Effect of Systemic Insecticides Applied via Drench on the Mortality of Diaphorina citri on Curry Leaf
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diaphorina citri Rearing Colony
2.2. Insecticides
2.3. Persistence of Systemic Insecticides Applied via Drench to Kill Diaphorina citri under Protected Cultivation and Field Conditions
2.4. Determination of Median Lethal Concentration (LC50) of Systemic Insecticides in Curry Leaf in Protected Cultivation
2.5. Sublethal Effects of Systemic Insecticides on Diaphorina citri Oviposition
2.6. Statistical Analysis
3. Results
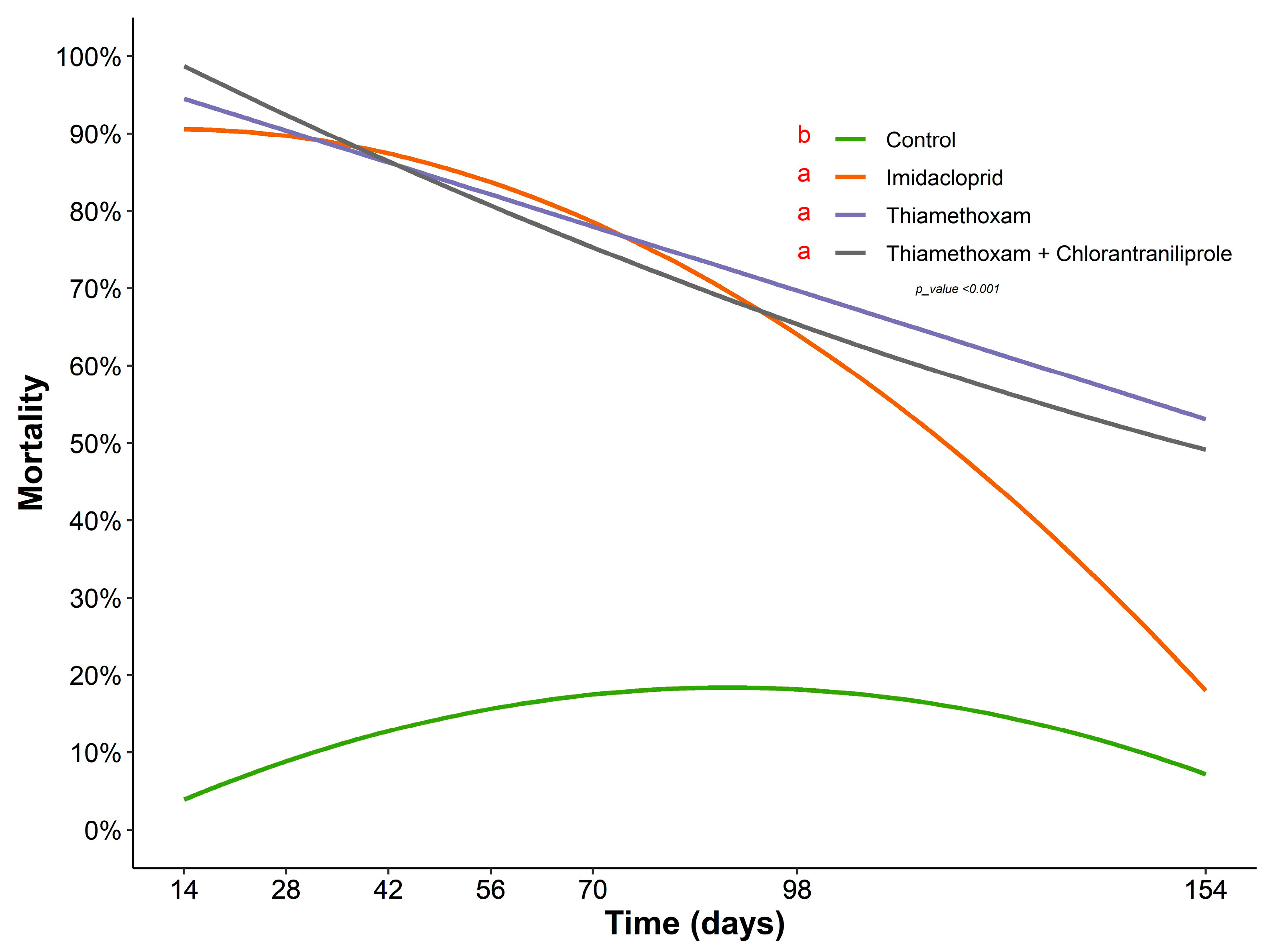
3.1. Persistence of Systemic Insecticides Applied via Drench Affecting the Mortality of Diaphorina citri under Protected and Field Conditions
3.2. Determination of Median Lethal Concentration (LC50) of Systemic Insecticides in Curry Leaf Trees
3.3. Sublethal Effect of Systemic Insecticides on Diaphorina citri Oviposition
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Leong, S.S.; Leong, S.C.; Beattie, G.A. Integrated Pest Management strategies for Asian Citrus Psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae) and Huanglongbing in Citrus for Sarawak, East Malaysia, Borneo. Insects 2022, 13, 960. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, P.T.; Alves, G.R.; Beloti, V.H. Manejo e controle do Huanglongbing (HLB) dos cítricos. Cienc. Investig. Agrar. 2014, 16, 69–82. [Google Scholar]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed]
- Bassanezi, R.B.; Lopes, S.A.; Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; AYRES, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Miranda, M.P.; Yamamoto, P.T.; Garcia, R.B.; Lopes, J.P. Thiamethoxam and imidacloprid drench applications on sweet orange nursery trees disrupt the feeding and settling behavior of Diaphorina citri (Hemiptera: Liviidae). Pest Manag. Sci. 2016, 72, 1785–1793. [Google Scholar] [CrossRef]
- Rogers, M.E.; Shawer, D.B. Effectiveness of several soil-applied systemic insecticides for managing the Asian Citrus Psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae). Proc. Fla. State Hort. Soc. 2007, 120, 116–119. [Google Scholar]
- Yamamoto, P.T.; Felippe, M.R.; Sanches, A.L.; Coelho, J.H.C.; Garbim, L.F.; Ximenes, N.L. Eficácia de inseticidas para o manejo de Diaphorina citri Kuwayama (Hemiptera: Psyllidae) em Citros. BioAssay 2009, 4, 1–9. [Google Scholar] [CrossRef]
- Ndakidemi, B.; Mtei, K.; Ndakidemi, P. Impacts of synthetic and botanical pesticides on beneficial insects. Agric. Sci. 2016, 7, 364–372. [Google Scholar] [CrossRef]
- Belasque, J.J.; Bassanezi, R.B.; Yamamoto, P.T.; Ayres, A.J.; Tachibana, A.; Violante, A.R.; Tank, A.J.; Giorgis, F.; Tersi, F.E.A.; Menezes, G.M.; et al. Lessons from Huanglongbing management in São Paulo State, Brazil. Plant Pathol. J. 2010, 92, 709–716. [Google Scholar]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinkski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1785–1793. [Google Scholar] [CrossRef]
