Effect of Ultraviolet-B Radiating Drosophila melanogaster as Host on the Quality of Trichopria drosophilae, a Pupal Parasitoid of Drosophila suzukii
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. UVB Radiation
2.3. Host Species Preference
2.4. Parasitic Efficiency Based on Generations
2.5. Outdoor Test
2.6. Data Analysis
3. Results
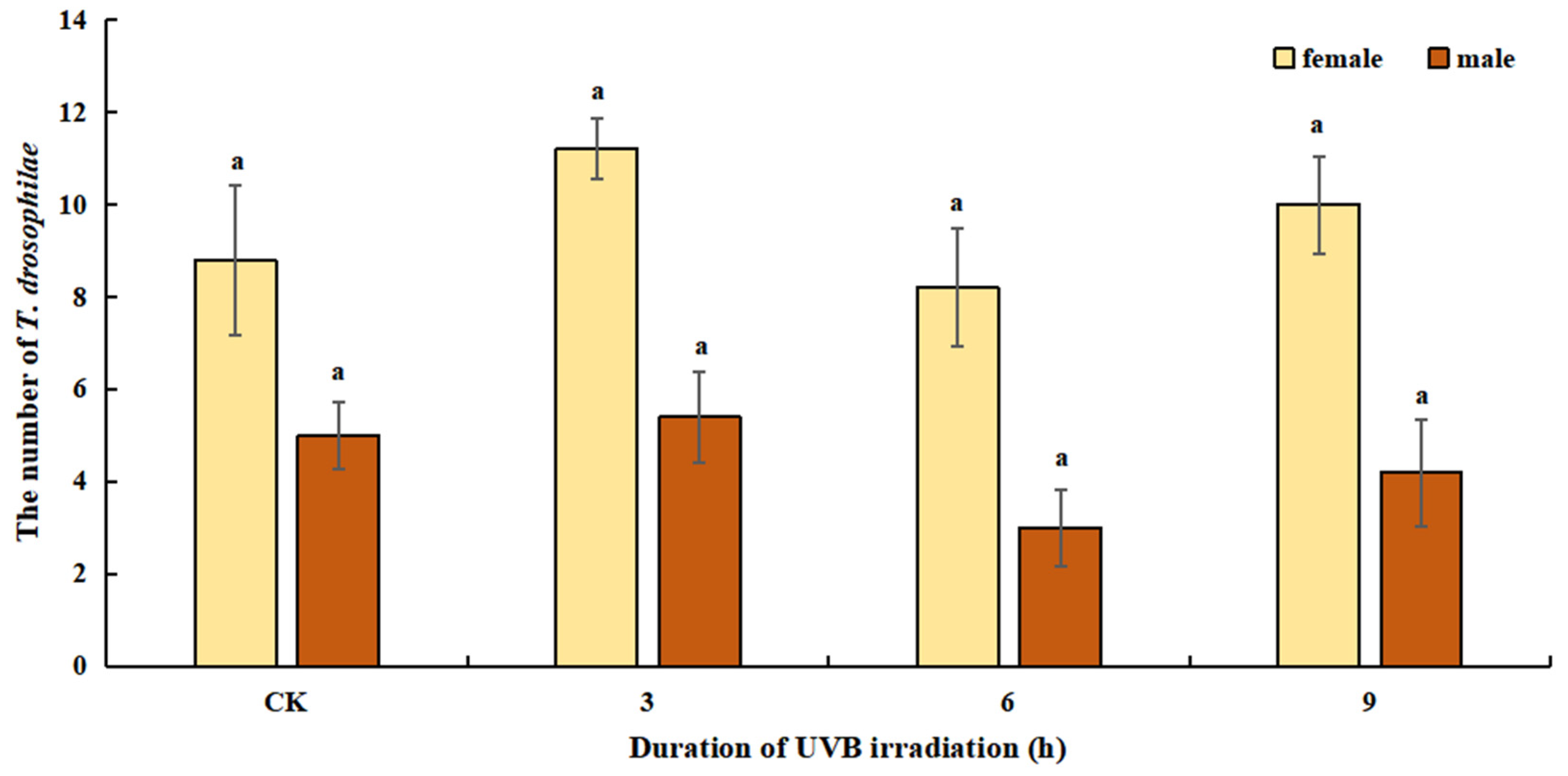
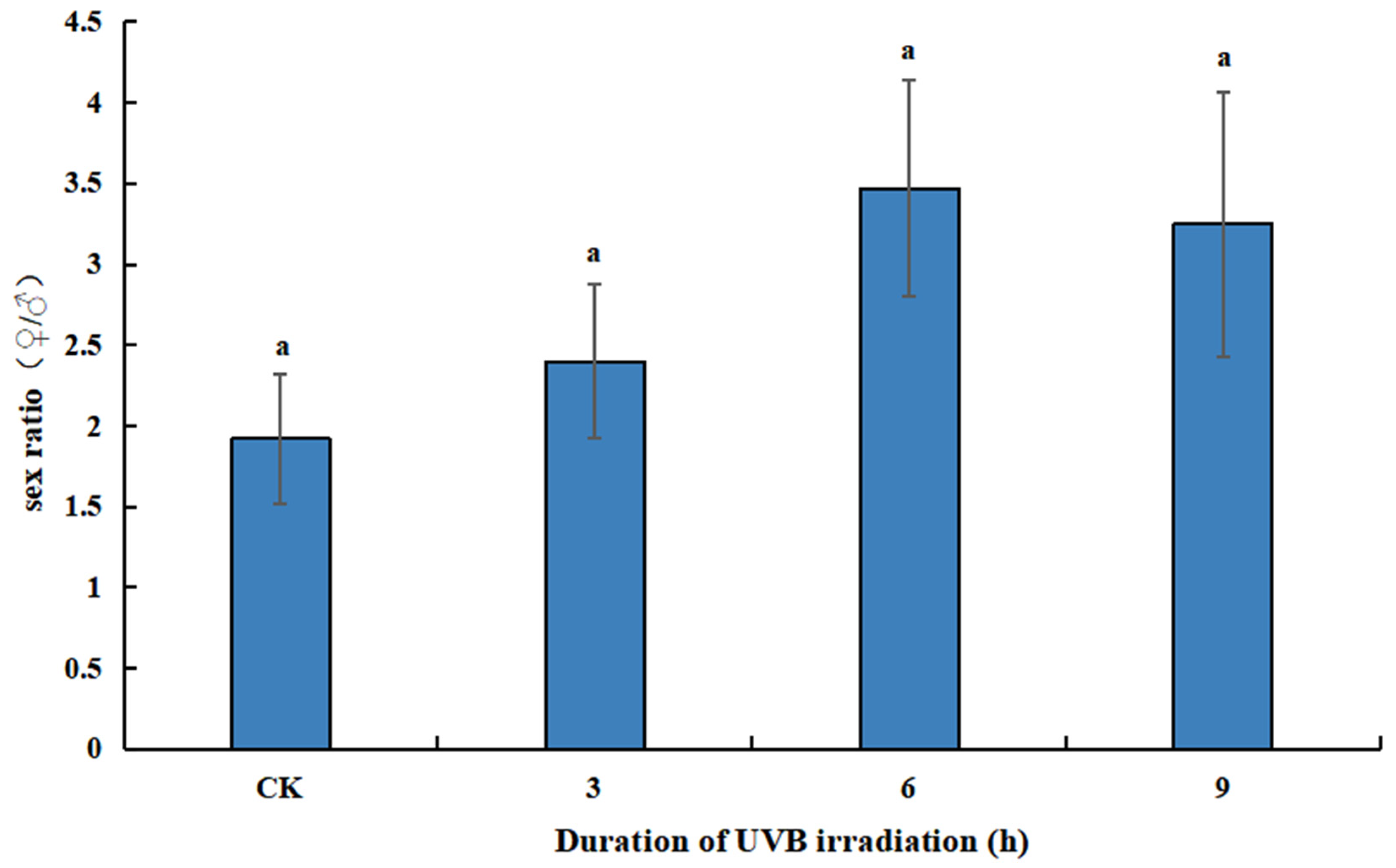
3.1. Host Species Preference
3.2. Parasitic Efficiency Based on Generations
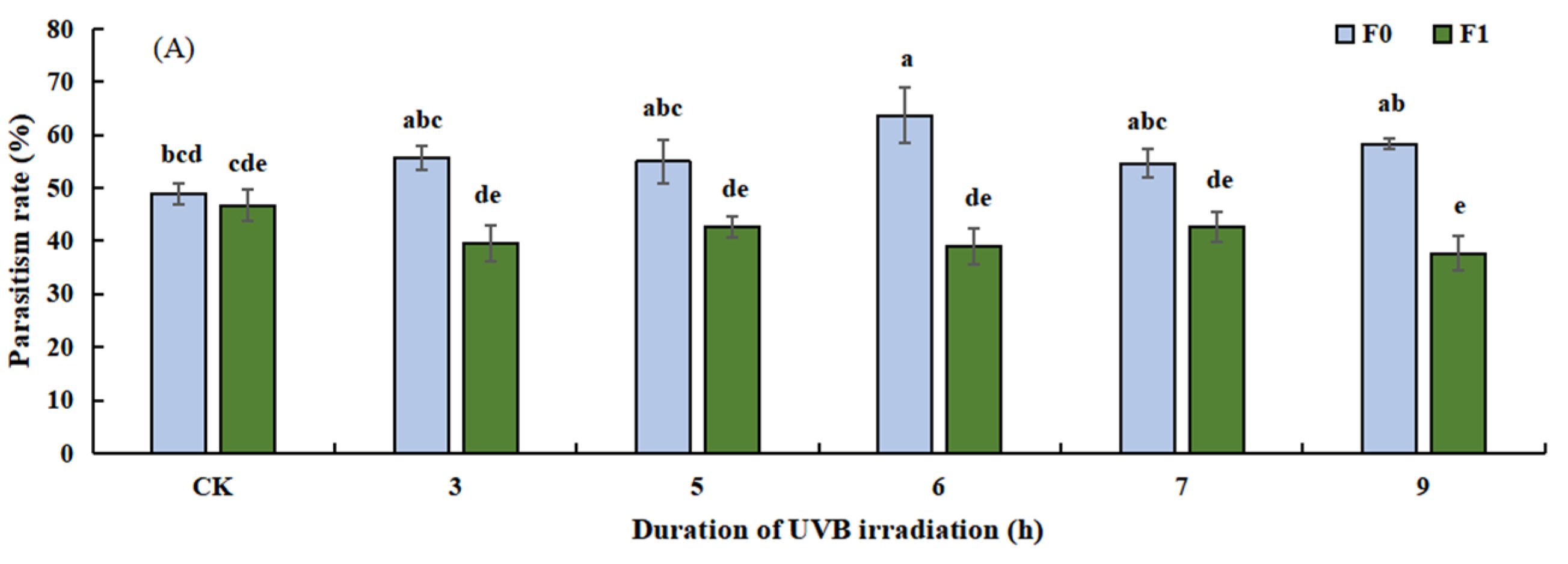
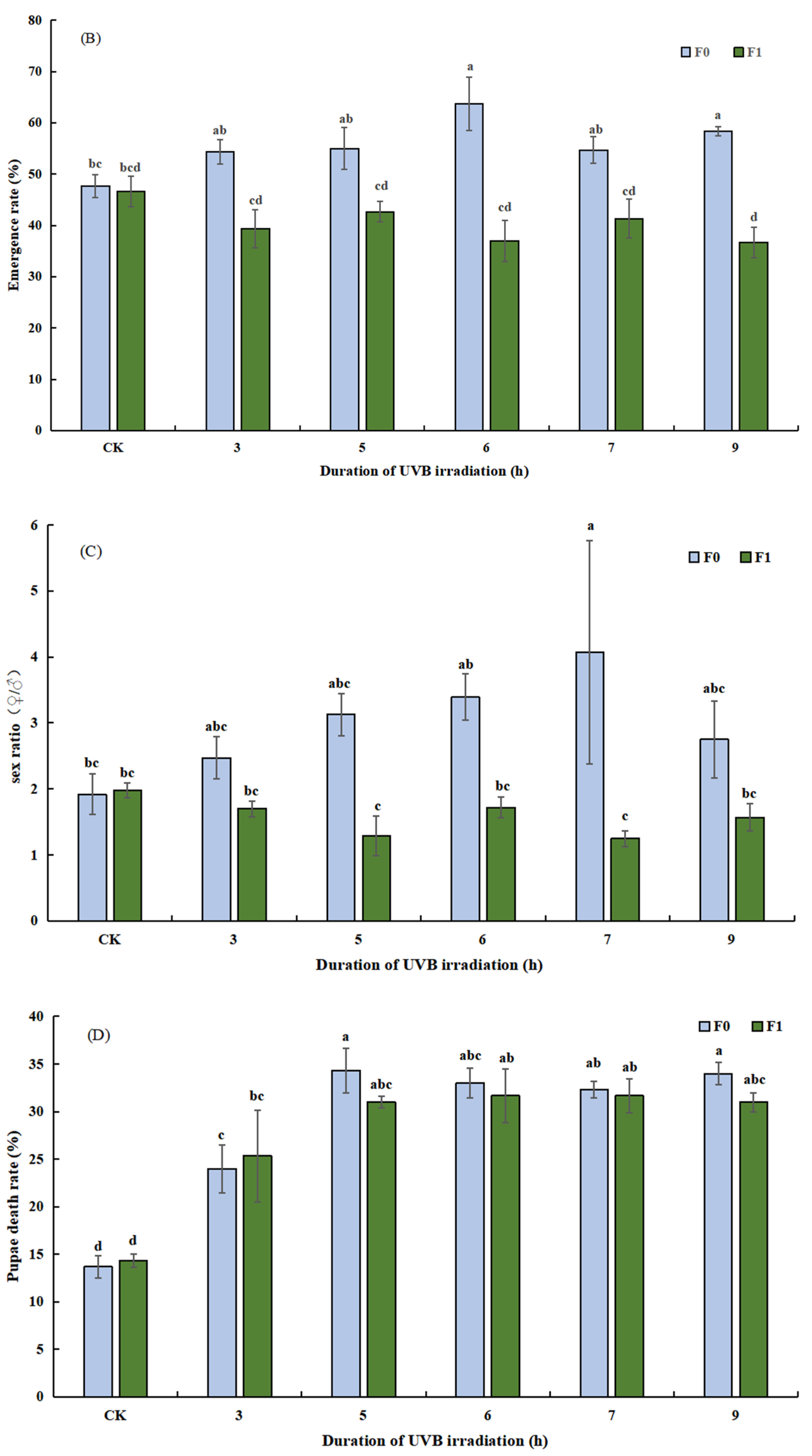
3.2.1. Effect of UVB-Irradiated Pupae on Parasitism Rate, Emergence Rate, Sex Ratio, and Pupal Death Rate of T. drosophilae
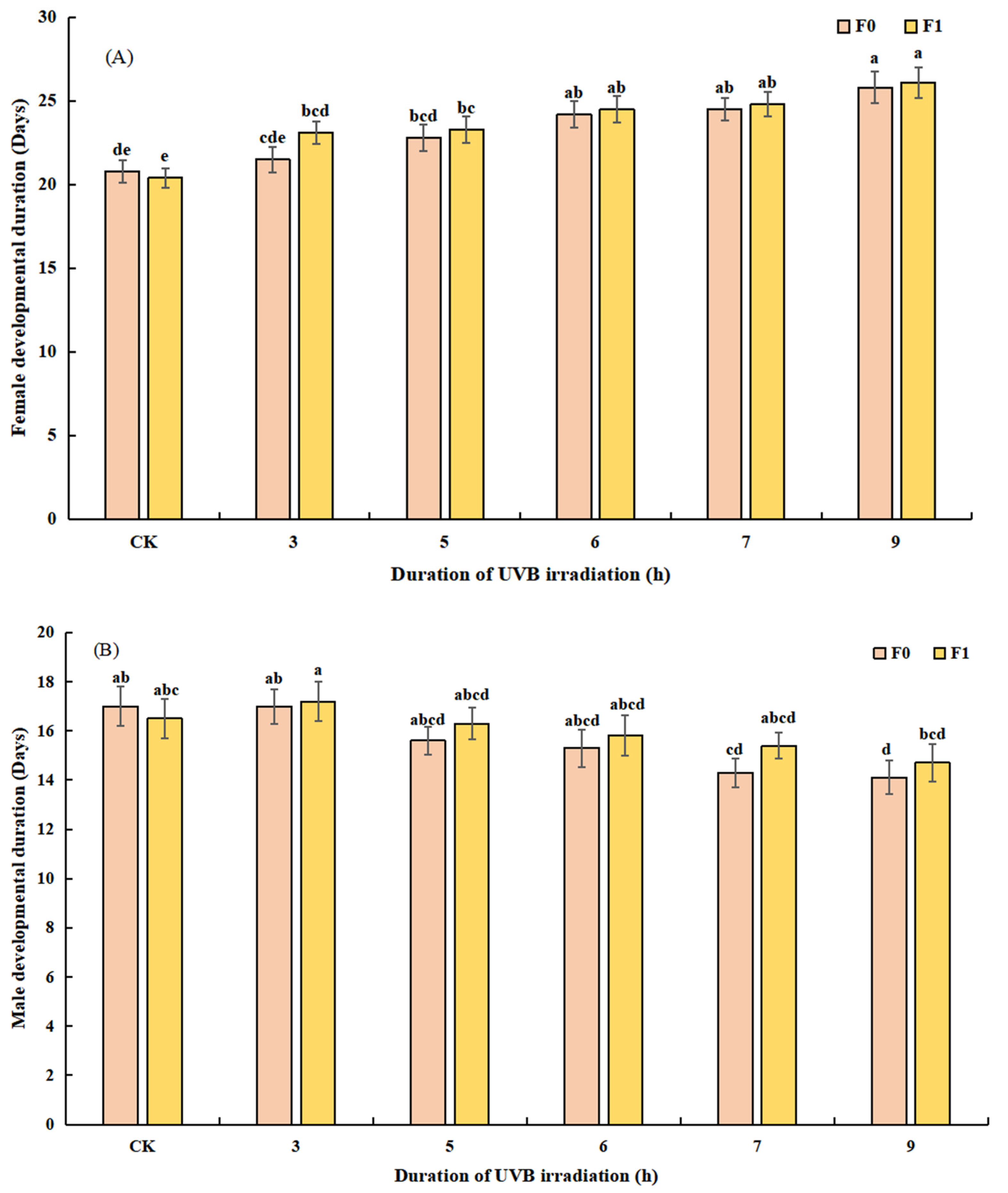
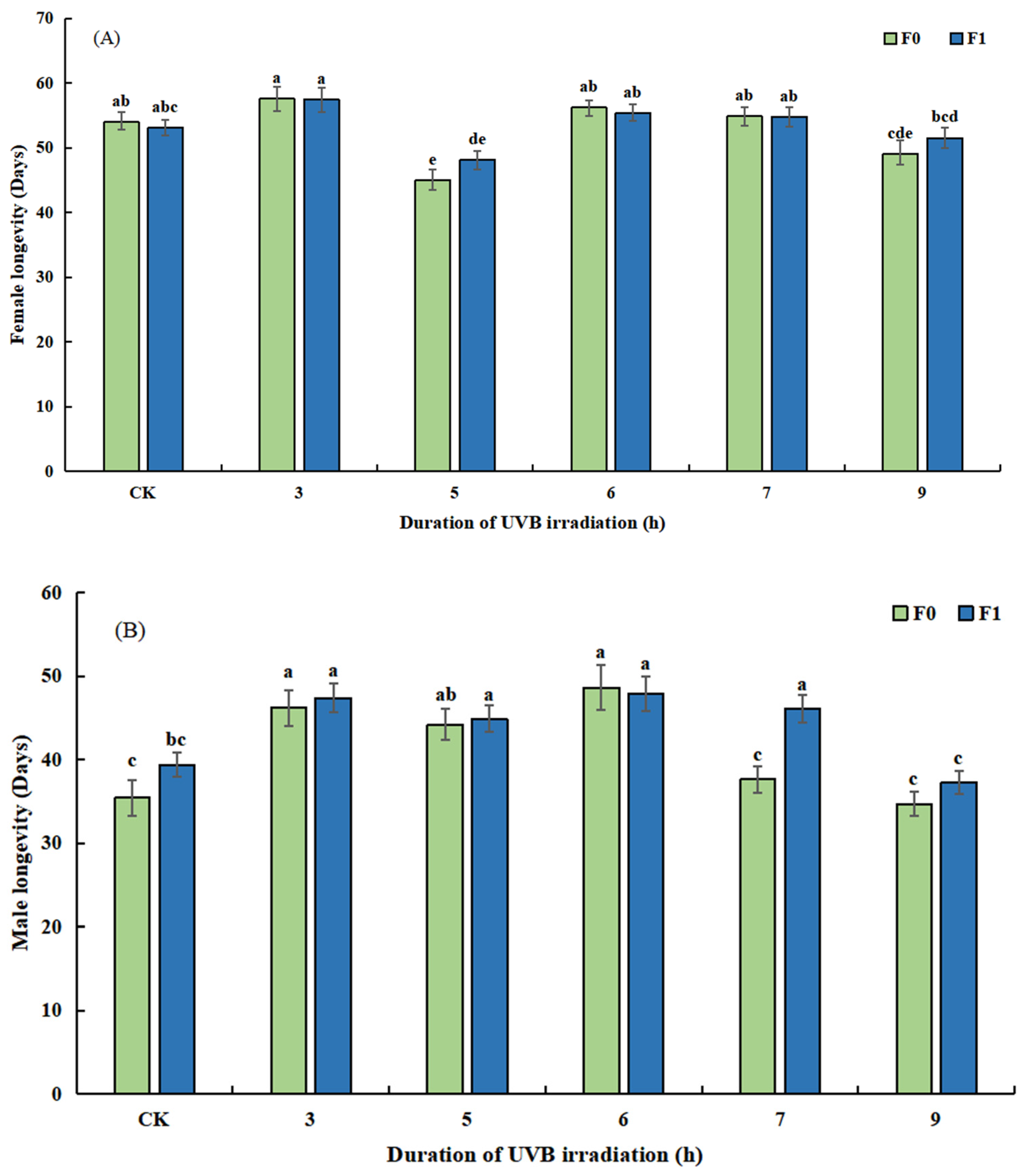
3.2.2. Effect of UVB-Irradiated Pupae on the Duration of Development and Longevity of T. drosophilae
3.3. Determination of the Outdoor Quality of Reared Irradiated Pupae Based on F1 and F2 T. drosophilae
3.3.1. Outdoor Quality of Reared UVB-Irradiated Pupae Based on Parasitism Rate, Emergence Rate, Sex Ratio, and Pupal Death Rate of F1 and F2 T. drosophilae
3.3.2. Outdoor Quality of Reared UVB-Irradiated Pupae Based on Duration of Development and Longevity of F1 and F2 T. drosophilae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, D. Using Drosophila as a model insect. Nat. Rev. Genet. 2000, 1, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mishra, A.K.; Mutsuddi, M.; Mukherjee, A. Mighty fly: An introduction to Drosophila. In Insights into Human Neurodegeneration: Lessons Learnt from Drosophila; Mutsuddi, M., Mukherjee, A., Eds.; Springer: Singapore, 2019; pp. 1–36. [Google Scholar]
- Garcia, F.R.M. Introduction to Drosophila suzukii management. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–9. [Google Scholar]
- Hamby, K.A.; Bellamy, D.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and abiotic factors impacting development, behavior, phenology, and reproductive biology of Drosophila suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive pest of ripening soft fruit expanding its geographic range and damage potential. J. Integr. Pest Manag. 2011, 106, 289–295. [Google Scholar] [CrossRef]
- Goodhue, R.E.; Bolda, M.; Farnsworth, D.; Williams, J.C.; Zalom, F.G. Spotted wing drosophila infestation of California strawberries and raspberries: Economic analysis of potential revenue losses and control costs. Pest Manag. Sci. 2011, 67, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.R.M.; Lasa, R.; Funes, C.F.; Buzzetti, K. Drosophila suzukii management in Latin America: Current status and perspectives. J. Econ. Entomol. 2022, 115, 1008–1023. [Google Scholar] [CrossRef]
- Tochen, S.; Dalton, D.T.; Wiman, N.; Hamm, C.; Shearer, P.W.; Walton, V.M. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environ. Entomol. 2014, 43, 501–510. [Google Scholar] [CrossRef]
