Relative Susceptibility of Brassicas to Cabbage Maggot (Diptera: Anthomyiidae) Infestation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site, Plants, and Insects
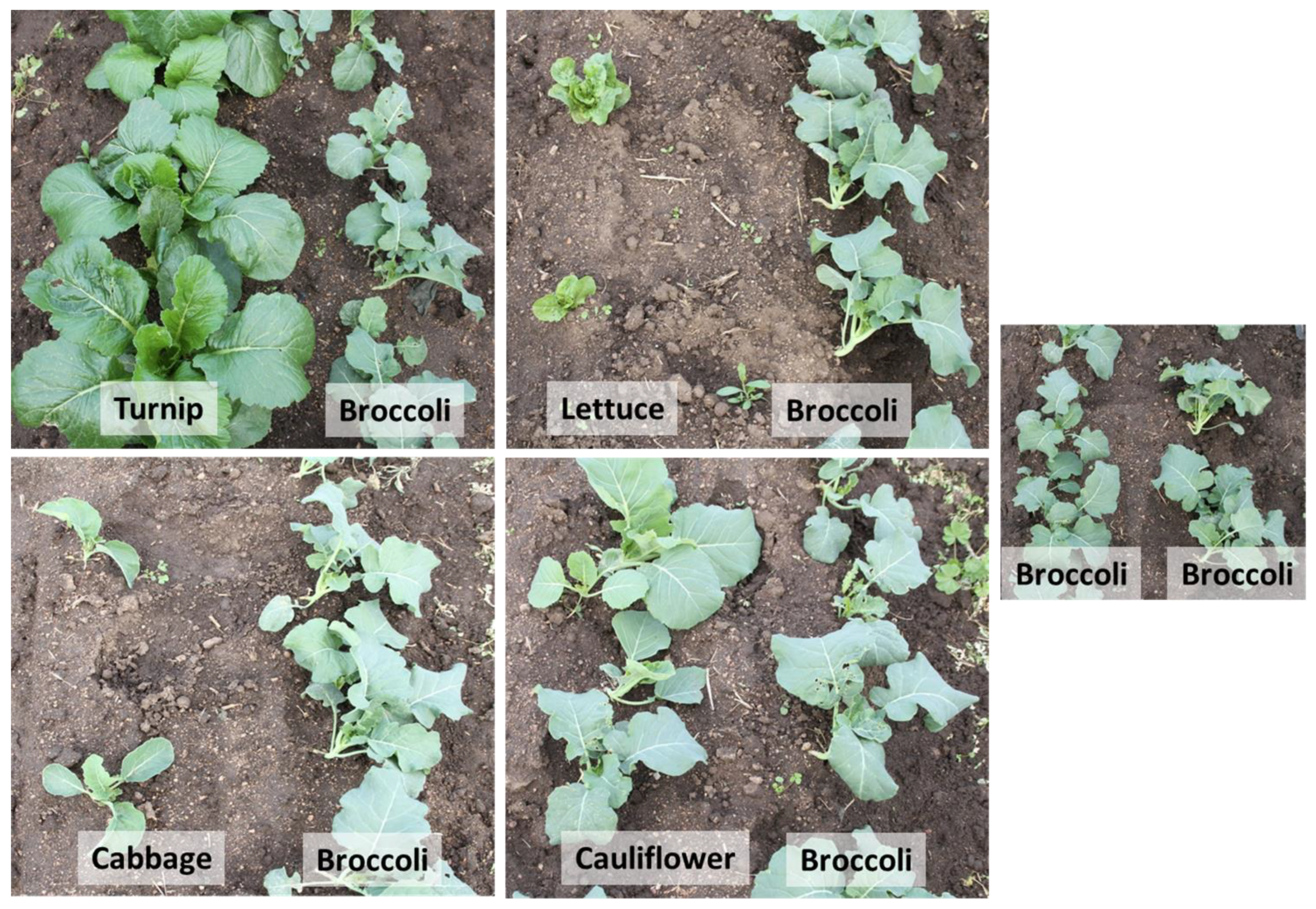
2.2. Experimental Design
2.3. Sampling and Evaluation
2.4. Statistical Analyses
3. Results
3.1. Delia spp. Adults
3.2. D. radicum Eggs
3.3. Severity of D. radicum Damage
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finch, S. Feeding and associated behaviour of the adult cabbage root fly Erioischia brassicae (Beh.) (Dipt., Anthomyiidae) under laboratory conditions. Bull. Entomol. Res. 1974, 63, 661–671. [Google Scholar] [CrossRef]
- Guerra, P.C.; Keil, C.B.; Stevenson, P.C.; Mina, D.; Samaniego, S.; Peralta, E.; Mazon, N.; Chancellor, T.C.B. Larval performance and adult attraction of Delia platura (Diptera: Anthomyiidae) in a native and an introduced crop. J. Econ. Entomol. 2017, 110, 186–191. [Google Scholar] [PubMed]
- Johnsen, S.; Gutierrez, A.P. Induction and termination of winter diapause in a Californian strain of the cabbage maggot (Diptera: Anthomyiidae). Environ. Entomol. 1997, 26, 84–90. [Google Scholar] [CrossRef]
- Joseph, S.V.; Martinez, J. Incidence of cabbage maggot (Diptera: Anthomyiidae) infestation and plant damage in seeded brassica fields in California’s central coast. Crop Prot. 2014, 62, 72–78. [Google Scholar] [CrossRef]
- San Benito County Crop Report, 2020. Office of Agricultural Commissioner- San Benito County, California. 2020. Available online: https://www.cosb.us/home/showpublisheddocument/7385/637693078344330000 (accessed on 31 December 2022).
