The Invasive Caucasian Populations of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Rapidly Adapt Their Ecophysiological Traits to the Local Environmental Conditions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
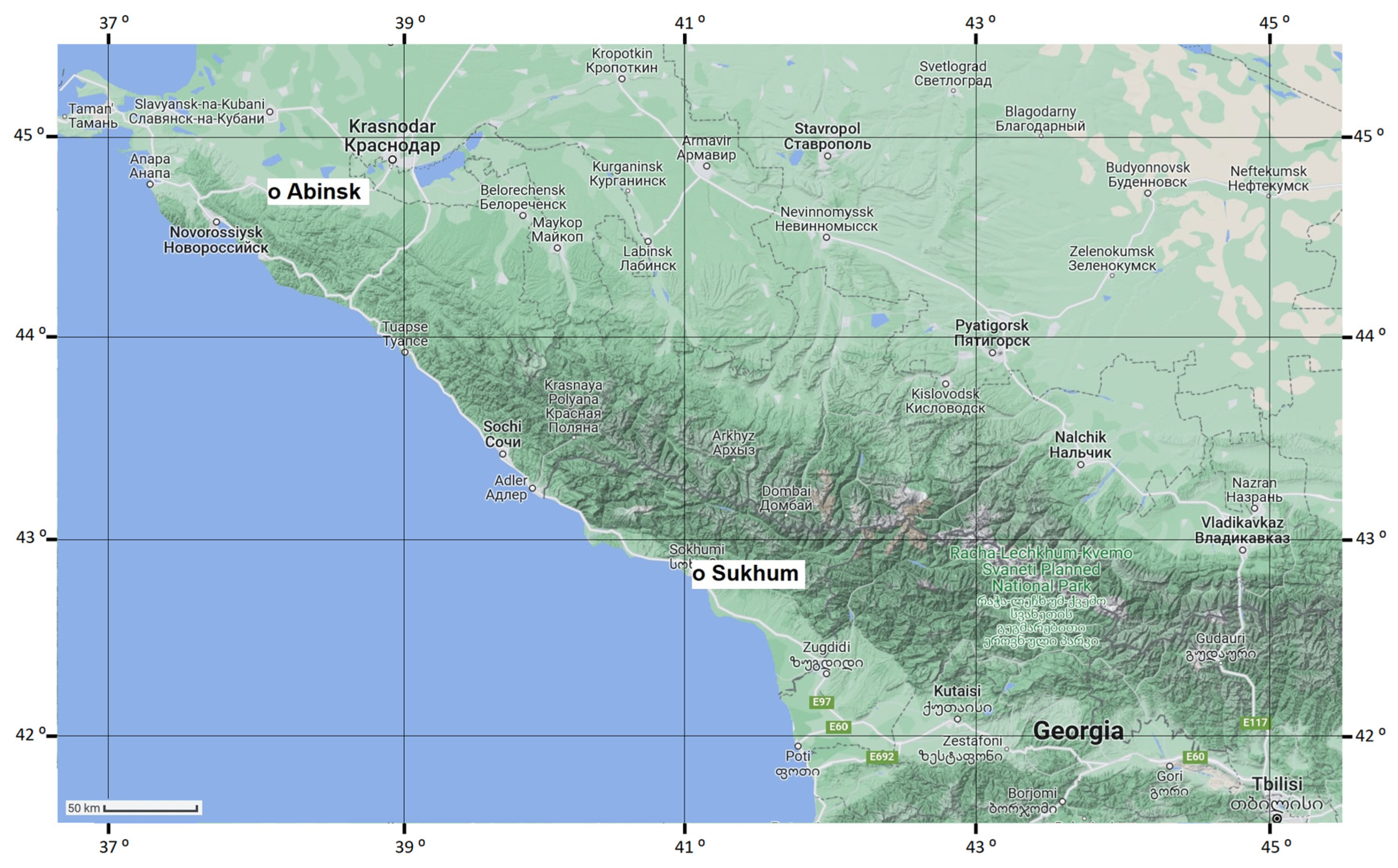
2.1. Insects, Collection Sites, and General Methods
- (1)
- Environs of Sukhum city, Abkhazia, ca. 42.95° N, 41.08° E, 20 m a.s.l., hereafter referred to as the Sukhum population;
- (2)
- Abinsk, Krasnodar Krai, Russia, ca. 44.87° N, 38.16° E, 41 m a.s.l., hereafter referred to as the Abinsk population.