- Wiman, N.G.; Walton, V.M.; Dalton, D.T.; Anfora, G.; Burrack, H.J.; Chiu, J.C.; Daane, K.M.; Grassi, A.; Miller, B.; Tochen, S.; et al. Integrating temperature-dependent life table data into a matrix projection model for Drosophila suzukii population estimation. PLoS ONE 2014, 9, e106909. [Google Scholar] [CrossRef]
- Dam, D.; Molitor, D.; Beyer, M. Natural compounds for controlling Drosophila suzukii. A review. Agron. Sustain. Dev. 2019, 39, 53. [Google Scholar] [CrossRef]
- Shawer, R. Chemical control of Drosophila suzukii. In Drosophila suzukii Management; Garcia, F.R.M., Ed.; Springer: Cham, Switzerland, 2020; pp. 133–142. [Google Scholar]
- Shawer, R.; Tonina, L.; Tirello, P.; Duso, C.; Mori, N. Laboratory and field trials to identify effective chemical control strategies for integrated management of Drosophila suzukii in European cherry orchards. Crop Prot. 2018, 103, 73–80. [Google Scholar] [CrossRef]
- Kruitwagen, A.; Beukeboom, L.W.; Wertheim, B. Optimization of native biocontrol agents, with parasitoids of the invasive pest Drosophila suzukii as an example. Evol. Appl. 2018, 11, 1473–1497. [Google Scholar] [CrossRef]
- Gabarra, R.; Riudavets, J.; Rodríguez, G.A.; Pujade-Villar, J.; Arnó, J. Prospects for the biological control of Drosophila suzukii. BioControl 2015, 60, 331–339. [Google Scholar] [CrossRef]
- Miller, B.; Anfora, G.; Buffington, M.; Daane, K.M.; Dalton, D.T.; Hoelmer, K.M.; Rossi Stacconi, M.V.; Grassi, A.; Ioriatti, C.; Loni, A.; et al. Seasonal occurrence of resident parasitoids associated with Drosophila suzukii in two small fruit production regions of Italy and the USA. Bull. Insectol. 2015, 68, 255–263. [Google Scholar]
- Cancino, M.D.G.; Hernández, A.G.; Cabrera, J.G.; Carrillo, G.M.; González, J.A.S.; Bernal, H.C.A. Parasitoides de Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) en Colima, México. Southwest. Entomol. 2015, 40, 855–858. [Google Scholar] [CrossRef]
- Wang, X.G.; Kaçar, G.; Biondi, A.; Daane, K.M. Life-history and host preference of Trichopria drosophilae, a pupal parasitoid of spotted wing drosophila. BioControl 2016, 61, 387–397. [Google Scholar] [CrossRef]
- Yi, C.D.; Cai, P.M.; Lin, J.; Liu, X.X.; Ao, G.F.; Zhang, Q.W.; Xia, H.M.; Yang, J.Q.; Ji, Q.E. Life history and host preference of Trichopria drosophilae from southern China, one of the effective pupal parasitoids on the Drosophila species. Insects 2020, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Kaçar, G.; Biondi, A.; Daane, K.M. Foraging efficiency and outcomes of interactions of two indigenous parasitoids attacking the invasive spotted wing drosophila. Biol. Control 2016, 96, 64–71. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.Y.; Xiong, Y.; Liu, S.N.; Hu, C.H.; Xiao, C.; Tang, G.W. Mating behavior of Trichopria drosophilae and the effect of male mating frequency on the production of female offspring. Chin. J. Appl. Entomol. 2017, 54, 749–754. [Google Scholar]
- Wang, X.G.; Nance, A.H.; Jones, J.M.L.; Hoelmer, K.A.; Daane, K.M. Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol. Control 2018, 121, 58–65. [Google Scholar] [CrossRef]
