Honeydew Is a Food Source and a Contact Kairomone for Aphelinus mali
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Material
2.2. Longevity
2.3. Infectivity of A. mali under Different Feeding Treatments
2.4. Choice Experiment
2.5. Inferring Feeding History of A. mali
2.5.1. Reference Profiles
2.5.2. Profiles of Field-Collected Parasitoids
2.5.3. Sugar Extraction for HPLC Analysis
2.6. Statistical Analysis
3. Results
3.1. Longevity
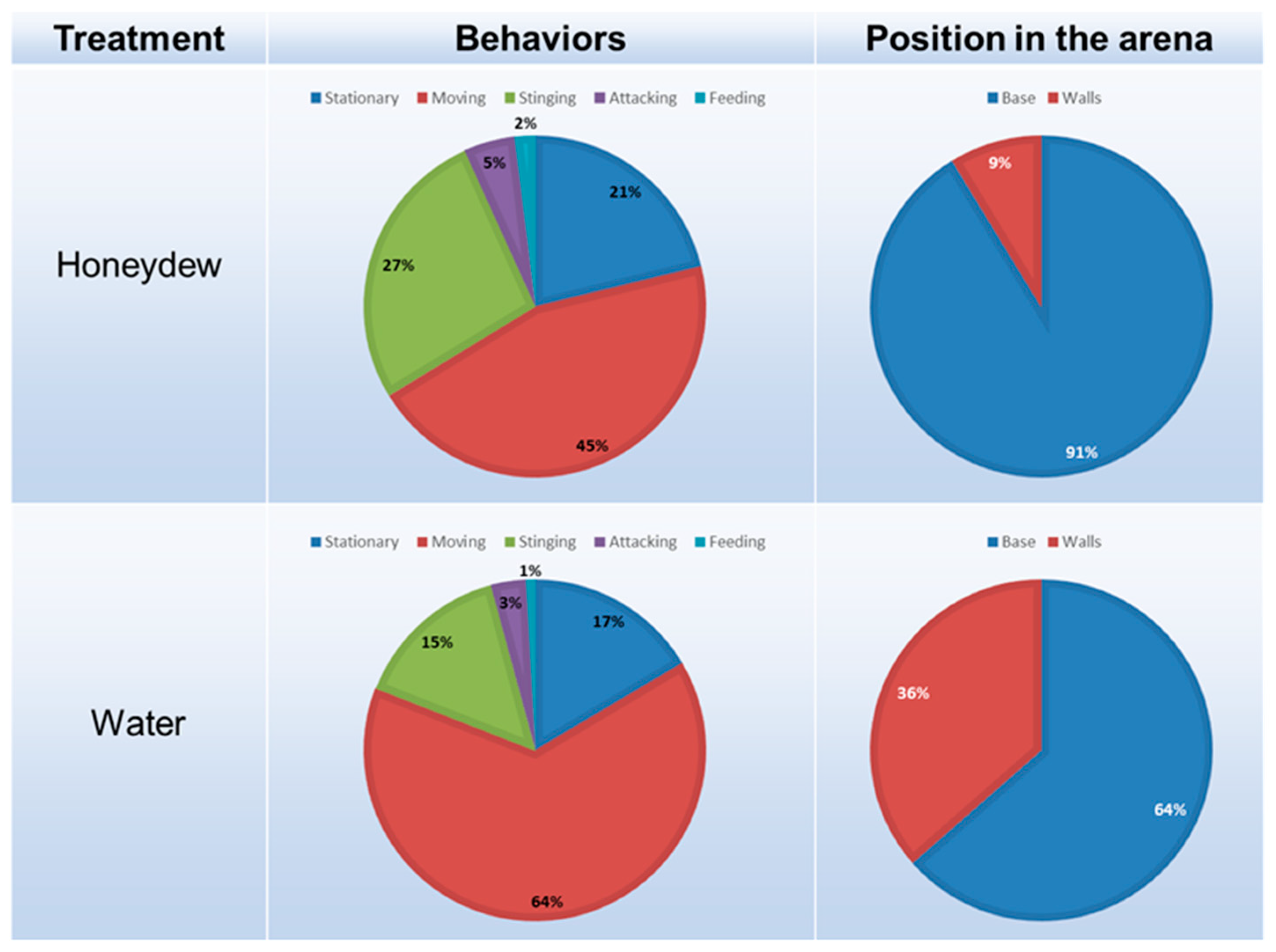
3.2. Infectivity of A. mali under Different Feeding Treatments
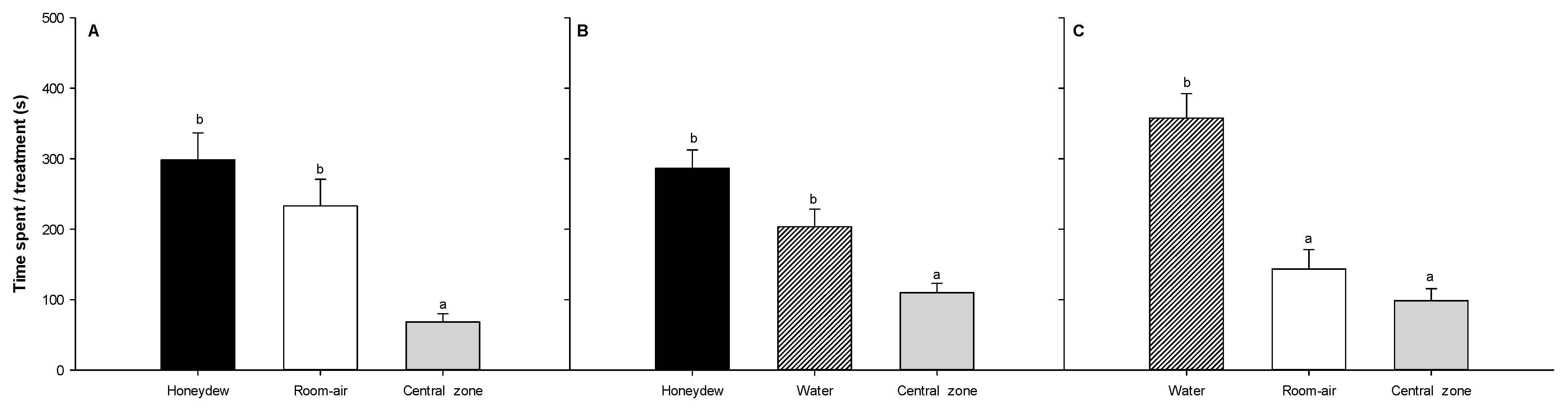
3.3. Choice Experiment
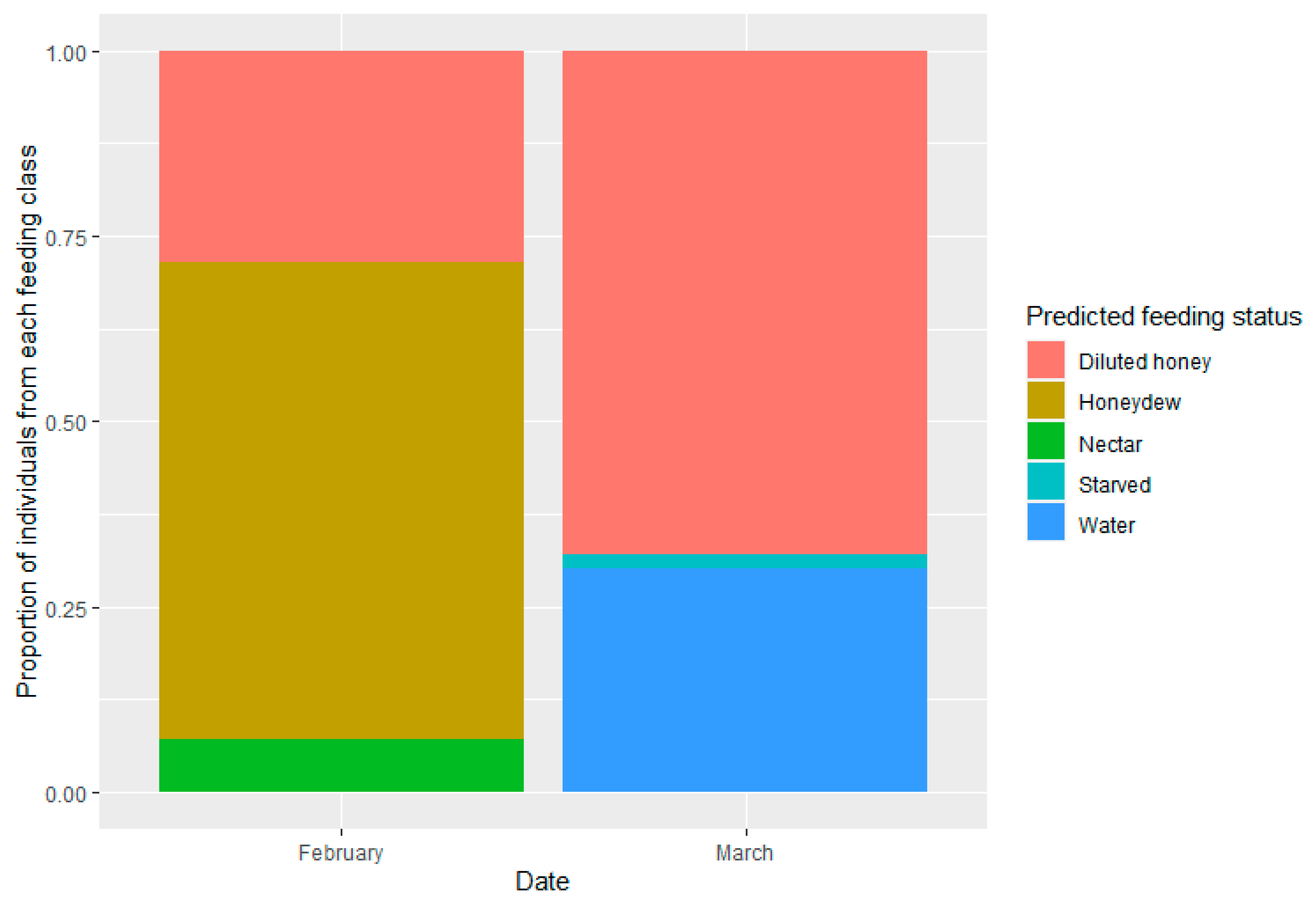
3.4. Inferring Feeding History of A. mali
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wäckers, F.; van Rijn, P.; Bruin, J. Plant-Provided Food for Carnivorous Insects: Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Hogervorst, P.A.M.; Wäckers, F.L.; Romeis, J. Detecting nutritional state and food source use in field-collected insects that synthesize honeydew oligosaccharides. Funct. Ecol. 2007, 21, 936–946. [Google Scholar] [CrossRef]
- Coll, M.; Guershon, M. Omnivory in Terrestrial Arthropods: Mixing Plant and Prey Diests. Ann. Rev. Entomol. 2002, 47, 267–297. [Google Scholar] [CrossRef]
- Wäckers, F.L.; van Rijn, P.C.; Heimpel, G.E. Honeydew as a food source for natural enemies: Making the best of a bad meal? Biol. Control 2008, 45, 176–184. [Google Scholar] [CrossRef]
- Tena, A.; Pekas, A.; Wäckers, F.L.; Urbaneja, A. Energy reserves of parasitoids depend on honeydew from non-hosts. Ecol. Èntomol. 2013, 38, 278–289. [Google Scholar] [CrossRef]
- Luquet, M.; Peñalver-Cruz, A.; Satour, P.; Anton, S.; Cortesero, A.-M.; Lavandero, B.; Jaloux, B. Aphid honeydew may be the predominant sugar source for Aphidius parasitoids even in nectar-providing intercrops. Biol. Control 2021, 158, 104596. [Google Scholar] [CrossRef]
- Tena, A.; Llácer, E.; Urbaneja, A. Biological control of a non-honeydew producer mediated by a distinct hierarchy of honeydew quality. Biol. Control 2013, 67, 117–122. [Google Scholar] [CrossRef]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Èntomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Faria, C.A.; Wäckers, F.L.; Turlings, T.C. The nutritional value of aphid honeydew for non-aphid parasitoids. Basic Appl. Ecol. 2008, 9, 286–297. [Google Scholar] [CrossRef]