- Monterey County Crop Report; Office of Agricultural Commissioner: Monterey County, CA, USA, 2021; Available online: https://www.co.monterey.ca.us/home/showpublisheddocument/113214 (accessed on 31 December 2022).
- Gutiérrez-Rodríguez, E.; Gundersen, A.; Sbodio, A.O.; Suslow, T.V. Variable agronomic practices, cultivar, strain source and initial contamination dose differentially affect survival of Escherichia coli on spinach. J. Appl. Microbiol. 2012, 112, 109–118. [Google Scholar] [CrossRef]
- Flint, M.L. Seedcorn Maggot, Hylema platura. pp. 36. In Integrated Management for Cole Crops and Lettuce; University of California Publication 3307: Riverside, CA, USA, 1985; p. 112. [Google Scholar]
- Joseph, S.V. Root maggot pest on brassicas in the Salinas Valley. Salinas Valley Agriculture. University of California Agriculture and Natural Resources. 2013. Available online: https://ucanr.edu/blogs/blogcore/postdetail.cfm?postnum=9804 (accessed on 25 April 2023).
- Natwick, E.T.; Joseph, S.V.; Dara, S.K. Cabbage maggot. UC IPM Pest Management Guidelines: Cole Crops. UC ANR Publication 3442. 2020. Available online: https://ipm.ucanr.edu/agriculture/cole-crops/cabbage-maggot/ (accessed on 25 April 2023).
- Tourte, L.; Smith, R.F.; Murdock, J.; Sumner, D.A. Sample Costs to Produce and Harvest Broccoli—Central Coast Region (Monterey, Santa Cruz, and San Benito Counties). University of California Agriculture and Natural Resources Cooperative Extension and Agricultural Issues Center. UC Davis Department of Agricultural and Resource Economics. 2017. Available online: https://coststudies.ucdavis.edu/en/current/ (accessed on 25 April 2023).