- Wang, X.G.; Serrato, M.A.; Son, Y.; Walton, V.M.; Hogg, B.N.; Daane, K.M. Thermal performance of two indigenous pupal parasitoids attacking the invasive Drosophila suzukii (Diptera: Drosophilidae). Environ. Entomol. 2018, 47, 764–772. [Google Scholar] [CrossRef]
- Chabert, S.; Allemand, R.; Poyet, M.; Eslin, P.; Gibert, P. Ability of European parasitoids (Hymenoptera) to control a new invasive Asiatic pest, Drosophila suzukii. Biol. Control 2012, 63, 40–47. [Google Scholar] [CrossRef]
- Kremmer, L.; Thaon, M.; Borowiec, N.; David, J.; Poirié, M.; Gatti, J.L.; Ris, N. Field monitoring of Drosophila suzukii and associated communities in south eastern France as a pre-requisite for classical biological control. Insects 2017, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Daane, K.M.; Wang, X.G.; Biondi, A.; Miller, B.; Miller, J.C.; Riedl, H.; Shearer, P.W.; Guerrieri, E.; Giorgini, M.; Buffington, M.; et al. First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. J. Pest Sci. 2016, 89, 823–835. [Google Scholar] [CrossRef]
- Boycheva, W.S.; Romeis, J.; Collatz, J. Influence of the rearing host on biological parameters of Trichopria drosophilae, a potential biological control agent of Drosophila suzukii. Insects 2019, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.Y.; Shi, H.M.; Jiang, X.L.; Liang, H.M.; Guo, Y.Y.; Gao, H.H. Host stage preference of Trichopria drosophilae to fruit fly pupae. Chin. J. Biol. Control 2019, 35, 977–981. [Google Scholar]
- Chen, J.N.; Zhou, S.C.; Wang, Y.; Shi, M.; Chen, X.X.; Huang, J.H. Biocontrol characteristics of the fruit fly pupal parasitoid Trichopria drosophilae (Hymenoptera: Diapriidae) emerging from different hosts. Sci. Rep. 2018, 8, 13323. [Google Scholar] [CrossRef]
- Rossi, S.M.V.; Grassi, A.; Ioriatti, C.; Anfora, G. Augmentative releases of Trichopria drosophilae for the suppression of early season Drosophila suzukii populations. BioControl 2019, 64, 9–19. [Google Scholar] [CrossRef]
- Zhu, C.J.; Li, J.; Wang, H.; Zhang, M.; Hu, H.Y. Demographic potential of the pupal parasitoid Trichopria drosophilae (Hymenoptera: Diapriidae) reared on Drosophila suzukii (Diptera: Drosophilidae). J. Asia-Pac. Entomol. 2017, 20, 747–751. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, F.; Yuan, B.; Yang, L.; Yang, Y.; Fang, Q.; Kuhn, J.H.; Song, Q.S.; Ye, G.Y. A novel cripavirus of an ectoparasitoid wasp increases pupal duration and fecundity of the wasp’s Drosophila melanogaster host. ISME J. 2021, 15, 3239–3257. [Google Scholar] [CrossRef]
- Huang, J.H.; Chen, J.N.; Fang, G.Q.; Pang, L.; Zhou, S.C.; Zhou, Y.N.; Pan, Z.Q.; Zhang, Q.C.; Sheng, Y.F.; Lu, Y.Q.; et al. Two novel venom proteins underlie divergent parasitic strategies between a generalist and a specialist parasite. Nat. Commun. 2021, 12, 234. [Google Scholar] [CrossRef]
- Chen, J.N.; Fang, G.Q.; Pang, L.; Sheng, Y.F.; Zhang, Q.C.; Zhou, Y.N.; Zhou, S.C.; Lu, Y.Q.; Liu, Z.G.; Zhang, Y.X.; et al. Neofunctionalization of an ancient domain allows parasites to avoid intraspecific competition by manipulating host behaviour. Nat. Commun. 2021, 12, 5489. [Google Scholar] [CrossRef]
