Giant Sex Chromosomes in Omophoita Species (Coleoptera, Chrysomelidae): Structural and Evolutionary Relationships Revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chromosomal Preparations
2.2. Comparative Genomic Hybridization (CGH)
2.3. Whole Chromosome Painting (WCP)
2.4. Image Analysis and Processing
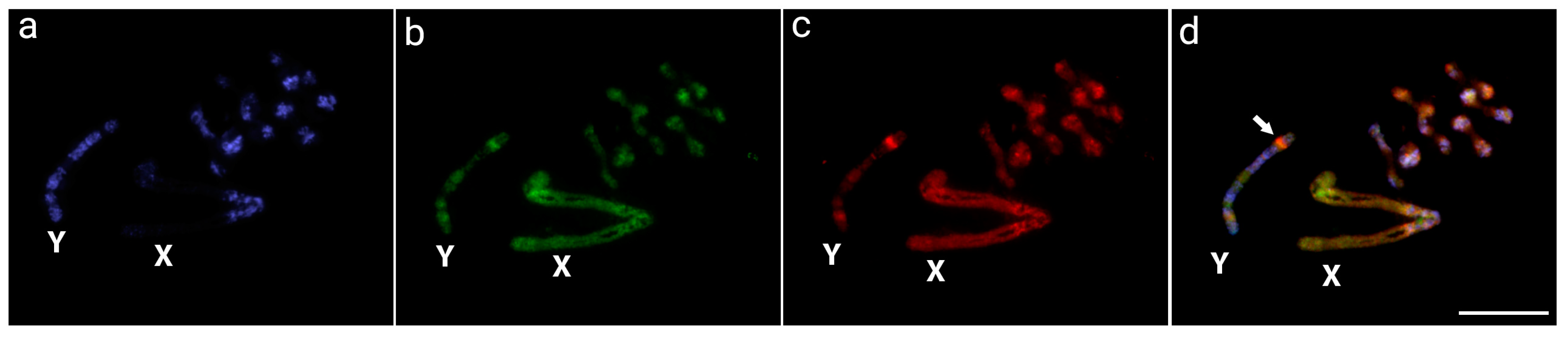
3. Results
3.1. Detection of the Male-Specific Region by CGH
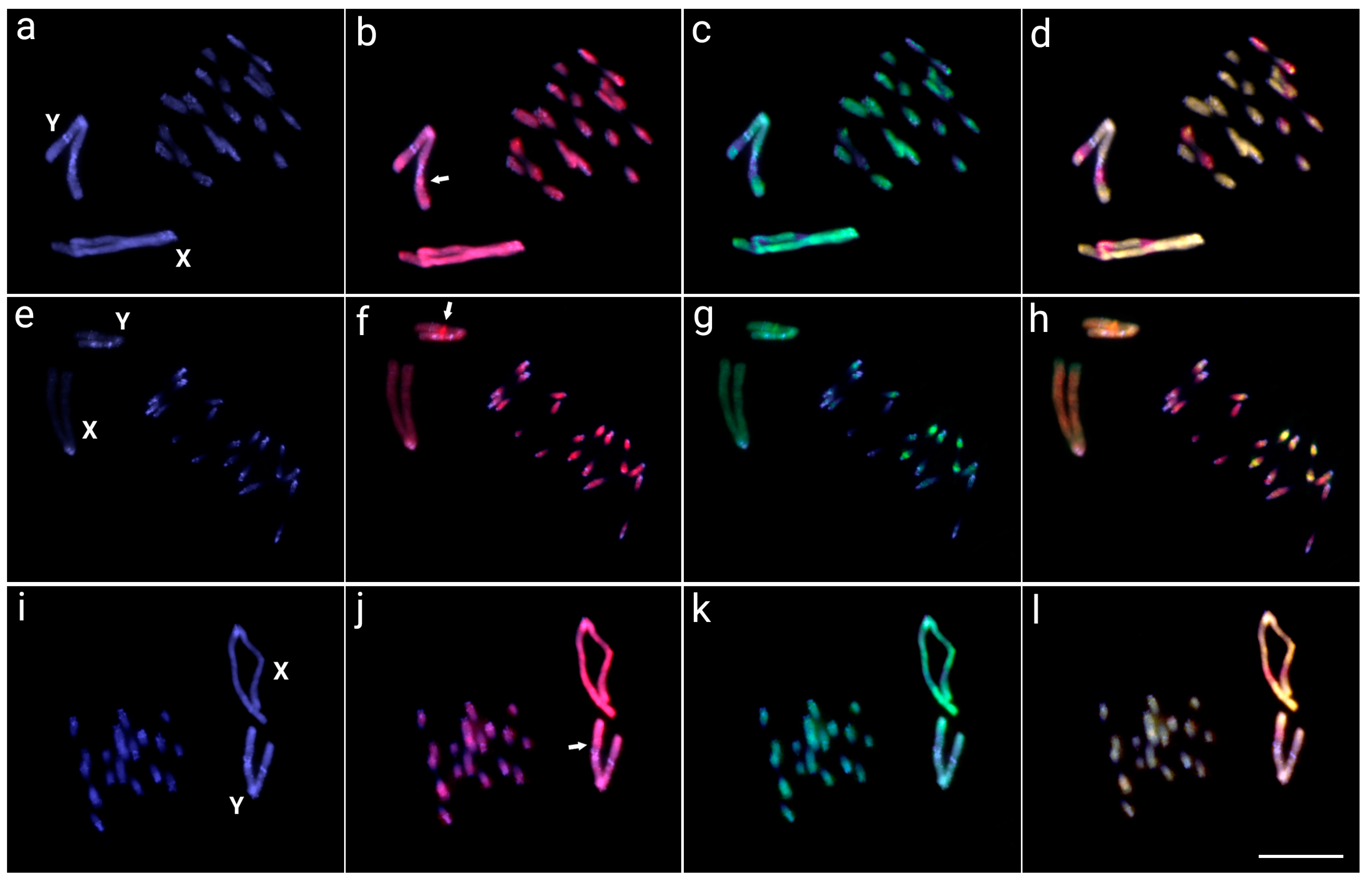
3.2. Interspecific Comparative Genomic Hybridization (CGH)
3.3. Whole Chromosome Painting (WCP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouchard, P.; Smith, A.B.T.; Douglas, H.; Gimmel, M.L.; Brunke, A.J.; Kanda, K. Biodiversity of coleoptera. In Insect Biodiversity: Science and Society, 2nd ed.; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 337–417. [Google Scholar] [CrossRef]
- Mckenna, D.D.; Wild, A.L.; Kanda, K.; Bellamy, C.L.; Beutel, R.G.; Caterino, M.S.; Farnum, C.W.; Hawks, D.C.; Ivie, M.A.; Jameson, M.L.; et al. The beetle tree of life reveals that Coleoptera survived end-P ermian mass extinction to diversify during the C retaceous terrestrial revolution. Syst. Entomol. 2015, 40, 835–880. [Google Scholar] [CrossRef]
- Coleoptera Karyotype Database. The Coleopterists Bulletin. Available online: https://evobir.shinyapps.io/ColeopteraDB (accessed on 5 February 2023).
- Ferreira, A.; Cella, D.; Tardivo, J.R.; Virkki, N. 2 pairs of chromossomes—A new low record for coleoptera. Rev. Bras. Genet. 1984, 7, 231–239. [Google Scholar]
- Lorite, P.; Carrillo, J.A.; Garnería, I.; Petitpierre, E.; Palomeque, T. Satellite DNA in the elm leaf beetle, Xanthogaleruca luteola (Coleoptera, Chrysomelidae): Characterization, interpopulation analysis, and chromosome location. Cytogenet. Genome Res. 2002, 98, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G. Evolutionary changes in the sex chromosomes of Coleoptera. Genetica 1951, 25, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Virkki, N. Sex chromosomes and karyotypes of the Alticidae (Coleoptera). Hereditas 1970, 64, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.G. Evolutionary changes in the sex chromosomes of Coleoptera. I. Wood borers of the genus Agrilus. Evolution 1949, 3, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Dutrillaux, A.-M.; Dutrillaux, B. Evolution of the sex chromosomes in beetles. I. The loss of the Y chromosome. Cytogenet. Genome Res. 2017, 152, 97–104. [Google Scholar] [CrossRef]
- Dutrillaux, A.-M.; Dutrillaux, B. Sex chromosome rearrangements in Polyphaga beetles. Sex. Dev. 2009, 3, 43–54. [Google Scholar] [CrossRef]
- Virkki, N. Sex chromosomes of Disonychina (Coleoptera, Alticinae): Xy + nX systems. Cytobios 1988, 53, 43–55. [Google Scholar]
- Virkki, N. Regular segregation of seven asynaptic sex chromosomes in the male of Asphaera daniela Bechyné (Coleoptera, Alticidae). Caryologia 1968, 21, 47–51. [Google Scholar] [CrossRef]