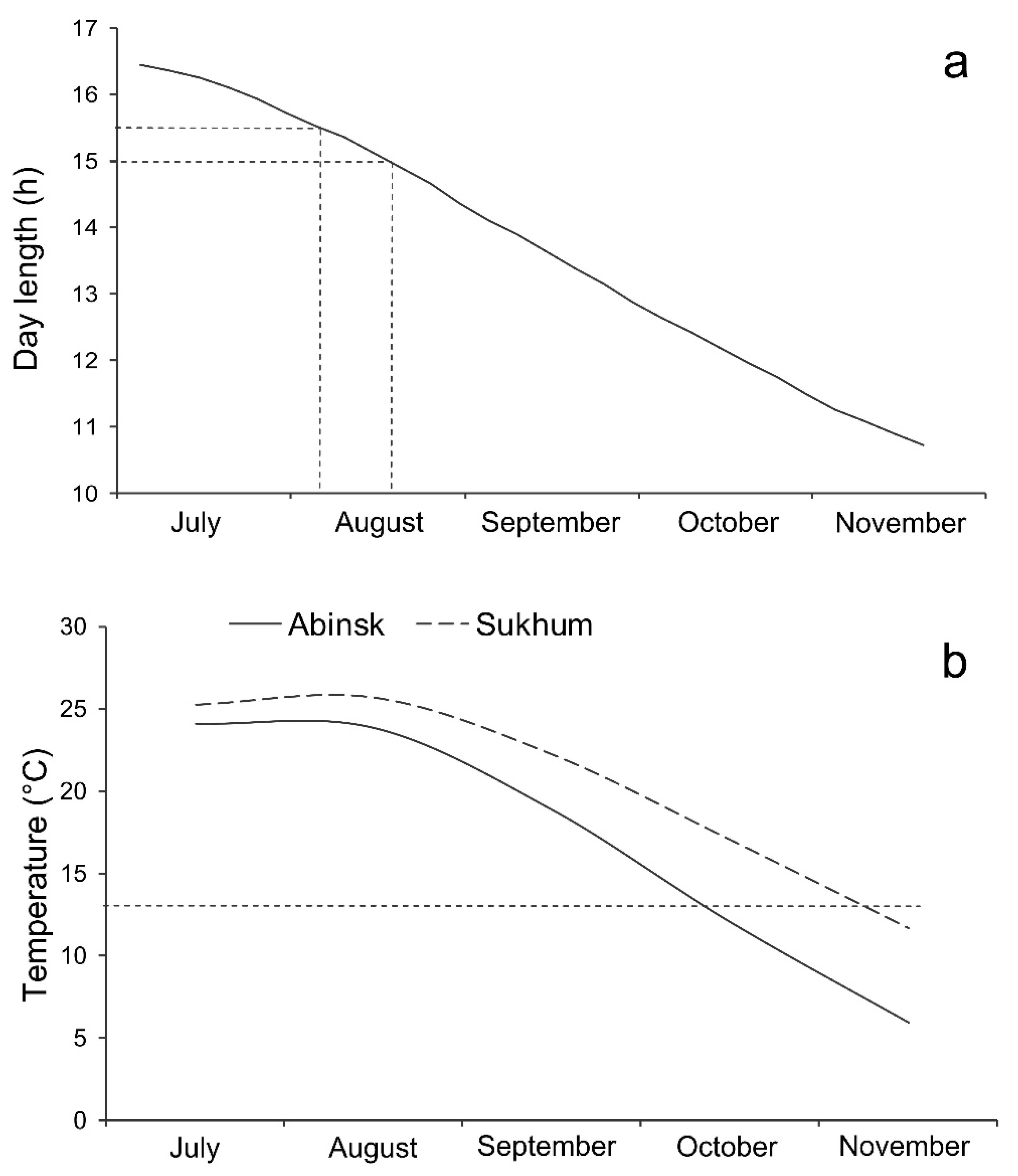
2.2. Nymphal Development
2.3. Reproductive Organs and Fat Body State of Adults
2.4. Statistical Analysis
3. Results
3.1. Nymphal Development
3.2. Reproductive Organs and Fat Body Development
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Danilevskii, A.S. Photoperiodism and Seasonal Development of Insects; Oliver & Boyd: London, UK, 1965. [Google Scholar]
- Zaslavski, V.A. Insect Development: Photoperiodic and Temperature Control; Springer: Berlin, Germany, 1988. [Google Scholar]
- Tauber, M.J.; Tauber, C.A.; Masaki, S. Seasonal Adaptations of Insects; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Denlinger, D.L. Regulation of diapause. Annu. Rev. Entomol. 2002, 47, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Denlinger, D.L. Insect Diapause; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar]
- Danks, H.V. Insect Dormancy: An Ecological Perspective; The Biological Survey of Canada: Ottawa, IL, Canada, 1987. [Google Scholar]
- Danks, H.V. The elements of seasonal adaptations in insects. Can. Entomol. 2007, 139, 1–44. [Google Scholar] [CrossRef]
- Tougeron, K. Diapause research in insects: Historical review and recent work perspectives. Entomol. Exp. Appl. 2019, 167, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Bradshaw, W.E.; Holzapfel, C.M. Evolution of animal photoperiodism. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 1–25. [Google Scholar] [CrossRef]
- Moran, E.V.; Alexander, J.M. Evolutionary responses to global change: Lessons from invasive species. Ecol. Lett. 2014, 17, 637–649. [Google Scholar] [CrossRef]
- Lindestad, O.; Wheat, C.W.; Nylin, S.; Gotthard, K. Local adaptation of photoperiodic plasticity maintains life cycle variation within latitudes in a butterfly. Ecology 2019, 100, e02550. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, G.A.; Waters, J.M. Rapid adaptation in a fast-changing world: Emerging insights from insect genomics. Glob. Change Biol. 2023, 29, 943–954. [Google Scholar] [CrossRef]
- Hendry, A.P.; Kinnison, M.T. Perspective: The pace of modern life: Measuring rates of contemporary microevolution. Evolution 1999, 53, 1637–1653. [Google Scholar] [CrossRef]
- Lee, C.E. Evolutionary genetics of invasive species. Trends Ecol. Evol. 2002, 17, 386–391. [Google Scholar] [CrossRef]
- Hairston, N.G., Jr.; Ellner, S.P.; Geber, M.A.; Yoshida, T.; Fox, J.A. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 2005, 8, 1114–1127. [Google Scholar] [CrossRef]
- Hufbauer, R.A.; Roderick, G.K. Microevolution in biological control: Mechanisms, patterns, and processes. Biol. Contr. 2005, 35, 227–239. [Google Scholar] [CrossRef]
- Lawson Handley, L.J.; Estoup, A.; Evans, D.M.; Thomas, C.E.; Lombaert, E.; Facon, B.; Aebi, A.; Roy, H.E. Ecological genetics of invasive alien species. BioControl 2011, 56, 409–428. [Google Scholar] [CrossRef] [Green Version]
- Colautti, R.I.; Lau, J.A. Contemporary evolution during invasion: Evidence for differentiation, natural selection, and local adaptation. Mol. Ecol. 2015, 24, 1999–2017. [Google Scholar] [CrossRef]
- Bean, D.W.; Dalin, P.; Dudley, T.L. Evolution of critical day length for diapause induction enables range expansion of Diorhabda carinulata, a biological control agent against tamarisk (Tamarix spp.). Evol. Appl. 2012, 5, 511–523. [Google Scholar] [CrossRef]