- Vázquez-García, M.; Velázquez-Monreal, J.; Medina-Urrutia, V.M.; Jesús Cruz-Vargas, C.; Sandoval-Salazar, M.; Virgen-Calleros, G.; Torres-Morán, J.P. Insecticide resistance in adult Diaphorina citri Kuwayama from lime orchards in Central West Mexico. Southwest. Entomol. 2013, 38, 579–596. [Google Scholar] [CrossRef]
- Beloti, V.H.; Alves, G.R.; Coletta-Filho, H.D.; Yamamoto, P.T. The Asian citrus psyllid host Murraya koenigii is immune to citrus huanglongbing pathogen ‘Candidatus Liberibacter asiaticus’. Phytopathology 2018, 108, 1089–1094. [Google Scholar] [CrossRef]
- Chen, X.D.; Ebert, T.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Fitness costs associated with thiamethoxam and imidacloprid resistance in three field populations of Diaphorina citri (Hemiptera: Liviidae) from Florida. Bull. Entomol. Res. 2020, 110, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Adler, L.S.; Hazzard, R.V. Comparison of perimeter trap crop varieties: Effects on herbivory, pollination, and yield in butternut squash. Environ. Entomol. 2009, 38, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ravanfar, S.A.; Achor, D.S.; Killiny, N.; Shilts, T.; Chen, Y.; El-Mohtar, C.; Stelinski, L.L.; Bonning, B.C.; Orbovic, V. Genetic modification of Bergera koenigii for expression of the bacterial pesticidal protein Cry1Ba1. Front. Plant Sci. 2022, 14, 899624. [Google Scholar] [CrossRef]
- Tomaseto, A.F.; Krugner, R.; Lopes, J.R.S. Effect of plant barriers and citrus leaf age on dispersal of Diaphorina citri (Hemiptera: Liviidae). J. Appl. Entomol. 2016, 140, 91–102. [Google Scholar] [CrossRef]
- Beloti, V.H.; Santos, F.; Alves, G.R.; Bento, J.M.S.; Yamamoto, P.T. Curry leaf smells better than citrus to females of Diaphorina citri (Hemiptera: Liviidae). Arthropod-Plant Interact. 2017, 11, 709–716. [Google Scholar] [CrossRef]
- Tomaseto, A.F.; Marques, R.N.; Fereres, A.; Zanardi, O.Z.; Volpe, H.X.L.; Alquézar, B.; Pena, L.; Miranda, M.P. Orange jasmine as a trap crop to control Diaphorina citri. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Hokkanen, H.M. Trap cropping in pest management. Annu. Rev. Entomol. 1991, 36, 119–138. [Google Scholar] [CrossRef]
- Sarkar, S.C.; Wang, E.; Wu, S.; Lei, Z. Application of trap cropping as companion plants for the management of agricultural pests: A review. Insects 2018, 9, 128. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Singha, A.; Haque, M.S.; Mondal, M.T.R.; Jiku, M.A.S.; Alam, M.A. Management of chili insect pests by using trap crops. Thai J. Agric. Sci. 2021, 54, 212–221. [Google Scholar]