- Baur, R.; Birch, A.N.E.; Hopkins, R.J.; Griffiths, D.W.; Simmonds, M.S.; Stadler, E. Oviposition and chemosensory stimulation of the root flies Delia radicum and D. floralis in response to plants and leaf surface extracts from resistant and susceptible Brassica genotypes. Entomol. Exp. Appl. 1996, 78, 61–75. [Google Scholar] [CrossRef]
- Joseph, S.V.; Iudice, S. Evaluation of seedling tray drench of insecticides for cabbage maggot (Diptera: Anthomyiidae) management in broccoli and cauliflower. Fla. Entomol. 2020, 103, 172–179. [Google Scholar] [CrossRef]
- Joseph, S.V.; Godfrey, L.D.; Bettiga, C. Influence of interval between post-harvest lettuce residue management and subsequent seeding of broccoli on cabbage maggot (Diptera: Anthomyiidae) infestation on broccoli. J. Econ. Entomol. 2017, 110, 2172–2179. [Google Scholar] [CrossRef]
- Joseph, S.V.; Zarate, J. Comparing efficacy of insecticides against cabbage maggot (Diptera: Anthomyiidae) in the laboratory. Crop Prot. 2015, 77, 148–156. [Google Scholar] [CrossRef]
- Joseph, S.V. Timing of insecticide application for cabbage maggot (Diptera: Anthomyiidae) control in seeded turnip in Central Coast of California. Southwest. Entomol. 2016, 41, 625–632. [Google Scholar] [CrossRef]
- Hunt, J.W.; Anderson, B.S.; Phillips, B.M.; Nicely, P.N.; Tjeerdema, R.S.; Puckett, H.M.; Stephenson, M.; Worcester, K.; de Vlaming, V. Ambient toxicity due to chlorpyrifos and diazinon in a central California coastal watershed. Environ. Monit. Assess. 2003, 82, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M.; Weston, D.P. Pyrethroid Pesticide Transport into Monterey Bay through Riverine Suspended Solids; UC Water Resources Center Technical Completion Report, Project No.WR1018; UC Water Resources Center: Davis, CA, USA, 2009. [Google Scholar]
- Schmidt, K.; Lopez, S.G.; Krone-Davis, P. Pesticides and Toxicity to Hyalella azteca in Sediments. In Central Coast Region Conditional Waiver Cooperative Monitoring Program; Follow-up Monitoring Report; Central Coast Water Quality Preservation, Inc.: Watsonville, CA, USA, 2010. [Google Scholar]
- Central Coast Water Board, Agricultural Order Adopted. 2021. Available online: https://www.waterboards.ca.gov/centralcoast/water_issues/programs/ilp/archive/ag_order4_renewal.html (accessed on 25 April 2023).
- [CCRWQCB] Central Coast Regional Water Quality Control Board. Salinas River Watershed Sediment Toxicity and Pyrethroid Pesticides in Sediment TMDL. 2016. Available online: http://www.waterboards.ca.gov/centralcoast/water_issues/programs/tmdl/docs/salinas/sed_tox/index.shtml (accessed on 25 April 2023).
- Hokkanen, H.M.T. Trap cropping in pest management. Annu. Rev. Entomol. 1991, 36, 119–138. [Google Scholar] [CrossRef]
- Shelton, A.M.; Badenes-Perez, F.R. Concepts and applications of trap cropping in pest management. Annu. Rev. Entomol. 2006, 51, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of trap cropping as companion plants for the management of agricultural pests: A review. Insects 2018, 9, 128. [Google Scholar] [CrossRef]
- George, D.R.; Collier, R.; Port, G. Testing and improving the effectiveness of trap crops for management of the diamondback moth Plutella xylostella (L.): A laboratory-based study. Pest. Manag. Sci. 2009, 65, 1219–1227. [Google Scholar] [CrossRef]
- Parsons, C.K.; Dixon, P.L.; Colbo, M. Relay cropping cauliflower with lettuce as a means to manage first-generation cabbage maggot (Diptera: Anthomyiidae) and minimize cauliflower yield loss. J. Econ. Entomol. 2007, 100, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Lamy, F.; Bellec, L.; Rusu-Stievenard, A.; Clin, P.; Ricono, C.; Olivier, D.; Mauger, S.; Poinsot, D.; Faloya, V.; Daniel, L.; et al. Oviposition preference of the cabbage root fly towards some Chinese cabbage cultivars: A search for future trap crop candidates. Insects 2020, 11, 127. [Google Scholar] [CrossRef]
- Brooks, A.R. Identification of root maggots attacking cruciferous garden crops in Canada with notes on biology and control. Can. Entomol. 1951, 83, 109–120. [Google Scholar] [CrossRef]
- SAS Institute, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2016.