- Almeida, M.C.; Campaner, C.; Cella, D.M. Cytogenetics of four Omophoita species (Coleoptera, Chrysomelidae, Alticinae): A comparative analysis using mitotic and meiotic cells submitted to the standard staining and C-banding technique. Micron 2009, 40, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.C.; Goll, L.G.; Artoni, R.F.; Nogaroto, V.; Matiello, R.R.; Vicari, M.R. Physical mapping of 18S rDNA cistron in species of the Omophoita genus (Coleoptera, Alticinae) using fluorescent in situ hybridization. Micron 2010, 41, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, M.; Rosolen, L.A.M.; Artoni, R.F.; Santos, M.H.; Almeida, M.C. Cytogenetic and molecular characterization of three mimetic species of the genus Alagoasa Bechyné 1955 (Coleoptera: Alticinae) from the Neotropical Region. Cytogenet. Genome Res. 2020, 160, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Goll, L.G.; Artoni, R.F.; Gross, M.C.; Mello, L.R.A.; Coelho, M.P.B.; Almeida, M.C.; Schneider, C.H. Comparative cytogenetics of Omophoita abbreviata and O. aequinoctialis (Coleoptera, Chrysomelidae, Alticini) from the Adolpho Ducke Forest Reserve in Brazilian Amazonia: Intrapopulation variation in karyotypes. Cytogenet. Genome Res. 2018, 156, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.R.A.; Tasior, D.; Goll, L.G.; Artoni, R.F.; Vicari, M.R.; Nogaroto, V.; Almeida, M.C. Physical map of repetitive DNA and karyotype evolution in three species of the genus Omophoita (Coleoptera: Alticinae). Ital. J. Zool. 2014, 81, 16–24. [Google Scholar] [CrossRef]
- Rosolen, L.A.M.; Vicari, M.R.; Almeida, M.C. Accumulation of transposable elements in autosomes and giant sex chromosomes of Omophoita (Chrysomelidae: Alticinae). Cytogenet. Genome Res. 2018, 156, 215–222. [Google Scholar] [CrossRef]
- Virkki, N. Banding of Oedionychina (Coleoptera, Alticinae) chromosomes. C-and Ag-bands. J. Agric. Univ. Puerto Rico 1983, 67, 221–225. [Google Scholar]
- Virkki, N. Prophase of spermatocyte I in Oedionychina (Coleoptera). J. Agric. Univ. Puerto Rico 1976, 60, 661–674. [Google Scholar]
- Virkki, N. Formation and maintenance of the distance sex bivalent in Oedionychina (Coleoptera, Alticidae). Hereditas 1971, 68, 305–312. [Google Scholar] [CrossRef]
- Virkki, N. Orientation and segregation of asynaptic multiple sex chromosomes in the male Omophoita clerica Erichson (Coleoptera: Alticidae). Hereditas 1967, 57, 275–288. [Google Scholar] [CrossRef]
- Virkki, N. High chromosome number and giant postreductional sex chromosomes in the beetle Walterianella venusta Schaufuss (Chrysomelidae, Alticinae). J. Agric. Univ. Puerto Rico 1963, 47, 154–163. [Google Scholar] [CrossRef]