- Sétamou, M.; Bartels, D.W. Living on the edges: Spatial niche occupation of Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae) in citrus groves. PLoS ONE 2015, 10, e0131917. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.R.S.; Parra, J.R.P.; Yamamoto, P.T.; Bento, J.M.S. Psilídeo-asiático-dos-citros, Diaphorina citri Kuwayama. In Pragas Introduzidas no Brasil: Insetos e Ácaros; Vilela, E.F., Zucchi, R.A., Eds.; FEALQ: Piracicaba, SP, Brazil, 2015; pp. 299–314. [Google Scholar]
- Holden, M.H.; Ellner, S.P.; Lee, D.H.; Nyrop, J.P.; Sanderson, J.P. Designing an effective trap cropping strategy: The effects of attraction, retention and plant spatial distribution. J. Appl. Entomol. 2012, 49, 715–722. [Google Scholar] [CrossRef]
- Langdon, K.W.; Rogers, M.E. Neonicotinoid-induced mortality of Diaphorina citri (Hemiptera: Liviidae) is affected by route of exposure. J. Econ. Entomol. 2017, 110, 2229–2234. [Google Scholar] [CrossRef]
- Mizell, R.F.; Sconyers, M.C. Toxicity of imidacloprid to selected arthropod predators in the laboratory. Fla. Entomol. 1992, 75, 277–280. [Google Scholar] [CrossRef]
- Parra, J.R.P.; Alves, G.R.; Diniz, A.J.F.; Vieira, J.M. Tamarixia radiata (Hymenoptera: Eulophidae) x Diaphorina citri (Hemiptera: Liviidae): Mass rearing and potential use of the parasitoid in Brasil. J. Integr. Pest Manag. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Nava, D.E.; Torres, M.L.G.; Rodrigues, M.D.L.; Bento, J.M.S.; Parra, J.R.P. Biology of Diaphorina citri (Hem., Psyllidae) on different hosts and at different temperatures. J. Appl. Entomol. 2007, 131, 709–715. [Google Scholar] [CrossRef]
- Alves, G.R.; Diniz, A.J.F.; Parra, J.R.P. Biology of the huanglongbing vector Diaphorina citri (Hemiptera: Liviidae) on different hosts plants. J. Econ. Entomol. 2014, 107, 691–696. [Google Scholar] [CrossRef]
- Carmo-Sousa, M.; Garcia, R.B.; Wulff, N.A.; Fereres, A.; Miranda, M.P. Drench application of systemic insecticides disrupts probing behavior of Diaphorina citri (Hemiptera: Liviidae) and inoculation of Candidatus Liberibacter asiaticus. Insects 2020, 11, 314. [Google Scholar] [CrossRef]
- Moral, R.A.; Hinde, J.; Demétrio, C.G. Half-normal plots and overdispersed models in R: The hnp package. J. Stat. Softw. 2017, 81, 1–23. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971; p. 333. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Random and mixed effects. In Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; pp. 271–300. [Google Scholar]
- Ichinose, K.; Bang, D.V.; Tuan, D.H.; Dien, L.Q. Effective use of neonicotinoids for protection of citrus seedlings from invasion by Diaphorina citri (Hemiptera: Psyllidae). J Econ. Entomol. 2010, 103, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bromilow, R.H.; Chamberlain, K.; Evans, A.A. Physicochemical aspects of phloem translocation of herbicides. Weed Sci. 1990, 38, 305–314. [Google Scholar] [CrossRef]