- Herz, A.; Dingeldey, E.; Englert, C. More power with flower for the pupal parasitoid Trichopria drosophilae: A candidate for biological control of the Spotted Wing Drosophila. Insects 2021, 12, 628. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Yi, C.D.; Ao, G.F.; Chen, S.; Ji, Q.E. Effects of three kinds of ultraviolet radiation on pupae of Drosophila melanogaster. Chin. J. Biol. Control 2021, 37, 228–234. [Google Scholar]
- Van Atta, K.J.; Potter, K.A.; Woods, H.A. Effects of UV-B on environmental preference and egg parasitization by Trichogramma wasps (Hymenoptera: Trichogrammatidae). J. Entomol. Sci. 2015, 50, 318–325. [Google Scholar]
- Alwandri, H.; Kusumawati, N.; Sudaryadi, I. The Effect of UV Radiation and Fruit Feedings (Banana and Guava) on the Survival Rate and Morphological Changes of Reproductive Organ of Fruit Fly (Drosophila melanogaster Meigen, 1830). In Proceedings of the 7th International Conference on Biological Science (ICBS 2021), Online, 14–15 October 2021; Atlantis: Amsterdam, The Netherlands, 2022; pp. 90–97. [Google Scholar]
- Ayvaz, A.; Karasu, E.; Karabörklü, S.; Tunçbilek, A.Ş. Effects of cold storage, rearing temperature, parasitoid age and irradiation on the performance of Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae). J. Stored Prod. Res. 2008, 44, 232–240. [Google Scholar] [CrossRef]
- Masry, S.H.; El-Wakeil, N. Egg parasitoid production and their role in controlling insect pests. In Cottage Industry of Biocontrol Agents and Their Applications; El-Wakeil, N., Saleh, M., Abu-hashim, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 3–47. [Google Scholar]
- Shinde, C.U.; Radadia, G.G.; Ghetiya, L.V.; Shah, K.D.; Gadhiya, V.C.; Patel, A.D. Effect of non-ionizing (UV) radiation on the development of egg parasitoid, Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae). Adv. Life Sci. 2016, 5, 10357–10361. [Google Scholar]
- St-Onge, M.; Cormier, D.; Todorova, S.; Lucas, E. Conservation of Ephestia kuehniella eggs as hosts for Trichogramma ostriniae. J. Appl. Entomol. 2016, 140, 218–222. [Google Scholar] [CrossRef]
- St-Onge, M.; Cormier, D.; Todorova, S.; Lucas, É. Comparison of Ephestia kuehniella eggs sterilization methods for Trichogramma rearing. Biol. Control 2014, 70, 73–77. [Google Scholar] [CrossRef]
- Xu, J.; Yang, X.; Lin, Y.; Zang, L.S.; Tian, C.Y.; Ruan, C.C. Effect of fertilized, unfertilized, and UV-irradiated hosts on parasitism and suitability for Trichogramma parasitoids. Entomol. Exp. Appl. 2016, 161, 50–56. [Google Scholar] [CrossRef]
- Mawela, K.V.; Kfir, R.; Krüger, K. Host suitability of UV-irradiated eggs of three Lepidoptera species for rearing Trichogrammatoidea lutea Girault (Hymenoptera: Trichogrammatidae). J. Appl. Entomol. 2010, 134, 737–744. [Google Scholar] [CrossRef]
- Foggo, A.; Higgins, S.; Wargent, J.J.; Coleman, R.A. Tri-trophic consequences of UV-B exposure: Plants, herbivores and parasitoids. Oecologia 2007, 154, 505–512. [Google Scholar] [CrossRef]