- Virkki, N.; Santiago-Blay, J.A.; Clark, S.M. Chromosomes of some Puerto Rican Disonychina and Oedionychina (Coleoptera: Chrysomelidae: Alticinae: Oedionychini): Evolutionary implications. Psyche 1991, 98, 373–390. [Google Scholar] [CrossRef]
- Virkki, N.; Santiago-Blay, J.A. Atypical cytology in some neotropical flea beetles (Coleoptera: Chrysomelidae: Alticinae: Oedionychina) from one of the most intense natural radiation sites known, Morro do Ferro (Brazil). Cytobios 1996, 85, 167–184. [Google Scholar]
- Virkki, N.; Santiago-Blay, J.A. Trends of karyotype evolution in neotropical Oedionychina (Coleoptera: Chrysomelidae: Alticinae). Hereditas 1993, 119, 263–283. [Google Scholar] [CrossRef]
- Wolski, M.A.V.; Artoni, R.F.; Santos, M.H.; Almeida, M.C. Cytogenetic, morphological and molecular characterization of two cryptic species of the genus Omophoita Chevrolat, 1837 (Coleoptera: Chrysomelidae: Galerucinae). Biologia 2021, 76, 2253–2262. [Google Scholar] [CrossRef]
- Haddad, S.; Mckenna, D.D. Phylogeny and evolution of the superfamily Chrysomeloidea (Coleoptera: Cucujiformia). Syst. Entomol. 2016, 41, 697–716. [Google Scholar] [CrossRef]
- Nie, R.-E.; Wei, J.; Zhang, S.-K.; Volger, A.P.; Wu, L.; Konstantinov, A.S.; Li, W.-Z.; Yang, X.-K.; Xue, H.-J. Diversification of mitogenomes in three sympatric Altica flea beetles (Insecta, Chrysomelidae). Zool. Scr. 2019, 48, 657–666. [Google Scholar] [CrossRef]
- Nie, R.-E.; Brreschoten, T.; Timmermans, M.J.T.N.; Nadein, K.; Xue, H.-J.; Bai, M.; Huang, Y.; Yang, X.-K.; Vogler, A.P. The phylogeny of Galerucinae (Coleoptera: Chrysomelidae) and the performance of mitochondrial genomes in phylogenetic inference compared to nuclear rRNA genes. Cladistics 2018, 34, 113–130. [Google Scholar] [CrossRef]
- Bechyné, J.; Bechyné, B.S. Evidenz der bisher bekannten. Entomol. Ts. Arg. 1966, 87, 142–170. [Google Scholar]
- Ge, D.; Gómez-Zurita, J.; Chesters, D.; Yang, X.; Vogler, A.P. Suprageneric systematics of flea beetles (Chrysomelidae: Alticinae) inferred from multilocus sequence data. Mol. Phylogenet. Evol. 2012, 62, 793–805. [Google Scholar] [CrossRef]
- Smith, S.G.; Virkki, N. Insecta 5. Coleoptera. In Animal cytogenetics; John, B., Ed.; Gebrüder Brontraeger: Stuttgart/Berlin, Germany, 1978; Volume 3, p. 366. [Google Scholar]
- Almeida, M.C.; Campaner, C.; Cella, D.M. Karyotype characterization, constitutive heterochromatin and nucleolus organizer regions of Paranaita opima (Coleoptera, Chrysomelidae, Alticinae). Genet. Mol. Biol 2006, 29, 475–481. [Google Scholar] [CrossRef]
- Virkki, N.; Denton, A. Silver staining of the elements of spermatogenesis in Oedionychina (Chrysomelidae: Alticinae). Hereditas 1987, 106, 37–49. [Google Scholar] [CrossRef]
- Gokhman, V.E.; Kuznetsova, V.G. FISH—In Insect Chromosomes. In Cytogenetics and Molecular Cytogenetics; Liehr, T., Ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 319–338. [Google Scholar] [CrossRef]