- Rand, T.A.; Waters, D.K. Aphid Honeydew Enhances Parasitoid Longevity to the Same Extent as a High-Quality Floral Resource: Implications for Conservation Biological Control of the Wheat Stem Sawfly (Hymenoptera: Cephidae). J. Econ. Èntomol. 2020, 113, 2022–2025. [Google Scholar] [CrossRef]
- Heimpel, G.E.; Jervis, M.A. Does Floral Nectar Improve Biological Control by Parasitoids? In Plant-Provided Food for Carnivorous Insects: A Protective Mutualism and Its Applications; Cambridge University Press: Cambridge, UK, 2005; pp. 267–304. ISBN 9780511542220. [Google Scholar]
- De Moraes, C.M.; Lewis, W.J.; Paré, P.W.; Alborn, H.T.; Tumlinson, J.H. Herbivore-infested plants selectively attract parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Soler, R.; Harvey, J.A.; Kamp, A.F.D.; Vet, L.E.M.; Van der Putten, W.H.; Van Dam, N.M.; Stuefer, J.F.; Gols, R.; Hordijk, C.A.; Bezemer, T.M. Root herbivores influence the behaviour of an aboveground parasitoid through changes in plant-volatile signals. Oikos 2007, 116, 367–376. [Google Scholar] [CrossRef]
- Erb, M.; Foresti, N.; Turlings, T.C. A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. BMC Plant Biol. 2010, 10, 247. [Google Scholar] [CrossRef]
- Leroy, P.D.; Sabri, A.; Heuskin, S.; Thonart, P.; Lognay, G.; Verheggen, F.J.; Francis, F.; Brostaux, Y.; Felton, G.W.; Haubruge, E. Microorganisms from aphid honeydew attract and enhance the efficacy of natural enemies. Nat. Commun. 2011, 2, 348. [Google Scholar] [CrossRef]
- Leroy, P.D.; Almohamad, R.; Attia, S.; Capella, Q.; Verheggen, F.J.; Haubruge, E.; Francis, F. Aphid honeydew: An arrestant and a contact kairomone for Episyrphus balteatus (Diptera: Syrphidae) larvae and adults. Eur. J. Èntomol. 2014, 111, 237–242. [Google Scholar] [CrossRef]