- Walgenbach, P. Laboratory studies on host plant selection by Hylemya brassicae (Bouché). Proc. North Cent. Branch Entomol. Soc. Am. 1979, 33, 19. [Google Scholar]
- Rousse, P.; Fournet, S.; Porteneuve, C.; Brunel, E. Trap cropping to control Delia radicum populations in cruciferous crops: First results and future applications. Entomol. Exp. Appl. 2003, 109, 133–138. [Google Scholar] [CrossRef]
- George, D.; Port, G.; Collier, R. Living on the edge: Using and improving trap crops for flea beetle management in small-scale cropping systems. Insects 2019, 10, 286. [Google Scholar] [CrossRef]
- Trdan, S.; Valic, N.; Znidarcic, D.; Vidrih, M.; Bergant, K.; Zlatic, E.; Milevoj, L. The role of Chinese cabbage as a trap crop for flea beetles (Coleoptera: Chrysomelidae) in production of white cabbage. Sci. Hortic. 2005, 106, 12–24. [Google Scholar] [CrossRef]
- Kostal, V.; Finch, S. Influence of background on host-plant selection and subsequent oviposition by the cabbage root fly (Delia radicum). Entomol. Exp. Appl. 1994, 70, 153–163. [Google Scholar] [CrossRef]
- Smith, J.G. Influence of crop backgrounds on aphids and other phytophagous insects of Brussels sprouts. Ann. Appl. Biol. 1976, 83, 1–13. [Google Scholar] [CrossRef]
- Ryan, J.; Ryan, M.F.; McNaeidhe, F. The effect of interrow plant cover on populations of the cabbage root fly, Delia brassicae (Wiedemann). J. Appl. Ecol. 1980, 17, 31–40. [Google Scholar] [CrossRef]
- Theunissen, J.; den Ouden, H. Effects of intercropping Spergula arvensis on pests of Brussels sprouts. Entomol. Exp. Appl. 1980, 27, 260–268. [Google Scholar] [CrossRef]
- Theunissen, J.; Booij, C.J.H.; Lotz, L.A.P. Effects of intercropping white cabbage with clovers on pest infestation and yield. Entomol. Exp. Appl. 1995, 74, 7–16. [Google Scholar] [CrossRef]
- Dixon, P.L.; Coady, J.R.; Larson, D.J.; Spaner, D. Undersowing rutabaga with white clover: Impact on Delia radicum (Diptera: Anthomyiidae) and its natural enemies. Can. Entomol. 2004, 136, 427–442. [Google Scholar] [CrossRef]
- Finch, S.; Kienegger, M.A. behavioral study to help clarify how undersowing with clover affects hostplant selection by pest insects of Brassica crops. Entomol. Exp. Appl. 1997, 84, 165–172. [Google Scholar] [CrossRef]
- Asman, K.; Ekbom, B.; Ramart, B. Effect of intercropping on oviposition and emigration behavior of the leek moth (Lepidoptera: Acrolepiidae) and the diamondback moth (Lepidoptera: Plutellidae). Environ. Entomol. 2001, 30, 288–294. [Google Scholar] [CrossRef]