- Cioffi, M.B.; Moreira-Filho, O.; Ráb, P.; Sember, A.; Molina, W.F.; Bertollo, L.A.C. Conventional cytogenetic approaches—Useful and indispensable tools in discovering fish biodiversity. Curr. Genet. Med. Rep. 2018, 6, 176–186. [Google Scholar] [CrossRef]
- Zwick, M.S.; Hanson, R.E.; Islam-Faridi, M.N.; Stelly, D.M.; Wing, R.A.; Price, H.J.; McKnight, T.D. A rapid procedure for the isolation of C 0 t-1 DNA from plants. Genome 1997, 40, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.M.C.; Toma, G.A.; Cioffi, M.B. FISH-in Fish Chromosomes. In Cytogenetics and Molecular Cytogenetics, 1st ed.; Liehr, T., Ed.; CRC Press: Boca Raton, FL, USA, 2022; Volume 1, pp. 281–297. [Google Scholar]
- Yang, F.; Graphodatsky, A.S. Animal probes and ZOO-fish. In Fluorescence In Situ Hybridization (FISH); Liehr, T., Ed.; Springer: Berlin, Germany, 2009; pp. 395–415. [Google Scholar]
- Yano, C.F.; Bertollo, L.A.C.; Cioffi, M. Fish-FISH: Molecular Cytogenetics in Fish Species. In Fluorescence In Situ Hybridization (FISH); Liehr, T., Ed.; Springer: Berlin, Germany, 2017; pp. 429–443. [Google Scholar] [CrossRef]
- Schmid, M.; Feichtinger, W.; Steinlein, C.; Rupprecht, A. Chromosome banding in Amphibia: XXIII. Giant W sex chromosomes and extremely small genomes in Eleutherodactylus euphronides and Eleutherodactylus shrevei (Anura, Leptodactylidae). Cytogenet. Genome Res. 2002, 97, 81. [Google Scholar] [CrossRef]
- Marchal, J.A.; Acosta, M.J.; Nietzel, H.; Sperling, K.; Bullejos, M.; Díaz de la Guardia, R.; Sánchez, A. X chromosome painting in Microtus: Origin and evolution of the giant sex chromosomes. Chromosome Res. 2004, 12, 767–776. [Google Scholar] [CrossRef]
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571. [Google Scholar] [CrossRef]
- Conte, M.A.; Clark, F.E.; Roberts, R.B.; Xu, L.; Tao, W.; Zhou, Q.; Wang, D.; Kocher, T.D. Origin of a giant sex chromosome. Mol. Biol. Evol. 2021, 38, 1554–1569. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B.; Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 2005, 95, 118–128. [Google Scholar] [CrossRef]
- Chalopin, D.; Volff, J.N.; Galiana, D.; Anderson, J.L.; Schartl, M. Transposable elements and early evolution of sex chromosomes in fish. Chromosome Res. 2015, 23, 545–560. [Google Scholar] [CrossRef]
- Douglas, H.B.; Konstantinov, A.S.; Brunke, A.J.; Moseyko, A.G.; Chapados, J.T.; Eyres, J.; Ritcher, R.; Savard, K.; Sears, E.; Prathapan, K.D.; et al. Phylogeny of the flea beetles (Galerucinae: Alticini) and the position of Aulacothorax elucidated through anchored phylogenomics (Coleoptera: Chrysomelidae: Alticini). Syst. Entomol. 2023, 1, 1–26. [Google Scholar] [CrossRef]
- Nokkala, S.; Grozeva, S. Achiasmatic male meiosis of achiasmatic type in Myrmedobia coleoptrata (Fn.) (Heteroptera, Microphysidae). Caryologia 2000, 53, 5–8. [Google Scholar] [CrossRef]