- Tena, A.; Wäckers, F.L.; Heimpel, G.E.; Urbaneja, A.; Pekas, A. Parasitoid nutritional ecology in a community context: The importance of honeydew and implications for biological control. Curr. Opin. Insect Sci. 2016, 14, 100–104. [Google Scholar] [CrossRef]
- Kaiser, L.; Ode, P.; van Nouhuys, S.; Calatayud, P.-A.; Colazza, S.; Cortesero, A.-M.; Thiel, A.; van Baaren, J. The Plant as a Habitat for Entomophagous Insects. Adv. Bot. Res. 2017, 81, 179–223. [Google Scholar] [CrossRef]
- Fischer, C.Y.; Detrain, C.; Thonart, P.; Haubruge, E.; Francis, F.; Verheggen, F.J.; Lognay, G.C. Bacteria may contribute to distant species recognition in ant-aphid mutualistic relationships. Insect Sci. 2016, 24, 278–284. [Google Scholar] [CrossRef]
- Bleeker, M.A.; Smid, H.M.; Van Aelst, A.C.; Van Loon, J.J.; Vet, L.E. Antennal sensilla of two parasitoid wasps: A comparative scanning electron microscopy study. Microsc. Res. Tech. 2004, 63, 266–273. [Google Scholar] [CrossRef]
- Li, C.; Roitberg, B.; Mackauer, M. Effects of contact kairomone and experience on initial giving-up time. Èntomol. Exp. Appl. 1997, 84, 101–104. [Google Scholar] [CrossRef]
- Iacovone, A.; French, A.S.; Tellier, F.; Cusumano, A.; Clément, G.; Gaertner, C.; Conti, E.; Salerno, G.; Marion-Poll, F. The role of contact chemoreception in the host location process of an egg parasitoid. J. Insect Physiol. 2016, 91–92, 63–75. [Google Scholar] [CrossRef]
- Howard, L. Aphelinus Mali and Its Travels. Ann. Entomol. Soc. Am. 1929, 22, 341–368. [Google Scholar] [CrossRef]
- Asante, S.K.; Danthanarayana, W. Sex ratios in natural populations ofAphelinus mali (Hym.: Aphelinidae) in relation to host size and host density. Biocontrol 1993, 38, 391–403. [Google Scholar] [CrossRef]
- Lundie, A.E. A Biological Study of Aphelinus Mali Hald., a Parasite of the Woolly Apple Aphid, Eriosoma Lanigera Hausm; Memoir Cornell University Agricultural Experiment Station: Ithaca, NY, USA, 1924; Volume 79, pp. 1–27. [Google Scholar]
- Evenhuis, H.H. Een oecologisch onderzoek over de appelbloedluis, Eriosoma lanigerum (Hausm.), en haar parasietAphelinus mali (Hald.) in Nederland. Eur. J. Plant Pathol. 1958, 64, 1–103. [Google Scholar] [CrossRef]