- McEvoy, P.B.; Higgs, K.M.; Coombs, E.M.; Karaçetin, E.; Ann Starcevich, L. Evolving while invading: Rapid adaptive evolution in juvenile development time for a biological control organism colonizing a high-elevation environment. Evol. Appl. 2012, 5, 524–536. [Google Scholar] [CrossRef] [Green Version]
- Szűcs, M.; Schaffner, U.; Price, W.J.; Schwarzländer, M. Post-introduction evolution in the biological control agent Longitarsus jacobaeae (Coleoptera: Chrysomelidae). Evol. Appl. 2012, 5, 858–868. [Google Scholar] [CrossRef]
- Urbanski, J.; Mogi, M.; O’Donnell, D.; DeCotiis, M.; Toma, T.; Armbruster, P. Rapid adaptive evolution of photoperiodic response during invasion and range expansion across a climatic gradient. Am. Nat. 2012, 179, 490–500. [Google Scholar] [CrossRef]
- Lehmann, P.; Lyytinen, A.; Piiroinen, S.; Lindström, L. Northward range expansion requires synchronization of both overwintering behaviour and physiology with photoperiod in the invasive Colorado potato beetle (Leptinotarsa decemlineata). Oecologia 2014, 176, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Grevstad, F.S.; Coop, L.B. The consequences of photoperiodism for organisms in new climates. Ecol. Appl. 2015, 25, 1506–1517. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Dolgovskaya, M.Y.; Ovchinnikov, A.N.; Belyakova, N.A. Weak photoperiodic response facilitates the biological invasion of the harlequin ladybird Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae). J. Appl. Entomol. 2015, 139, 241–249. [Google Scholar] [CrossRef]
- Ittonen, M.; Hagelin, A.; Wiklund, C.; Gotthard, K. Local adaptation to seasonal cues at the fronts of two parallel, climate-induced butterfly range expansions. Ecol. Lett. 2022, 25, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Bu, W.; Gao, Y.; Liu, G. Potential geographic distribution of brown marmorated stink bug invasion (Halyomorpha halys). PLoS ONE 2012, 7, e31246. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.B.; Bergh, C.J.; Bergmann, E.J.; Biddinger, D.J.; Dieckhoff, C.; Dively, G.; Fraser, H.; Gariepy, T.; Hamilton, G.; Haye, T.; et al. Biology, ecology, and management of brown marmorated stink bug (Hemiptera: Pentatomidae). J. Integr. Pest Manag. 2014, 5, A1–A13. [Google Scholar] [CrossRef]
- Haye, T.; Gariepy, T.; Hoelmer, K.; Rossi, J.P.; Streito, J.C.; Tassus, X.; Desneux, N. Range expansion of the invasive brown marmorated stinkbug, Halyomorpha halys: An increasing threat to field, fruit and vegetable crops worldwide. J. Pest Sci. 2015, 88, 665–673. [Google Scholar] [CrossRef]
- Lee, D.H. Current status of research progress on the biology and management of Halyomorpha halys (Hemiptera: Pentatomidae) as an invasive species. Appl. Entomol. Zool. 2015, 50, 277–290. [Google Scholar] [CrossRef]
- Hamilton, G.C.; Ahn, J.J.; Bu, W.; Leskey, T.C.; Nielsen, A.L.; Park, Y.L.; Rabitsch, W.; Hoelmer, K.A. Halyomorpha halys (Stål). In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 243–292. [Google Scholar] [CrossRef]
- Musolin, D.L.; Konjević, A.; Karpun, N.N.; Protsenko, V.Y.; Ayba, L.Y.; Saulich, A.K. Invasive brown marmorated stink bug Halyomorpha halys (Stål) (Heteroptera: Pentatomidae) in Russia, Abkhazia, and Serbia: History of invasion, range expansion, early stages of establishment, and first records of damage to local crops. Arthropod-Plant Interact. 2018, 12, 517–529. [Google Scholar] [CrossRef]
