar]
- Rao, C.; Shivankar, V.; Deole, S.; Dhengre, V. Effect of organic manures on the incidence of Asian citrus psyllid, Diaphorina citri Kuwayama. Pest Manag. Hortic. Ecosyst. 2013, 19, 92–94. [Google Scholar]
- Li, W.; Hartung, J.S.; Levy, L. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J. Microbiol. Methods 2006, 66, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.A.; Backus, E.A.; Cid, M.; Fereres, A.; Rogers, M.E. A new SAS program for behavioral analysis of electrical penetration graph data. Comput. Electron. Agric. 2015, 116, 80–87. [Google Scholar] [CrossRef]
- Miranda, M.P.; Yamamoto, P.T.; Garcia, R.B.; Lopes, J.P.A.; Lopes, J.R.S. Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behaviour of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2016, 72, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Tjallingii, W.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus Persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Razmjou, J.; Vorburger, C.; Mohammadi, M.; Hassanpour, M. Influence of vermicompost and cucumber cultivar on population growth of Aphis gossypii Glover. J. Appl. Entomol. 2012, 136, 568–575. [Google Scholar] [CrossRef]
- Bonani, J.P.; Fereres, A.; Garzo, E.; Miranda, M.P.; Appezzato-Da-Gloria, B.; Lopes, J.R.S. Characterization of electrical penetration graphs of the Asian citrus psyllid, Diaphorina citri, in sweet orange seedlings. Entomol. Exp. Appl. 2010, 134, 35–49. [Google Scholar] [CrossRef]
- Gao, J.; Arthurs, S.; Mao, R.Q. Asymmetric interaction between Aphis spiraecola and Toxoptera citricida on sweet orange induced by pre-infestation. Insects 2020, 11, 414. [Google Scholar] [CrossRef]
- Guo, H.; Sun, Y.; Li, Y.; Tong, B.; Harris, M.; Zhu-Salzman, K.; Ge, F. Pea aphid promotes amino acid metabolism both in M edicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Glob. Chang. Biol. 2013, 19, 3210–3223. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Qian, L.X.; Wang, X.W.; Shao, R.X.; Hong, Y.; Liu, S.S.; Wang, X.W. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef]
- Feng, J.L.; Zhang, J.; Yang, J.; Zou, L.P.; Fang, T.T.; Xu, H.L.; Cai, Q.N. Exogenous salicylic acid improves resistance of aphid-susceptible wheat to the grain aphid, Sitobion avenae (F.) (Hemiptera: Aphididae). Bull. Entomol. Res. 2021, 111, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Aslam, H.; Mushtaq, S.; Maalik, S.; Bano, N.; Eed, E.M.; Bibi, A.; Tahir, A.; Ijaz, I.; Tanwir, S.; Khalifa, A.S. Exploring the effect of jasmonic acid for aphids control for improving the yield of Triticum aestivum varieties. PeerJ 2022, 10, e14018. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Van der Westhuizen, A.; Qian, X.M.; Botha, A.M. β-1, 3-Glucanases in wheat and resistance to the Russian wheat aphid. Physiol. Plant. 1998, 103, 125–131. [Google Scholar] [CrossRef]
- Losvik, A.; Beste, L.; Glinwood, R.; Ivarson, E.; Stephens, J.; Zhu, L.-H.; Jonsson, L. Overexpression and down-regulation of barley lipoxygenase LOX2. 2 affects jasmonate-regulated genes and aphid fecundity. Int. J. Mol. Sci. 2017, 18, 2765. [Google Scholar] [CrossRef]