- Bennett, S.H. The behaviour of systemic insecticides applied to plants. Annu. Rev. Entomol. 1957, 2, 279–296. [Google Scholar] [CrossRef]
- Lemoine, R.; Camera, S.L.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, L.; Faucher, M.; Girousse, C.; Lemonnier, P.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Sevanto, S. Drought impacts on phloem transport. Curr. Opin. Plant Biol. 2018, 43, 76–81. [Google Scholar] [CrossRef]
- Beloti, V.H. Utilização de Murraya koenigii Como Planta-Isca Visando o Manejo do Psilídeo Diaphorina citri (Hemiptera: Liviidae). Ph.D. Thesis, Universidade de São Paulo, Piracicaba, SP, Brazil, 2018. [Google Scholar]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef]
- Byrne, F.J.; Daugherty, M.P.; Grafton-Cardwell, E.E.; Bethke, J.A.; Morse, J.G. Evaluation of systemic neonicotinoid insecticides for the management of the Asian citrus psyllid Diaphorina citri on containerized citrus. Pest Manag. Sci. 2017, 73, 506–514. [Google Scholar] [CrossRef]
- Fletcher, E.; Morgan, K.T.; Qureshi, J.A.; Leiva, J.A.; Nkedi-Kizza, P. Imidacloprid soil movement under micro-sprinkler irrigation and soil-drench applications to control Asian citrus psyllid (ACP) and citrus leafminer (CLM). PLoS ONE 2018, 13, e0192668. [Google Scholar] [CrossRef]
- Boina, D.R.; Salyani, M.; Stelinski, L.L. Chemical control of the Asian citrus psyllid, Diaphorina citri Kuwayama. Proc. Fla. State Hort. Soc. 2009, 122, 176–180. [Google Scholar]
- Boina, D.R.; Onagbola, E.O.; Salyani, M.; Stelinski, L.L. Antifeedant and sublethal effects of imidacloprid on Asian citrus psyllid, Diaphorina citri. Pest Manag. Sci. 2009, 65, 870–877. [Google Scholar] [CrossRef]
- Dmitriew, C.; Rowe, L. The effects of larval nutrition on reproductive performance in a food-limited adult environment. PLoS ONE 2011, 6, e17399. [Google Scholar] [CrossRef] [PubMed]
- Damsteegt, V.D.; Postnikova, E.N.; Stone, A.L.; Kuhlmann, M.; Wilson, C.; Sechler, A.; Schaad, N.W.; Brlansky, R.H.; Schneider, W.L. Murraya paniculata and related species as potential hosts and inoculum reservoirs of ‘Candidatus Liberibacter asiaticus’, causal agent of huanglongbing. Plant Dis. 2010, 94, 528–533. [Google Scholar] [CrossRef]
- Marsh, A.S. Use of Curry Leaf (Murraya koenigii) and Volkamer Lemon (Citrus volkameriana) as Potential Trap Crops for the Asian Citrus Psyllid (Diaphorina citri) in a Commercial Citrus Grove. Ph.D. Thesis, California State Polytechnic University, Pomona, CA, USA, 2016. [Google Scholar]
- Medina, P.; Morales, J.J.; Budia, F.; Adan, A.; Del Estal, P.; Vinuela, E. Compatibility of endoparasitoid Hyposoter didymator (Hymenoptera: Ichneumonidae) protected stages with five selected insecticides. J. Econ. Entomol. 2007, 100, 1789–1796. [Google Scholar] [CrossRef] [PubMed]