- Musolin, D.L.; Dolgovskaya, M.Y.; Zakharchenko, V.Y.; Karpun, N.N.; Haye, T.; Saulich, A.K.; Reznik, S.Y. Flying over Eurasia: Geographic variation of photoperiodic control of nymphal development and adult diapause induction in native and invasive populations of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 522. [Google Scholar] [CrossRef]
- Watanabe, M. Ecology and extermination of Halyomorpha halys. 4. The relationship between day length and ovarian development. Ann. Rep. Toyama Inst. Health 1979, 3, 33–37. (In Japanese) [Google Scholar]
- Yanagi, T.; Hagihara, Y. Ecology of the brown marmorated stink bug. Plant Prot. 1980, 34, 315–321. (In Japanese) [Google Scholar]
- Nielsen, A.L.; Hamilton, G.C. Life history of the invasive species Halyomorpha halys (Hemiptera: Pentatomidae) in northeastern United States. Ann. Entomol. Soc. Am. 2009, 102, 608–616. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.L.; Chen, S.; Fleischer, S.J. Coupling developmental physiology, photoperiod, and temperature to model phenology and dynamics of an invasive heteropteran, Halyomorpha halys. Front. Physiol. 2016, 7, 165. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.L.; Fleischer, S.; Hamilton, G.C.; Hancock, T.; Krawczyk, G.; Lee, J.C.; Ogburn, E.; Pote, J.M.; Raudenbush, A.; Rucker, A.; et al. Phenology of brown marmorated stink bug described using female reproductive development. Ecol. Evol. 2017, 7, 6680–6690. [Google Scholar] [CrossRef] [Green Version]
- Musolin, D.L.; Dolgovskaya, M.Y.; Protsenko, V.Y.; Karpun, N.N.; Reznik, S.Y.; Saulich, A.K. Photoperiodic and temperature control of nymphal growth and adult diapause induction in the invasive Caucasian population of the brown marmorated stink bug, Halyomorpha halys. J. Pest Sci. 2019, 92, 621–631. [Google Scholar] [CrossRef]
- Saulich, A.K.; Musolin, D.L. Seasonal cycles of Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 565–607. [Google Scholar] [CrossRef]
- Reznik, S.Y.; Karpun, N.N.; Zakharchenko, V.Y.; Shoshina, Y.I.; Dolgovskaya, M.Y.; Saulich, A.K.; Musolin, D.L. To every thing there is a season: Phenology and photoperiodic control of seasonal development in the invasive Caucasian population of the brown marmorated stink bug, Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae). Insects 2022, 13, 580. [Google Scholar] [CrossRef]
- Mityushev, I.M. First record of Halyomorpha halys in Russia. Plant Prot. Quar. 2016, 3, 48. Available online: https://elibrary.ru/download/elibrary_25606587_22412850.pdf (accessed on 28 April 2023). (In Russian).
- Gapon, D.A. First records of the brown marmorated stink bug Halyomorpha halys (Stål, 1855) (Heteroptera, Pentatomidae) in Russia, Abkhazia, and Georgia. Entomol. Rev. 2016, 96, 1086–1088. [Google Scholar] [CrossRef]
- Gulia, V.O.; Orlovskaya, T.V.; Adzinba, Z.I.; Chitanava, S.M. Physical features of Abkhazia (Report 1). Int. J Appl. Fundam. Stud. 2014, 11, 35–38. (In Russian) [Google Scholar]
- Gulia, V.O.; Orlovskaya, T.V.; Adzinba, Z.I.; Chitanava, S.M. Physical features of Abkhazia (Report 2). Int. J Appl. Fundam. Stud. 2014, 11, 593–596. (In Russian) [Google Scholar]
- Belyuchenko, I.S. Ecology of Kuban. Part I.; KubGAU Publishing House: Krasnodar, Russia, 2005. (In Russian) [Google Scholar]
- Time and Date. Available online: https://www.timeanddate.com/sun/georgia/sukhumi (accessed on 7 July 2022).