- Boughton, A.J.; Hoover, K.; Felton, G.W. Impact of chemical elicitor applications on greenhouse tomato plants and population growth of the green peach aphid, Myzus persicae. Entomol. Exp. Appl. 2006, 120, 175–188. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.H. Exogenous treatment with salicylic acid attenuates occurrence of citrus canker in susceptible navel orange (Citrus sinensis Osbeck). J. Plant Physiol. 2012, 169, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Fang, W.; Lu, H.; Zhu, R.; Lu, L.; Zheng, X.; Yu, T. Inhibition of green mold disease in mandarins by preventive applications of methyl jasmonate and antagonistic yeast Cryptococcus laurentii. Postharvest Biol. Technol. 2014, 88, 72–78. [Google Scholar] [CrossRef]
- Killiny, N.; Nehela, Y. Metabolomic response to Huanglongbing: Role of carboxylic compounds in Citrus sinensis response to ‘Candidatus liberibacter asiaticus’ and its vector, Diaphorina citri. Mol. Plant Microbe Interact. 2017, 30, 666–678. [Google Scholar] [CrossRef]
- Cao, H.H.; Wang, S.H.; Liu, T.X. Jasmonate-and salicylate-induced defenses in wheat affect host preference and probing behavior but not performance of the grain aphid, Sitobion avenae. Insect Sci. 2014, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Peng, Z.; Tong, H.; Xie, W.; Wang, S.; Wu, Q.; Zhang, J.; Li, C.; Zhang, Y. A salivary ferritin in the whitefly suppresses plant defenses and facilitates host exploitation. J. Exp. Bot. 2019, 70, 3343–3355. [Google Scholar] [CrossRef]
- Boquel, S.; Ameline, A.; Giordanengo, P. Assessing aphids potato virus Y-transmission efficiency: A new approach. J. Virol. Methods 2011, 178, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; de Blas, C.; Barrios, L.; Fereres, A. Correlation between whitefly (Homoptera: Aleyrodidae) feeding behavior and transmission of tomato yellow leaf curl virus. Ann. Entomol. Soc. Am. 2000, 93, 573–579. [Google Scholar] [CrossRef]
- Raj Boina, D.; Youn, Y.; Folimonova, S.; Stelinski, L.L. Effects of pymetrozine, an antifeedant of Hemiptera, on Asian citrus psyllid, Diaphorina citri, feeding behavior, survival and transmission of Candidatus Liberibacter asiaticus. Pest Manag. Sci. 2011, 67, 146–155. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Garcia, R.B.; Wulff, N.A.; Fereres, A.; Miranda, M.P. Drench application of systemic insecticides disrupts probing behavior of Diaphorina citri (Hemiptera: Liviidae) and inoculation of Candidatus Liberibacter asiaticus. Insects 2020, 11, 314. [Google Scholar] [CrossRef] [PubMed]
| EPG Variables | Control | 20% Vermicompost | 40% Vermicompost | 60% Vermicompost | F | df | p |
|---|---|---|---|---|---|---|---|
| Np | 1.25 ± 0.18 a | 1.33 ± 0.26 a | 1.33 ± 0.33 a | 1.17 ± 0.11 a | 0.12 | 44 | 0.95 |
| C | 6.20 ± 1.63 a | 3.33 ± 0.65 a | 3.33 ± 0.50 a | 3.25 ± 0.79 a | 2.20 | 44 | 0.10 |
| D | 5.25 ± 1.32 a | 2.50 ± 0.57 a | 2.58 ± 0.53 a | 2.500 ± 0.73 a | 2.58 | 44 | 0.07 |
| E1 | 5.25 ± 1.32 a | 2.50 ± 0.57 a | 2.58 ± 0.53 a | 2.500 ± 0.73 a | 2.58 | 44 | 0.07 |
| E2 | 2.8 ± 0.55 a | 1.75 ± 0.43 a | 1.83 ± 0.51 a | 1.50 ± 0.20 a | 1.53 | 44 | 0.22 |