| Active Ingredient | Trade Name | Manufacturer Company | Chemical Group | IRAC MoA | Concentration (g a.i./Plant) |
|---|---|---|---|---|---|
| Thiamethoxam | Actara® 25 WG | Syngenta® São Paulo, SP–Brazil | Neonicotinoid | 4A | 0.25 |
| Thiamethoxam + Chlorantraniliprole * | Durivo® 30 SC | Syngenta® São Paulo, SP-Brazil | Neonicotinoid + Diamide | 4A + 28 | 0.2 + 0.1 |
| Imidacloprid | Provado 20 SC | Bayer® São Paulo, SP-Brazil | Neonicotinoid | 4A | 0.3 |
| Time (Days) | N | LC10 (CI95%) * | LC50 (CI95%) * | Slope (±SE) * | χ2 (df) * | p |
|---|---|---|---|---|---|---|
| 1 | 320 | 0.035 (0.023–0.052) | 0.375 (0.131–1.074) | 1.24 (±0.32) | 5.45 (5) | 0.36 |
| 2 | 320 | 0.010 (0.005–0.021) | 0.135 (0.076–0.239) | 1.15 (±0.25) | 8.94 (5) | 0.11 |
| 3 | 240 | 0.007 (0.005–0.012) | 0.031 (0.025–0.038) | 2.09 (±0.28) | 2.39 (4) | 0.66 |
| 5 | 320 | 0.004 (0.002–0.008) | 0.021 (0.016–0.027) | 1.75 (±0.26) | 7.54 (5) | 0.18 |
| 7 | 250 | 0.003 (0.001–0.007) | 0.015 (0.010–0.022) | 1.68 (±0.36) | 8.17 (4) | 0.09 |
| Time (Days) | N | LC10 (CI95%) * | LC50 (CI95%) * | Slope (±SE) * | χ2 (df) * | p |
|---|---|---|---|---|---|---|
| 1 | 280 | 0.005 (0.002–0.018) | 0.197 (0.081–0.476) | 0.81 (±0.21) | 2.56 (4) | 0.63 |
| 2 | 280 | 0.007 (0.004–0.012) | 0.041 (0.032–0.052) | 1.65 (±0.23) | 8.63 (4) | 0.07 |
| 3 | 270 | 0.006 (0.003–0.009) | 0.028 (0.022–0.035) | 1.87 (±0.24) | 7.85 (4) | 0.10 |
| 5 | 280 | 0.004 (0.002–0.008) | 0.022 (0.017–0.029) | 1.76 (±0.24) | 5.91 (4) | 0.21 |
| 7 | 280 | 0.003 (0.001–0.007) | 0.015 (0.010–0.022) | 1.67 (±0.24) | 5.88 (4) | 0.21 |
| Insecticides | Concentration (a.i. Plant–1) | Oviposition (Mean ± SE) * |
|---|---|---|
| Control | - | 13.4 ± 5.4 a |
| Thiamethoxam | 0.0025 g | 0 ± 0 b |
| Thiamethoxam + Chlorantraniliprole | 0.0020 + 0.0010 g | 0 ± 0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, J.G.A.; Santana, E.D.R.; Thiesen, L.V.; Matioli, T.F.; Yamamoto, P.T. Effect of Systemic Insecticides Applied via Drench on the Mortality of Diaphorina citri on Curry Leaf. Insects 2023, 14, 422. https://doi.org/10.3390/insects14050422
Vieira JGA, Santana EDR, Thiesen LV, Matioli TF, Yamamoto PT. Effect of Systemic Insecticides Applied via Drench on the Mortality of Diaphorina citri on Curry Leaf. Insects. 2023; 14(5):422. https://doi.org/10.3390/insects14050422
Chicago/Turabian StyleVieira, Julia Gabriela Aleixo, Emile Dayara Rabelo Santana, Leonardo Vinicius Thiesen, Thaís Fagundes Matioli, and Pedro Takao Yamamoto. 2023. "Effect of Systemic Insecticides Applied via Drench on the Mortality of Diaphorina citri on Curry Leaf" Insects 14, no. 5: 422. https://doi.org/10.3390/insects14050422