- Weather and Climate. Available online: http://www.pogodaiklimat.ru/history/37002.htm (accessed on 20 March 2023). (In Russian).
- Zakharchenko, V.E. Bioecological Peculiarities and Control Measures for the Brown Marmorated Stink Bug (Halyomorpha halys Stål) in Wet Subtropical of Russia. Ph.D. Thesis, Russian State Agrarian University, Moscow, Russia, 2021. (In Russian). [Google Scholar]
- Watanabe, M. Study of the life cycle of the brown marmorated stink bug, Halyomorpha mista. Insectarium 1980, 17, 168–173. (In Japanese) [Google Scholar]
- Nakamura, K.; Numata, H. Seasonal life cycle of Aelia fieberi (Hemiptera: Pentatomidae) in relation to the phenology of its host plants. Ann. Entomol. Soc. Am. 1997, 90, 625–630. [Google Scholar] [CrossRef]
- Musolin, D.L.; Numata, H. Photoperiodic and temperature control of diapause induction and colour change in the southern green stink bug Nezara viridula. Physiol. Entomol. 2003, 28, 65–74. [Google Scholar] [CrossRef] [Green Version]
- SYSTAT 10.2. Available online: https://systat.informer.com/10.2/ (accessed on 20 March 2023).
- Niva, C.C.; Takeda, M. Effects of photoperiod, temperature and melatonin on nymphal development, polyphenism and reproduction in Halyomorpha halys (Heteroptera: Pentatomidae). Zool. Sci. 2003, 20, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haye, T.; Abdallah, S.; Gariepy, T.; Wyniger, D. Phenology, life table analysis and temperature requirements of the invasive brown marmorated stink bug, Halyomorpha halys, in Europe. J. Pest Sci. 2014, 87, 407–418. [Google Scholar] [CrossRef]
- Baek, S.; Hwang, A.; Kim, H.; Lee, H.; Lee, J.H. Temperature-dependent development and oviposition models of Halyomorpha halys (Hemiptera: Pentatomidae). J. Asia-Pac. Entomol. 2017, 20, 367–375. [Google Scholar] [CrossRef]
- Nielsen, A.L.; Hamilton, G.C.; Matadha, D. Developmental rate estimation and life table analysis for Halyomorpha halys (Hemiptera: Pentatomidae). Environ. Entomol. 2008, 37, 348–355. [Google Scholar] [CrossRef]
- Musolin, D.L.; Saulich, A.K. Diapause in Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 497–564. [Google Scholar] [CrossRef]
- Saunders, D.S. A diapause induction-termination asymmetry in the photoperiodic responses of the Linden bug, Pyrrhocoris apterus and an effect of near-critical photoperiods on development. J. Insect Physiol. 1983, 29, 399–405. [Google Scholar] [CrossRef]
- Numata, H.; Saulich, A.H.; Volkovich, T.A. Photoperiodic responses of the linden bug, Pyrrhocoris apterus, under conditions of constant temperature and under thermoperiodic conditions. Zool. Sci. 1993, 10, 521–527. Available online: https://ci.nii.ac.jp/naid/110003323090/ (accessed on 24 April 2023).