| E2 > 10 min | 1.67 ± 0.28 a | 1.33 ± 0.19 ab | 0.83 ± 0.21 b | 0.75 ± 0.13 b | 4.25 | 44 | 0.01 |
| G | 0 a | 0.17 ± 0.112 a | 0 a | 0 a | 2.20 | 44 | 0.10 |
| EPG Variables (min) | Control | 20% Vermicompost | 40% Vermicompost | 60% Vermicompost | F | df | p |
|---|---|---|---|---|---|---|---|
| Np | 17.78 ± 10.92 | 4.86 ± 1.899 a | 2.60 ± 0.83 a | 1.92 ± 0.75 a | 1.78 | 44 | 0.17 |
| C | 314.31 ± 34.41 b | 349.08 ± 23.80 b | 432.44 ± 9.70 a | 451.37 ± 4.26 a | 9.26 | 44 | <0.001 |
| D | 4.57 ± 1.32 a | 2.76 ± 1.01 a | 2.43 ± 0.61 a | 2.66 ± 0.66 a | 1.09 | 44 | 0.36 |
| E1 | 5.23 ± 1.26 a | 5.80 ± 2.43 a | 2.21 ± 0.53 a | 3.25 ± 1.31 a | 1.19 | 44 | 0.33 |
| E2 | 138.12 ± 29.17 a | 116.11 ± 20.46 a | 40.32 ± 9.75 b | 20.41 ± 3.63 b | 9.48 | 44 | <0.001 |
| E2 > 10 min | 133.53 ± 30.21 a | 115.11 ± 20.46 a | 52.44 ± 9.83 ab | 24.31 ± 3.24 b | 5.53 | 37 | 0.003 |
| G | NA | 8.43 ± 0.55 | NA | NA | - | - | - |
| EPG Variables (min) | Control | 20% Vermicompost | 40% Vermicompost | 60% Vermicompost | F | df | p |
|---|---|---|---|---|---|---|---|
| Time to first probe from start of recording | 10. 50 ± 5.47 a | 14.86 ± 10.98 a | 2.10 ± 0.76 a | 1.66 ± 0.71 a | 1.11 | 44 | 0.36 |
| Minimum duration of C before first E1 | 112.34 ± 21.58 b | 135.77 ± 32.21 ab | 150.60 ± 26.50 ab | 257.61 ± 40.18 a | 4.33 | 44 | 0.01 |
| First E1 from start of recording | 130.75 ± 21.76 b | 152.87 ± 31.13 ab | 151.33 ± 26.49 ab | 259.11 ± 40.33 a | 3.56 | 44 | 0.02 |
| First E2 from start of recording | 138.36 ± 23.30 b | 154.12 ± 31.11 ab | 171.66 ± 31.73 ab | 270.01 ± 38.22 a | 3.53 | 44 | 0.02 |
| Average duration of E2 | 84.17 ± 29.07 b | 82.46 ± 17.08 ab | 30.46 ± 9.46 ab | 18.83 ± 4.04 a | 3.92 | 44 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, T.; Wang, Z.; Mao, R.; Hussain, M.; Arthurs, S.P.; Ye, F.; An, X.; Gao, J. Vermicompost Amendments Disrupt Feeding Behavior of Diaphorina citri Kuwayama and Boost Activities of Salicylic Acid and Jasmonic Acid Pathway-Related Enzymes in Citrus. Insects 2023, 14, 410. https://doi.org/10.3390/insects14050410
Tao T, Wang Z, Mao R, Hussain M, Arthurs SP, Ye F, An X, Gao J. Vermicompost Amendments Disrupt Feeding Behavior of Diaphorina citri Kuwayama and Boost Activities of Salicylic Acid and Jasmonic Acid Pathway-Related Enzymes in Citrus. Insects. 2023; 14(5):410. https://doi.org/10.3390/insects14050410
Chicago/Turabian StyleTao, Tonglai, Zhaohong Wang, Runqian Mao, Mubasher Hussain, Steven P. Arthurs, Fengxian Ye, Xincheng An, and Jing Gao. 2023. "Vermicompost Amendments Disrupt Feeding Behavior of Diaphorina citri Kuwayama and Boost Activities of Salicylic Acid and Jasmonic Acid Pathway-Related Enzymes in Citrus" Insects 14, no. 5: 410. https://doi.org/10.3390/insects14050410
APA StyleTao, T., Wang, Z., Mao, R., Hussain, M., Arthurs, S. P., Ye, F., An, X., & Gao, J. (2023). Vermicompost Amendments Disrupt Feeding Behavior of Diaphorina citri Kuwayama and Boost Activities of Salicylic Acid and Jasmonic Acid Pathway-Related Enzymes in Citrus. Insects, 14(5), 410. https://doi.org/10.3390/insects14050410