- Finch, S.; Collier, R.H. Host-plant selection by insects—A theory based on appropriate/inappropriate landings by pest insects of cruciferous plants. Entomol. Exp. Appl. 2000, 96, 91–102. [Google Scholar] [CrossRef]
- Hokkanen, H.M.T. Biological and agrotechnical control of the rape blossom beetle Meligethes aeneus (Coleoptera: Nitidulidae). Acta Entomol. Fenn. 1989, 53, 25–30. [Google Scholar]





| Week a after Seedling Emergence | 2013 | 2014 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggs | Severity of Injury | Eggs | Severity of Injury | |||||||||
| F | df | P | F | df | P | F | df | P | F | df | P | |
| 1 b | 1.0 | 4,12 | 1.000 | 7.6 | 4,12 | 0.002 | - d | - | - | - d | - | - |
| 2 | 0.1 | 4,12 | 0.972 | 3.1 | 4,12 | 0.047 | 0.0 | 3,12 | 0.999 | - | - | - |
| 3 | - c | - | - | 19.5 | 4,12 | <0.001 | 2.6 | 3,12 | 0.099 | 1.0 | 3,12 | 0.426 |
| 4 | 0.9 | 4,12 | 0.451 | 11.9 | 4,12 | <0.001 | 1.1 | 3,12 | 0.377 | 26.5 | 3,12 | <0.001 |
| 5 | 19.0 | 4,12 | <0.001 | 28.5 | 4,12 | <0.001 | 0.3 | 3,12 | 0.840 | 12.3 | 3,12 | <0.001 |
| 6 | 10.5 | 4,12 | <0.001 | 17.7 | 4,12 | <0.001 | 0.7 | 3,12 | 0.593 | 5.2 | 3,12 | 0.015 |
| 7 | 8.9 | 4,12 | 0.001 | 8.9 | 4,12 | <0.001 | 2.3 | 3,12 | 0.129 | 0.9 | 3,12 | 0.473 |
| 8 | 3.5 | 4,12 | 0.042 | 20.5 | 4,12 | <0.001 | 1.4 | 3,12 | 0.292 | 8.5 | 3,12 | 0.003 |
| Week a after Seedling Emergence | Broccoli:Turnip | Broccoli:Lettuce | Broccoli:Cauliflower | Broccoli:Cabbage | Broccoli:Broccoli | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t | df | P | t | df | P | t | df | P | t | df | P | t | df | P | |
| 2013 | |||||||||||||||
| 3 b | −1.9 | 6 | 0.097 | - c | - | - | 2.1 | 6 | 0.085 | 0.2 | 6 | 0.842 | 0.9 | 6 | 0.412 |
| 4 | −1.9 | 6 | 0.103 | 2.7 | 6 | 0.035 | 1.3 | 6 | 0.235 | −0.9 | 6 | 0.371 | −0.9 | 6 | 0.395 |
| 5 | −2.8 | 6 | 0.033 | 3.3 | 6 | 0.017 | 2.1 | 6 | 0.086 | 0.4 | 6 | 0.675 | 0.6 | 6 | 0.571 |
| 6 | −0.9 | 6 | 0.422 | 4.5 | 6 | 0.004 | 1.3 | 6 | 0.241 | −0.7 | 6 | 0.509 | −1.4 | 6 | 0.222 |
| 2014 | |||||||||||||||
| 5 b | - d | - | - | 0 | 6 | 1.000 | - d | - | - | - e | - | - | −1 | 6 | 0.356 |
| 6 | −0.5 | 6 | 0.633 | 1.4 | 6 | 0.207 | 0.3 | 6 | 0.801 | - | - | - | 0.8 | 6 | 0.477 |
| 7 | −0.5 | 6 | 0.620 | 3.6 | 6 | 0.012 | 0.0 | 6 | 1.000 | - | - | - | 0.0 | 6 | 1.000 |
| 8 | −0.6 | 6 | 0.571 | 8.7 | 6 | <0.001 | −0.3 | 6 | 0.750 | - | - | - | 1.9 | 6 | 0.114 |
| Treatment Pairs | Total D. radicum Eggs a | |
|---|---|---|
| 2013 | 2014 | |
| Broccoli | 330.3 ± 69.9b | 22.5 ± 1.0b |
| Broccoli + Turnip | 965.3 ± 235.9a | 53.3 ± 2.6a |
| t | −2.9 | −11.1 |
| P | 0.042 | <0.001 |