- Lopatina, E.B.; Balashov, S.V.; Kipyatkov, V.E. First demonstration of the influence of photoperiod on the thermal requirements for development in insects and in particular the linden-bug, Pyrrhocoris apterus (Heteroptera: Pyrrhocoridae). Eur. J. Entomol. 2007, 104, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Kutcherov, D.; Lopatina, E.B.; Balashov, S. Convergent photoperiodic plasticity in developmental rate in two species of insects with widely different thermal phenotypes. Eur. J. Entomol. 2018, 115, 624–631. [Google Scholar] [CrossRef] [Green Version]
- Lopatina, E.B.; Gusev, I.A. A novel form of phenotypic plasticity of the thermal reaction norms for development in the bug Graphosoma lineatum (L.) (Heteroptera, Pentatomidae). Entomol. Rev. 2019, 99, 417–436. [Google Scholar] [CrossRef]
- Kidokoro, T.; Masaki, S. Photoperiodic response in relation to variable voltinism in the ground cricket, Pteronemobius fascipes Walker (Orthoptera: Gryllidae). Jpn. J. Ecol. 1978, 28, 291–298. [Google Scholar]
- Gomi, T. Geographic variation in critical photoperiod for diapause induction and its temperature dependence in Hyphantria cunea Drury (Lepidoptera: Arctiidae). Oecologia 1997, 111, 160–165. [Google Scholar] [CrossRef]
- Saunders, D.S.; Steel, C.G.H.; Vafopoulou, X.; Lewis, R.D. Insect Clocks; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Goto, S.G.; Numata, H. Insect photoperiodism. In Insect Molecular Biology and Ecology; Hoffmann, K.H., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 217–244. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Musolin, D.L.; Konjević, A.; Karpun, N.N.; Zakharchenko, V.Y.; Zhuravleva, E.N.; Tavella, L.; Bruin, A.; Haye, T. Diversity and distribution of cytochrome oxidase I (COI) haplotypes of the brown marmorated stink bug, Halyomorpha halys Stål (Hemiptera, Pentatomidae), along the eastern front of its invasive range in Eurasia. NeoBiota 2021, 68, 53–77. [Google Scholar] [CrossRef]
- Gariepy, T.D.; Bruin, A.; Haye, T.; Milonas, P.; Vétek, G. Occurrence and genetic diversity of new populations of Halyomorpha halys in Europe. J. Pest Sci. 2015, 88, 451–460. [Google Scholar] [CrossRef]