- Dhal, M.K.; Seth, R.K. Effect of ionizing (gamma) and non-ionizing (UV) radiation on Lepidopteran host eggs for the efficacy of egg parasitoid, Trichogramma Chilonis Ishii (Hymenoptera: Trichogrammatidae). Int. J. Res. Biosci. Agric. Technol. 2017, V, 223–235. [Google Scholar]
- Little, C.M.; Rizzato, A.R.; Charbonneau, L.; Chapman, T.; Hillier, N.K. Color preference of the spotted wing Drosophila, Drosophila suzukii. Sci. Rep. 2019, 9, 16051. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.; Iglesias, L.E.; Bonneau, P.; Liburd, O.E. Trapping system comparisons for and factors affecting populations of Drosophila suzukii and Zaprionus indianus in winter-grown strawberry. Pest Manag. Sci. 2018, 74, 2076–2088. [Google Scholar] [CrossRef] [PubMed]
- Renkema, J.M.; Buitenhuis, R.; Hallett, R.H. Optimizing trap design and trapping protocols for Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2014, 107, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, L.E.; Nyoike, T.W.; Liburd, O.E. Effect of trap design, bait type, and age on captures of Drosophila suzukii (Diptera: Drosophilidae) in berry crops. J. Econ. Entomol. 2014, 107, 1508–1518. [Google Scholar] [CrossRef]
- Zapater, M.C.; Andiarena, C.E.; Camargo, G.P.; Bartoloni, N. Use of irradiated Musca domestica pupae to optimize mass rearing and commercial shipment of the parasitoid Spalangia endius (Hymenoptera: Pteromalidae). Biocontrol Sci. Technol. 2009, 19, 261–270. [Google Scholar] [CrossRef]
- Peng, W.; Li, Y.X.; Weng, S.H.; Zhou, R.H.; Pan, X.; Li, J.Y.; Han, B.Y. Progress and prospects of sex-separation techniques for dipteran insects. Acta Entomol. Sin. 2020, 63, 902–912. [Google Scholar]
- Zhang, X.; Yi, P.; Chu, P.F.; Yuan, X.Q.; Zhan, E.L.; Leng, C.M.; Li, Y.; Hu, D.; Li, Y.P. Effects of microwave irradiation on the growth, development andreproduction of the green peach aphid, Myzus persicae (Hemiptera: Aphididae). Acta Entomol. Sin. 2020, 63, 1215–1222. [Google Scholar]
- Ben-Yakir, D.; Fereres, A. The effects of UV radiation on arthropods: A review of recent publications (2010–2015). In International Symposium on Light in Horticulture; ISHS Acta Horticulturae: Leuven, Belgium, 2016; Volume 1134, pp. 335–342. [Google Scholar]
- Tilton, E.W.; Brower, J.H. Radiation effects on arthropods. In Preservation of Food by Ionizing Radiation; CRC: Boca Raton, FL, USA, 2018; pp. 269–316. [Google Scholar]
- Tuncbilek, A.S.; Kusmus, A.; Canpolat, U.A.; Ercan, F.S. Effect of UV radiation on Bracon Hebetor (Hymenoptera: Braconidae) and its host larvae, Ephestia Kuehniella (Lepidoptera: Pyralidae). Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2011, 41, 270–278. [Google Scholar]
- Cui, H.Y.; Zeng, Y.Y.; Reddy, G.V.; Gao, F.; Li, Z.H.; Zhao, Z.H. UV radiation increases mortality and decreases the antioxidant activity in a tephritid fly. Food Energy Secur. 2021, 10, e297. [Google Scholar] [CrossRef]
- Espo, E.; Eyidozehi, K.; Ravan, S. Influence of gamma and ultraviolet irradiation on pest control. MAGNT Res. Rep. 2015, 3, 319–326. [Google Scholar]