- Grozeva, S.; Simov, N.; Nokkala, S. Achiasmatic male meiosis in three Micronecta species (Heteroptera: Nepomorpha: Micronectidae). Comp. Cytogenet. 2008, 2, 73–78. [Google Scholar]
- Satomura, K.; Tamura, K. Ancient male recombination shaped genetic diversity of neo-Y chromosome in Drosophila albomicans. Mol. Biol. Evol. 2016, 33, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Blackmon, H.; Demuth, J.P. Estimating tempo and mode of Y chromosome turnover: Explaining Y chromosome loss with the fragile Y hypothesis. Genetics 2014, 197, 561–572. [Google Scholar] [CrossRef]
- Bracewell, R.; Tran, A.; Chatla, K.; Bachtrog, D. Sex chromosome evolution in beetles. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dutrillaux, B.; Dutrillaux, A.-M. Why Are X Autosome Rearrangements so Frequent in Beetles? A Study of 50 Cases. Genes 2023, 14, 150. [Google Scholar] [CrossRef]
- Traut, W.; Sahara, K.; Otto, T.; Marec, F. Molecular differentiation of sex chromosomes probed by comparative genomic hybridization. Chromosoma 1999, 108, 173–180. [Google Scholar] [CrossRef]
- Natri, H.M.; Merilä, J.; Shikano, T. The evolution of sex determination associated with a chromosomal inversion. Nat. Commun. 2019, 10, 145. [Google Scholar] [CrossRef]
- Ohno, S. Sex. Chromosome and Sex-Linked Genes, 1st ed.; Springer: New York, NY, USA, 1967; p. 192. [Google Scholar]
- Vicoso, B. Molecular and evolutionary dynamics of animal sex-chromosome turnover. Nat. Ecol. Evol. 2019, 3, 1632–1641. [Google Scholar] [CrossRef]
- Lamelas, L.; Arroyo, M.; Fernández, F.J.; Marchal, J.A.; Sánchez, A. Structural and evolutionary relationships in the giant sex chromosomes of three Microtus species. Genes 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.A.; Acosta, M.J.; Bullejos, M.; Díaz de La Guardia, R.; Sánchez, A. Sex chromosomes, sex determination, and sex-linked sequences in Microtidae. Cytogenet. Genome Res. 2003, 101, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Rovatsos, M.; Marchal, J.A.; Giagia-Athanasopoulou, E.; Sánchez, A. Molecular composition of heterochromatin and its contribution to chromosome variation in the Microtus thomasi/Microtus atticus species complex. Genes 2021, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.J.; Romero-Fernández, I.; Sánchez, A.; Marchal, J.A. Comparative analysis by chromosome painting of the sex chromosomes in arvicolid rodents. Cytogenet. Genome Res. 2011, 132, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Marchal, J.A.; Acosta, M.J.; Bullejos, M.; Puerma, E.; Díaz de la Guardia, R.; Sánchez, A. Distribution of L1-retroposons on the giant sex chromosomes of Microtus cabrerae (Arvicolidae, Rodentia): Functional and evolutionary implications. Chromosome Res. 2006, 14, 177–186. [Google Scholar] [CrossRef]