- Morrison, W.R.; Milonas, P.; Kapantaidaki, D.E.; Cesari, M.; Di Bella, E.; Guidetti, R.; Haye, T.; Maistrello, L.; Moraglio, S.T.; Piemontese, L.; et al. Attraction of Halyomorpha halys (Hemiptera: Pentatomidae) haplotypes in North America and Europe to baited traps. Sci. Rep. 2017, 7, e16941. [Google Scholar] [CrossRef] [Green Version]
- Valentin, R.E.; Nielsen, A.L.; Wiman, N.G.; Lee, D.-H.; Fonseca, D.M. Global invasion network of the brown marmorated stink bug, Halyomorpha halys. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Maistrello, L.; Piemontese, L.; Bonini, R.; Dioli, P.; Lee, W.; Park, C.-G.; Partsinevelos, G.K.; Rebecchi, L.; Guidetti, R. Genetic diversity of the brown marmorated stink bug Halyomorpha halys in the invaded territories of Europe and its patterns of diffusion in Italy. Biol. Invasions 2018, 20, 1073–1092. [Google Scholar] [CrossRef]
- Schuler, H.; Elsler, D.; Fischnaller, S. Population genetics of the brown marmorated stink bug Halyomorpha halys in the early phase of invasion in South Tyrol (Northern Italy). Bull. Entomol. Res. 2020, 111, 394–401. [Google Scholar] [CrossRef]
- Yan, J.; Pal, C.; Anderson, D.; Vétek, G.; Farkas, P.; Burne, A.; Fan, Q.-H.; Zhang, J.; Gunawardana, D.N.; Balan, R.K. Genetic diversity analysis of brown marmorated stink bug, Halyomorpha halys based on mitochondrial COI and COII haplotypes. BMC Genomic Data 2021, 22, 7. [Google Scholar] [CrossRef]
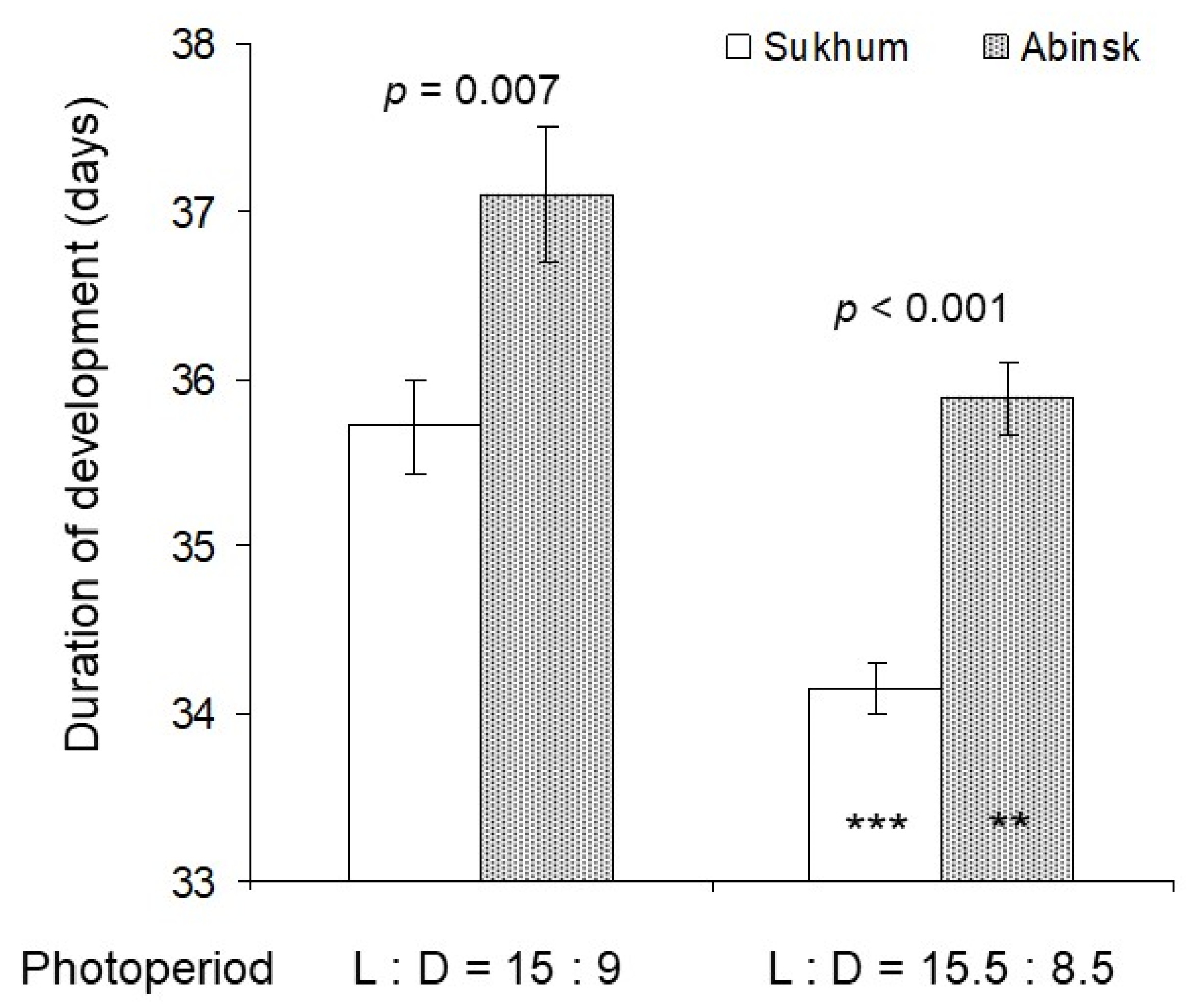
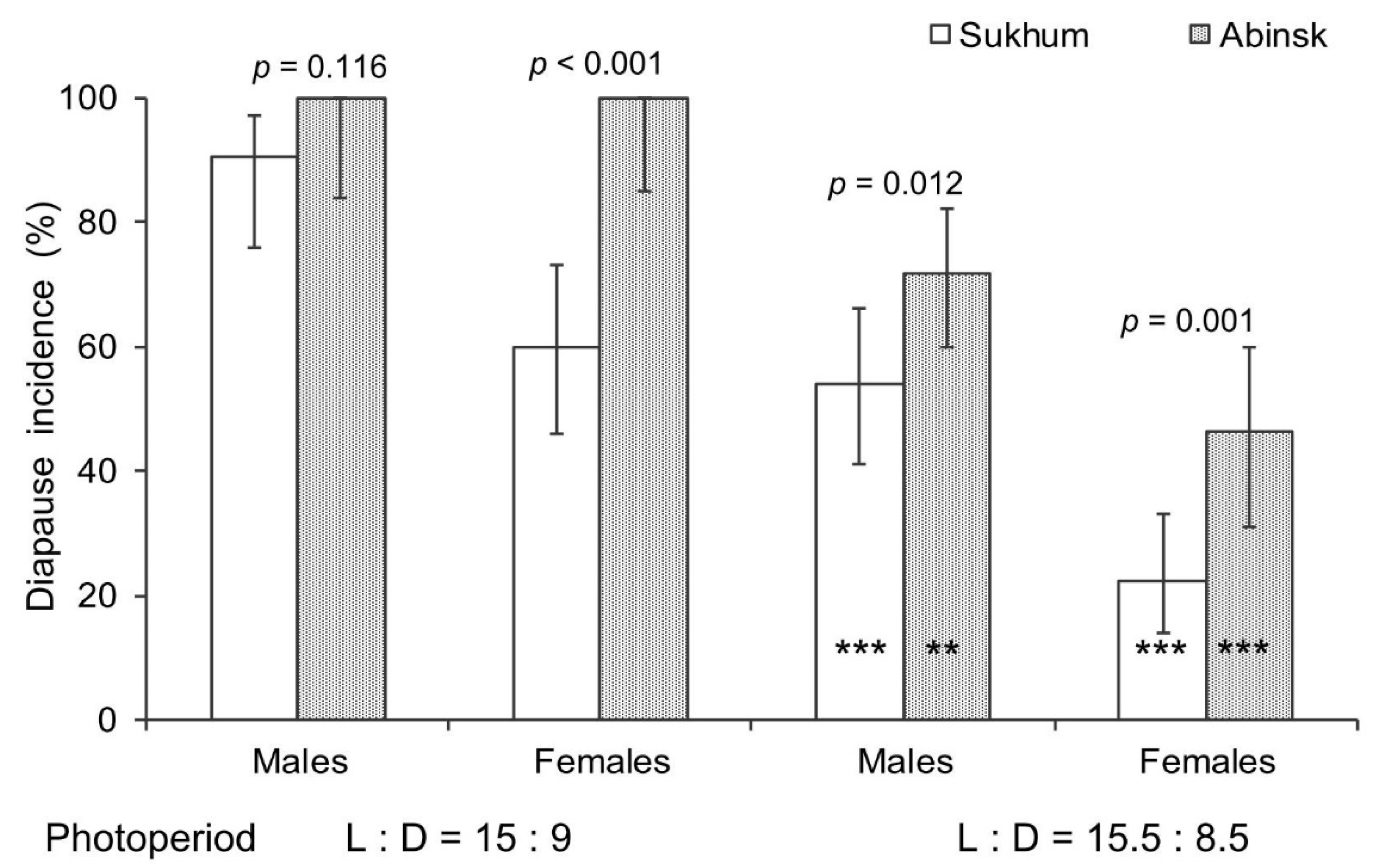
| Factor | Estimated Coefficients (C ± S.E.) and Their Effects (p) | |||
|---|---|---|---|---|
| Influence on the Proportion of Individuals with Fully Developed Reproductive Organs | Influence on the Proportion of Individuals with Fully Developed Fat Body | |||
| Males | Females | Males | Females | |
| Photoperiod | 2.64 ± 0.51, p < 0.001 | 2.47 ± 0.37, p < 0.001 | −1.04 ± 0.38, p = 0.008 | −2.62 ± 0.36, p < 0.001 |
| Population | −0.53 ± 0.18, p = 0.004 | −0.89 ± 0.19, p < 0.001 | 0.03 ± 0.17, p = 0.853 | 0.70 ± 0.18, p < 0.001 |
| Fat body | Reproductive Organs | |||
|---|---|---|---|---|
| Males | Females | |||
| Poorly Developed | Fully Developed | Poorly Developed | Fully Developed | |
| Sukhum population, L:D = 15:9 | ||||
| Poorly developed (%) | 3.8 | 7.7 | 2.9 | 31.4 |