| Broccoli | 438.0 ± 54.1a | 20.3 ± 1.9a |
| Broccoli + Lettuce | 443.8 ± 53.7a | 20.3 ± 1.9a |
| t | −0.10 | 1.00 |
| P | 0.943 | 0.000 |
| Broccoli | 346.3 ± 43.4b | 18.0 ± 1.0b |
| Broccoli + Cauliflower | 658.0 ± 82.8a | 35.0 ± 2.0a |
| t | −3.33 | −7.5 |
| P | 0.016 | <0.001 |
| Broccoli | 255.3 ± 45.4b | - |
| Broccoli + Cabbage b | 546.0 ± 94.2a | - |
| t | −2.8 | - |
| P | 0.032 | - |
| Broccoli | 464.8 ± 68.4b | 19.5 ± 1.8b |
| Broccoli + Broccoli | 927.8 ± 111.1a | 40.8 ± 2.1a |
| t | −3.6 | −7.7 |
| P | 0.012 | <0.001 |
| Week a after Seedling Emergence | Broccoli:Turnip | Broccoli:Lettuce | Broccoli:Cauliflower | Broccoli:Cabbage | Broccoli:Broccoli | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | F | df | P | F | df | P | F | df | P | |
| 2013 | |||||||||||||||
| 1 b | −3.0 | 6 | 0.023 | - | - | - | 1.5 | 6 | 0.178 | 0.0 | 6 | 1.000 | 0.3 | 6 | 0.766 |
| 2 | −2.4 | 6 | 0.055 | 3.0 | 6 | 0.023 | 0.4 | 6 | 0.736 | −1.2 | 6 | 0.295 | −0.8 | 6 | 0.482 |
| 3 | −1.7 | 6 | 0.148 | 8.6 | 6 | <0.001 | 2.2 | 6 | 0.069 | −0.7 | 6 | 0.520 | −0.3 | 6 | 0.786 |
| 4 | −3.0 | 6 | 0.023 | 20.5 | 6 | <0.001 | 4.9 | 6 | 0.003 | −0.9 | 6 | 0.364 | −0.8 | 6 | 0.435 |
| 5 | −1.9 | 6 | 0.104 | 20.7 | 6 | <0.001 | 4.6 | 6 | 0.004 | −0.9 | 6 | 0.385 | −0.8 | 6 | 0.412 |
| 6 | −0.7 | 6 | 0.512 | 8.9 | 6 | <0.001 | 6.1 | 6 | 0.001 | −2.2 | 6 | 0.071 | −0.2 | 6 | 0.835 |
| 7 | −0.1 | 6 | 0.951 | 6.6 | 6 | 0.001 | 0.4 | 6 | 0.735 | −1.6 | 6 | 0.168 | −1.1 | 6 | 0.302 |
| 8 | −0.9 | 6 | 0.378 | 7.9 | 6 | <0.001 | 1.0 | 6 | 0.344 | −0.9 | 6 | 0.390 | −0.9 | 6 | 0.387 |
| 2014 | |||||||||||||||
| 5 | 5.0 | 6 | 0.002 | - c | - | - | - | - | - d | - | - | 0.0 | 6 | 1.000 | |
| 6 | −0.8 | 6 | 0.477 | −2.8 | 6 | 0.031 | 1.1 | 6 | 0.327 | - | - | - | 1.6 | 6 | 0.152 |
| 7 | −1.1 | 6 | 0.328 | −5.8 | 6 | 0.001 | −2.4 | 6 | 0.055 | - | - | - | −1.3 | 6 | 0.252 |
| 8 | −0.7 | 6 | 0.534 | −6.4 | 6 | <0.001 | −0.5 | 6 | 0.640 | - | - | - | −0.6 | 6 | 0.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joseph, S.V. Relative Susceptibility of Brassicas to Cabbage Maggot (Diptera: Anthomyiidae) Infestation. Insects 2023, 14, 411. https://doi.org/10.3390/insects14050411
Joseph SV. Relative Susceptibility of Brassicas to Cabbage Maggot (Diptera: Anthomyiidae) Infestation. Insects. 2023; 14(5):411. https://doi.org/10.3390/insects14050411
Chicago/Turabian StyleJoseph, Shimat V. 2023. "Relative Susceptibility of Brassicas to Cabbage Maggot (Diptera: Anthomyiidae) Infestation" Insects 14, no. 5: 411. https://doi.org/10.3390/insects14050411