- Jervis, M.A.; Kidd, N.A.C. Host-feeding strategies in hymenopteran parasitoids. Biol. Rev. 1986, 61, 395–434. [Google Scholar] [CrossRef]
- Ortiz-Martínez, S.A.; Ramírez, C.C.; Lavandero, B. Host acceptance behavior of the parasitoid Aphelinus mali and its aphid-host Eriosoma lanigerum on two Rosaceae plant species. J. Pest Sci. 2013, 86, 659–667. [Google Scholar] [CrossRef]
- Pettersson, J. An Aphid Sex Attractant. Insect Syst. Evol. 1970, 1, 63–73. [Google Scholar] [CrossRef]
- Vet, L.E.M.; VAN Lenteren, J.C.; Heymans, M.; Meelis, E. An airflow olfactometer for measuring olfactory responses of hymenopterous parasitoids and other small insects. Physiol. Èntomol. 1983, 8, 97–106. [Google Scholar] [CrossRef]
- Luquet, M.; Tritto, O.; Cortesero, A.-M.; Jaloux, B.; Anton, S. Early Olfactory Environment Influences Antennal Sensitivity and Choice of the Host-Plant Complex in a Parasitoid Wasp. Insects 2019, 10, 127. [Google Scholar] [CrossRef] [PubMed]
- Luquet, M.; Parisey, N.; Hervé, M.; Desouhant, E.; Cortesero, A.; Peñalver-Cruz, A.; Lavandero, B.; Anton, S.; Jaloux, B. Inferring insect feeding patterns from sugar profiles: A comparison of statistical methods. Ecol. Èntomol. 2020, 46, 19–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Tena, A.; Senft, M.; Desneux, N.; Dregni, J.; Heimpel, G.E. The influence of aphid-produced honeydew on parasitoid fitness and nutritional state: A comparative study. Basic Appl. Ecol. 2018, 29, 55–68. [Google Scholar] [CrossRef]
- Milosavljević, I.; Morgan, D.J.; Massie, R.E.; Hoddle, M.S. Density dependent mortality, climate, and Argentine ants affect population dynamics of an invasive citrus pest, Diaphorina citri, and its specialist parasitoid, Tamarixia radiata, in Southern California, USA. Biol. Control 2021, 159, 104627. [Google Scholar] [CrossRef]
- Wyckhuys, K.A.; Strange-George, J.E.; Kulhanek, C.A.; Wäckers, F.L.; Heimpel, G.E. Sugar feeding by the aphid parasitoid Binodoxys communis: How does honeydew compare with other sugar sources? J. Insect Physiol. 2008, 54, 481–491. [Google Scholar] [CrossRef]
- Völkl, W. Aphids or Their Parasitoids: Who Actually Benefits from Ant-Attendance? J. Anim. Ecol. 1992, 61, 273–281. [Google Scholar] [CrossRef]