- Shimoda, M.; Honda, K.I. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Nakagawa, S.; Farias, G.J.; Steiner, L.F. Response of female Mediterranean fruit flies to male lures in the relative absence of males. J. Econ. Entomol. 1970, 63, 227–229. [Google Scholar] [CrossRef]
- Jarrett, B.J.; Linder, S.; Fanning, P.D.; Isaacs, R.; Szűcs, M. Experimental adaptation of native parasitoids to the invasive insect pest, Drosophila suzukii. Biol. Control 2022, 167, 104843. [Google Scholar] [CrossRef]
- Lee, J.C.; Wang, X.G.; Daane, K.M.; Hoelmer, K.A.; Isaacs, R.; Sial, A.A.; Walton, V.M. Biological control of spotted-wing Drosophila (Diptera: Drosophilidae)-current and pending tactics. J. Integr. Pest Manag. 2019, 10, 13. [Google Scholar] [CrossRef]
- Wolf, S.; Boycheva-Woltering, S.; Romeis, J.; Collatz, J. Trichopria drosophilae parasitizes Drosophila suzukii in seven common non-crop fruits. J. Pest Sci. 2020, 93, 627–638. [Google Scholar] [CrossRef]
- Häussling, B.J.; Mautner, M.; Stökl, J. Below ground efficiency of a parasitic wasp for Drosophila suzukii biocontrol in different soil types. Sci. Rep. 2022, 12, 9130. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, F.; Lei, Z.L. Effects of UV radiation on biological characteristics of Plutella xylostella. Stud. Insects Cent. China 2015, 11, 155–159. [Google Scholar]
- Carton, Y.; Poirié, M.; Nappi, A.J. Insect immune resistance to parasitoids. Insect Sci. 2008, 15, 67–87. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Mateos, M.; Winter, L.; Winter, C.; Higareda-Alvear, V.M.; Martinez-Romero, E.; Xie, J. Independent origins of resistance or susceptibility of parasitic wasps to a defensive symbiont. Ecol. Evol. 2016, 6, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Ventura, I.M.; Martins, A.B.; Lyra, M.L.; Andrade, C.A.; Carvalho, K.A.; Klaczko, L.B. Spiroplasma in Drosophila melanogaster populations: Prevalence, male-killing, molecular identification, and no association with Wolbachia. Microb. Ecol. 2012, 64, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Van, G.J.; Herre, E.A.; Gómez, A.; Nason, J.D. Extraordinarily precise nematode sex ratios: Adaptive responses to vanishingly rare mating opportunities. Proc. R. Soc. B 2022, 289, 20211572. [Google Scholar]
- Krüger, A.P.; Scheunemann, T.; Vieira, J.G.A.; Morais, M.C.; Bernardi, D.; Nava, D.E.; Garcia, F.R.M. Effects of extrinsic, intraspecific competition and host deprivation on the biology of Trichopria anastrephae (Hymenoptera: Diapriidae) reared on Drosophila suzukii (Diptera: Drosophilidae). Neotrop. Entomol. 2019, 48, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Linder, S.; Jarrett, B.J.; Fanning, P.; Isaacs, R.; Szűcs, M. Limited gains in native parasitoid performance on an invasive host beyond three generations of selection. Evol. Appl. 2022, 15, 2113–2124. [Google Scholar] [CrossRef]
- Wolf, S.; Barmettler, E.; Eisenring, M.; Romeis, J.; Collatz, J. Host searching and host preference of resident pupal parasitoids of Drosophila suzukii in the invaded regions. Pest Manag. Sci. 2021, 77, 243–252. [Google Scholar] [CrossRef]