- Hall, A.B.; Basu, S.; Jiang, X.; Qi, Y.; Timoshevskiy, V.A.; Biedler, J.K.; Sharakhova, M.V.; Elahi, R.; Anderson, M.A.E.; Chen, X.G.; et al. A male-determining factor in the mosquito Aedes aegypti. Science 2015, 348, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Krzywinska, E.; Dennison, N.J.; Lycett, G.J.; Krzywinski, J. A maleness gene in the malaria mosquito Anopheles gambiae. Science 2016, 353, 67–69. [Google Scholar] [CrossRef]
- Sharma, A.; Heinze, S.D.; Wu, Y.; Kohlbrenner, T.; Morilla, I.; Brunner, C.; Wimmer, E.A.; Van De Zande, L.; Robinson, M.D.; Beukeboom, L.W.; et al. Male sex in houseflies is determined by Mdmd, a paralog of the generic splice factor gene CWC22. Science 2017, 356, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Meccariello, A.; Salvemini, M.; Primo, P.; Hall, B.; Koskinioti, P.; Dalíková, M.; Gravina, A.; Gucciardino, M.A.; Forlenza, F.; Gregoriou, M.E.; et al. Maleness-on-the-Y (MoY) orchestrates male sex determination in major agricultural fruit fly pests. Science 2019, 365, 1457–1460. [Google Scholar] [CrossRef]
- Laslo, M.; Just, J.; Angelini, D.R. Theme and variation in the evolution of insect sex determination. J. Exp. Zool. B Mol. Dev. Evol. 2023, 340, 162–181. [Google Scholar] [CrossRef]
- Jolivet, P. Food habits and food selection of Chrysomelidae: Bionomic and evolutionary perspectives. In Biology of Chrysomelidae, 1st ed.; Jolivet, P., Petitpierre, E., Hsiao, T., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 1988; pp. 1–24. [Google Scholar] [CrossRef]
- Kapatos, E.T. Integrated pest management systems of Dacus oleae. In Fruit Flies: Their Biology, Natural Enemies and Control, 3rd ed.; Rombinson, A.S., Hooper, G.H.S., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; pp. 391–398. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, J.A.D.; Sassi, F.d.M.C.; de Moraes, R.L.R.; Artoni, R.F.; Liehr, T.; Cioffi, M.B.; de Almeida, M.C. Giant Sex Chromosomes in Omophoita Species (Coleoptera, Chrysomelidae): Structural and Evolutionary Relationships Revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Insects 2023, 14, 440. https://doi.org/10.3390/insects14050440
Vidal JAD, Sassi FdMC, de Moraes RLR, Artoni RF, Liehr T, Cioffi MB, de Almeida MC. Giant Sex Chromosomes in Omophoita Species (Coleoptera, Chrysomelidae): Structural and Evolutionary Relationships Revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH). Insects. 2023; 14(5):440. https://doi.org/10.3390/insects14050440
Chicago/Turabian StyleVidal, Jhon A. D., Francisco de M. C. Sassi, Renata L. R. de Moraes, Roberto F. Artoni, Thomas Liehr, Marcelo B. Cioffi, and Mara C. de Almeida. 2023. "Giant Sex Chromosomes in Omophoita Species (Coleoptera, Chrysomelidae): Structural and Evolutionary Relationships Revealed by Zoo-FISH and Comparative Genomic Hybridization (CGH)" Insects 14, no. 5: 440. https://doi.org/10.3390/insects14050440