- Wahab, W. Observations on the biology and behaviour of Aphelinus abdominalis Dalm. (Hym., Aphelinidae), a parasite of aphids1. Z. Für Angew. Entomol. 1985, 100, 290–296. [Google Scholar] [CrossRef]
- Miñarro, M.; Fernández-Mata, G.; Medina, P. Role of ants in structuring the aphid community on apple. Ecol. Èntomol. 2010, 35, 206–215. [Google Scholar] [CrossRef]
- Lester, P.J.; Baring, C.W.; Longson, C.G.; Hartley, S. Argentine and other ants (Hymenoptera: Formicidae) in New Zealand horticultural ecosystems: Distribution, hemipteran hosts, and review. New Zealand Èntomol. 2003, 26, 79–89. [Google Scholar] [CrossRef]
- Wäckers, F.L. Do Oligosaccharides Reduce the Suitability of Honeydew for Predators and Parasitoids? A Further Facet to the Function of Insect-Synthesized Honeydew Sugars. Oikos 2000, 90, 197–201. [Google Scholar] [CrossRef]
- Winkler, K.; Wäckers, F.L.; Kaufman, L.V.; Larraz, V.; van Lenteren, J. Nectar exploitation by herbivores and their parasitoids is a function of flower species and relative humidity. Biol. Control 2009, 50, 299–306. [Google Scholar] [CrossRef]
- Heimpel, G.E. Linking parasitoid nectar feeding and dispersal in conservation biological control. Biol. Control 2019, 132, 36–41. [Google Scholar] [CrossRef]
- Budenberg, W.J. Honeydew as a contact kairomone for aphid parasitoids. Èntomol. Exp. Appl. 1990, 55, 139–148. [Google Scholar] [CrossRef]
- Romeis, J.; Zebitz, C. Searching behaviour of Encarsia formosa as mediated by colour and honeydew. Èntomol. Exp. Appl. 1997, 82, 299–309. [Google Scholar] [CrossRef]
- Tang, Y.-Z.; Kuang, R.-P.; Zhao, W.-Y. A Simulation of Interspecific Relationship between Woolly Apple Aphid, Eriosoma Lanigerum (Haus.) and Its Parasite, Aphelinus Mali (Hald.) II. A Simulation and Sensitive Analysis of Natural Systems of Host-Parasite. Acta Ecol. Sin. 1991, 11, 130Á. [Google Scholar]