- Stacconi, M.V.R.; Panel, A.; Baser, N.; Ioriatti, C.; Pantezzi, T.; Anfora, G. Comparative life history traits of indigenous Italian parasitoids of Drosophila suzukii and their effectiveness at different temperatures. Biol. Control 2017, 112, 20–27. [Google Scholar] [CrossRef]
- Liu, X. Study on Reproductive Biology of Spalangia endius Walker. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2017. [Google Scholar]
- Wang, J.Q.; Xu, L.Y.; Li, F.C.; Zheng, Y.P.; Deng, Y.X.; Zhang, Y.K.; Zhu, G.Y.; Li, G.H. Effect of temperature on emergence rate and sex ratio of Diversinervus elegans Silvestri. J. Environ. Entomol. 2019, 41, 161–166. [Google Scholar]
- Hallouti, A.; Ait, H.M.; Tazi, H.; Ait, H.R.; Zahidi, A.; Ait, B.A.A.; Boubaker, H. Pathogenicity of Fusarium spp. isolates against the Mediterranean fruit fly (Ceratitis capitata) and their responses to ultraviolet-B radiation and water stress. Entomol. Exp. Appl. 2021, 169, 424–436. [Google Scholar] [CrossRef]
- Ghosh, E.; Ballal, C.R. Short-term storage of the egg parasitoids, Trichogramma and Trichogrammatoidea. Egypt. J. Biol. Pest Control 2018, 28, 34. [Google Scholar] [CrossRef]
- László, Z.; Dénes, A.L.; Király, L.; Tóthmérész, B. Biased parasitoid sex ratios: Wolbachia, functional traits, local and landscape effects. Basic Appl. Ecol. 2018, 31, 61–71. [Google Scholar] [CrossRef]
- Tang, Y.L.; Wang, L.N.; Wang, Y.Q.; Zhang, Y.L.; Wang, X.Y.; Wei, K. Effects of different foundress densities on sex ratio of the offspring of Bethylid wasps. Sci. Silvae Sin. 2022, 58, 161–168. [Google Scholar]
- Huang, J.; Zhi, F.Y.; Lv, Y.B. Fitness of Aenasius bambawalei (Hymenoptera: Encyrtidae) on a new host species. Acta Entomol. Sin. 2019, 62, 1427–1434. [Google Scholar]
- Hu, S.; Luo, L.P.; Wang, X.Y. Wing dimorphism and sex ratio changes in progeny of various sister broods in parasitoid Sclerodermus pupariae (Hymenoptera: Bethylidae). For. Res. 2019, 32, 21–26. [Google Scholar]
- Krylova, A.E.; Chaplygina, A.V.; Vekshin, N.L. Formation of Lipofuscin in Drosophila after exposure to elevated temperatures and UV radiation. Biophysics 2020, 65, 58–62. [Google Scholar] [CrossRef]
- Bath, E.; Edmunds, D.; Norman, J.; Atkins, C.; Harper, L.; Rostant, W.G.; Chapman, T.; Wigby, S.; Perry, J.C. Sex ratio and the evolution of aggression in fruit flies. Proc. R. Soc. B 2021, 288, 20203053. [Google Scholar] [CrossRef]
- Alvarenga, C.D.; Dias, V.; Stuhl, C.; Sivinski, J. Contrasting brood-sex ratio flexibility in two Opiine (Hymenoptera: Braconidae) parasitoids of tephritid (Diptera) fruit flies. J. Insect Behav. 2016, 29, 25–36. [Google Scholar] [CrossRef]
- West, S.A.; Reece, S.E.; Sheldon, B.C. Sex ratios. Heredity 2002, 88, 117–124. [Google Scholar] [CrossRef]
- Werren, J.H. Sex ratio adaptations to local mate competition in a parasitic wasp. Science 1980, 208, 1157–1159. [Google Scholar] [CrossRef]