| Fully developed (%) | 86.6 | 1.9 | 57.1 | 8.6 |
| Correlation between development of fat body and reproductive organs | ρ = −0.699 ± 0.163, χ2 = 25.4, p < 0.001, n = 52 | ρ = −0.762 ± 0.078, χ2 = 40.6, p < 0.001, n = 70 | ||
| Sukhum population, L:D = 15.5:8.5 | ||||
| Poorly developed (%) | 12.6 | 27.6 | 15.3 | 67.3 |
| Fully developed (%) | 41.4 | 18.4 | 7.2 | 10.2 |
| Correlation between development of fat body and reproductive organs | ρ = −0.372 ± 0.100, χ2 = 12.0, p = 0.001, n = 87 | ρ = −0.206 ± 0.115, χ2 = 4.1, p = 0.042, n = 98 | ||
| Abinsk population, L:D = 15:9 | ||||
| Poorly developed (%) | 33.3 | 0.0 | 15.4 | 0.0 |
| Fully developed (%) | 66.7 | 0.0 | 84.6 | 0.0 |
| Correlation between development of fat body and reproductive organs | – a n = 24 | – a n = 26 | ||
| Abinsk population, L:D = 15.5:8.5 | ||||
| Poorly developed (%) | 12.5 | 17.7 | 20.9 | 37.3 |
| Fully developed (%) | 59.4 | 10.4 | 25.4 | 16.4 |
| Correlation between development of fat body and reproductive organs | ρ = −0.446 ± 0.100, χ2 = 19.1, p < 0.001, n = 96 | ρ = −0.245 ± 0.119, χ2 = 4.0, p = 0.044, n = 67 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reznik, S.Y.; Dolgovskaya, M.Y.; Karpun, N.N.; Zakharchenko, V.Y.; Saulich, A.K.; Musolin, D.L. The Invasive Caucasian Populations of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Rapidly Adapt Their Ecophysiological Traits to the Local Environmental Conditions. Insects 2023, 14, 424. https://doi.org/10.3390/insects14050424
Reznik SY, Dolgovskaya MY, Karpun NN, Zakharchenko VY, Saulich AK, Musolin DL. The Invasive Caucasian Populations of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Rapidly Adapt Their Ecophysiological Traits to the Local Environmental Conditions. Insects. 2023; 14(5):424. https://doi.org/10.3390/insects14050424
Chicago/Turabian StyleReznik, Sergey Ya., Margarita Yu. Dolgovskaya, Natalia N. Karpun, Vilena Ye. Zakharchenko, Aida Kh. Saulich, and Dmitrii L. Musolin. 2023. "The Invasive Caucasian Populations of the Brown Marmorated Stink Bug Halyomorpha halys (Hemiptera: Heteroptera: Pentatomidae) Rapidly Adapt Their Ecophysiological Traits to the Local Environmental Conditions" Insects 14, no. 5: 424. https://doi.org/10.3390/insects14050424