- Bourdais, D.; Vernon, P.; Krespi, L.; Le Lannic, J.; Van Baaren, J. Antennal structure of male and femaleAphidius rhopalosiphi DeStefani-Peres (Hymenoptera:Braconidae): Description and morphological alterations after cold storage or heat exposure. Microsc. Res. Tech. 2006, 69, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.-S.; Lee, C.-Y. Water balance profiles, humidity preference and survival of two sympatric cockroach egg parasitoids Evania appendigaster and Aprostocetus hagenowii (Hymenoptera: Evaniidae; Eulophidae). J. Insect Physiol. 2015, 77, 45–54. [Google Scholar] [CrossRef]
- Segoli, M.; Rosenheim, J.A. Spatial and temporal variation in sugar availability for insect parasitoids in agricultural fields and consequences for reproductive success. Biol. Control 2013, 67, 163–169. [Google Scholar] [CrossRef]
- Kishinevsky, M.; Keasar, T. Sugar feeding by parasitoids inside and around vineyards varies with season and weed management practice. Agric. Ecosyst. Environ. 2020, 307, 107229. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Alvarez, D.; Lavandero, B. Do hedgerows influence the natural biological control of woolly apple aphids in orchards? J. Pest Sci. 2019, 93, 219–234. [Google Scholar] [CrossRef]
- Fischer, M.K.; Völkl, W.; Schopf, R.; Hoffmann, K.H. Age-Specific Patterns in Honeydew Production and Honeydew Composition in the Aphid Metopeurum Fuscoviride: Implications for Ant-Attendance. J. Insect Physiol. 2002, 48, 319–326. [Google Scholar] [CrossRef]
- James, A.; Dungan, R.; Plank, M.; Ito, R. A dynamical model of honeydew droplet production by sooty-beech scale insects (Ultracoelostoma spp.) in New Zealand Nothofagus forest. Ecol. Model. 2007, 209, 323–332. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peñalver-Cruz, A.; Satour, P.; Jaloux, B.; Lavandero, B. Honeydew Is a Food Source and a Contact Kairomone for Aphelinus mali. Insects 2023, 14, 426. https://doi.org/10.3390/insects14050426
Peñalver-Cruz A, Satour P, Jaloux B, Lavandero B. Honeydew Is a Food Source and a Contact Kairomone for Aphelinus mali. Insects. 2023; 14(5):426. https://doi.org/10.3390/insects14050426
Chicago/Turabian StylePeñalver-Cruz, Ainara, Pascale Satour, Bruno Jaloux, and Blas Lavandero. 2023. "Honeydew Is a Food Source and a Contact Kairomone for Aphelinus mali" Insects 14, no. 5: 426. https://doi.org/10.3390/insects14050426