- Ghana, S.; Suleman, N.; Compton, S.G. Factors influencing realized sex ratios in fig wasps: Double oviposition and larval mortalities. J. Insect Behav. 2012, 25, 254–263. [Google Scholar] [CrossRef]
- Rempoulakis, P.; Castro, R.; Nemny-Lavy, E.; Nestel, D. Effects of radiation on the fertility of the Ethiopian fruit fly, Dacus ciliatus. Entomol. Exp. Appl. 2015, 155, 117–122. [Google Scholar] [CrossRef]
- Paithankar, J.G.; Deeksha, K.; Patil, R.K. Gamma radiation tolerance in different life stages of the fruit fly Drosophila melanogaster. Int. J. Radiat. Biol. 2017, 93, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.R.; Weldon, C.W.; Banos, C.; Taylor, P.W. Effects of irradiation dose rate on quality and sterility of Queensland fruit flies, Bactrocera tryoni (Froggatt). J. Appl. Entomol. 2008, 132, 398–405. [Google Scholar] [CrossRef]
- Mastrangelo, T.; da Silva, F.F.; Mascarin, G.M.; da Silva, C.B. Multispectral imaging for quality control of laboratory-reared Anastrepha fraterculus (Diptera: Tephritidae) pupae. J. Appl. Entomol. 2019, 143, 1072–1079. [Google Scholar] [CrossRef]
| Indexes | Treatments and Generations | ||
|---|---|---|---|
| CK | F1 | F2 | |
| Parasitism rate (%) | 48.00 ± 0.01 b | 59.67 ± 0.02 a | 51.67 ± 0.01 b |
| Emergence rate (%) | 46.67 ± 0.00 b | 59.67 ± 0.02 a | 51.67 ± 0.01 a |
| Sex ratio (♀/♂) | 1.84 ± 0.18 b | 3.20 ± 0.25 a | 2.46 ± 0.18 b |
| Pupal death rate (%) | 13.33 ± 0.01 b | 29.67 ± 0.02 a | 13.33 ± 0.00 b |
| Indexes (Days) | Treatments and Generations | |||
|---|---|---|---|---|
| F1 | F2 | |||
| CK | Ultraviolet-B | CK | Ultraviolet-B | |
| Female DD | 21.30 ± 0.67 b | 23.80 ± 0.96 a | 21.30 ± 0.67 b | 20.70 ± 0.88 b |
| Male DD | 17.30 ± 0.83 a | 16.10 ± 0.78 a | 17.30 ± 0.83 a | 17.50 ± 0.93 a |
| Female L | 52.10 ± 1.55 a | 54.93 ± 1.33 a | 52.10 ± 1.55 a | 53.07 ± 1.67 a |
| Male L | 35.87 ± 1.84 a | 40.87 ± 1.75 a | 35.87 ± 1.84 a | 37.93 ± 1.91 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yang, Y.; Fan, Q.; Zhang, Q.; Ji, Q. Effect of Ultraviolet-B Radiating Drosophila melanogaster as Host on the Quality of Trichopria drosophilae, a Pupal Parasitoid of Drosophila suzukii. Insects 2023, 14, 423. https://doi.org/10.3390/insects14050423
Liu X, Yang Y, Fan Q, Zhang Q, Ji Q. Effect of Ultraviolet-B Radiating Drosophila melanogaster as Host on the Quality of Trichopria drosophilae, a Pupal Parasitoid of Drosophila suzukii. Insects. 2023; 14(5):423. https://doi.org/10.3390/insects14050423
Chicago/Turabian StyleLiu, Xuxiang, Yongbang Yang, Qingwen Fan, Qinyuan Zhang, and Qinge Ji. 2023. "Effect of Ultraviolet-B Radiating Drosophila melanogaster as Host on the Quality of Trichopria drosophilae, a Pupal Parasitoid of Drosophila suzukii" Insects 14, no. 5: 423. https://doi.org/10.3390/insects14050423
APA StyleLiu, X., Yang, Y., Fan, Q., Zhang, Q., & Ji, Q. (2023). Effect of Ultraviolet-B Radiating Drosophila melanogaster as Host on the Quality of Trichopria drosophilae, a Pupal Parasitoid of Drosophila suzukii. Insects, 14(5), 423. https://doi.org/10.3390/insects14050423





