The Leg Sensilla of Insects from Different Habitats—Comparison of Strictly Aquatic and Riparian Bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Gross Morphology of the First Pair of Legs
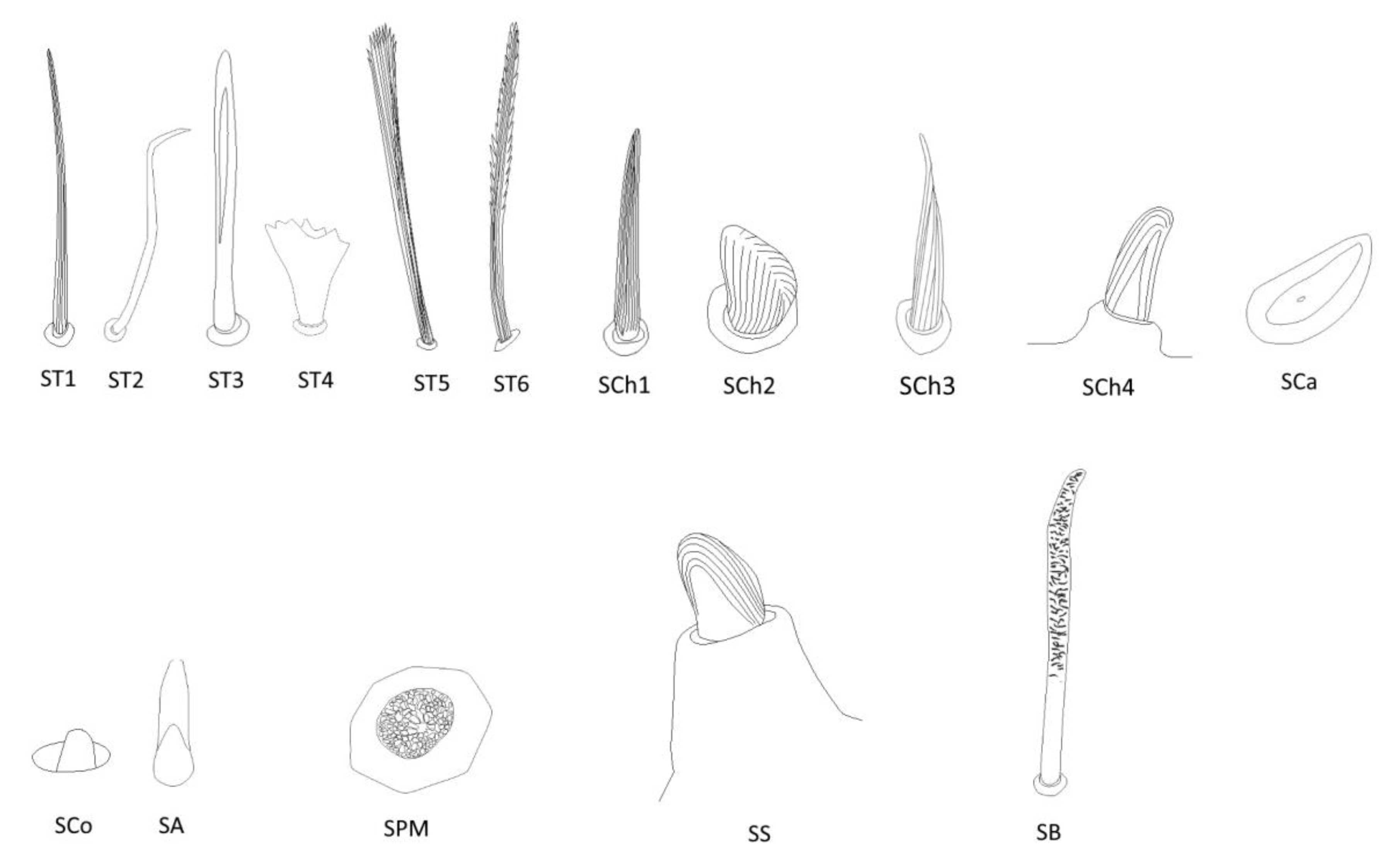
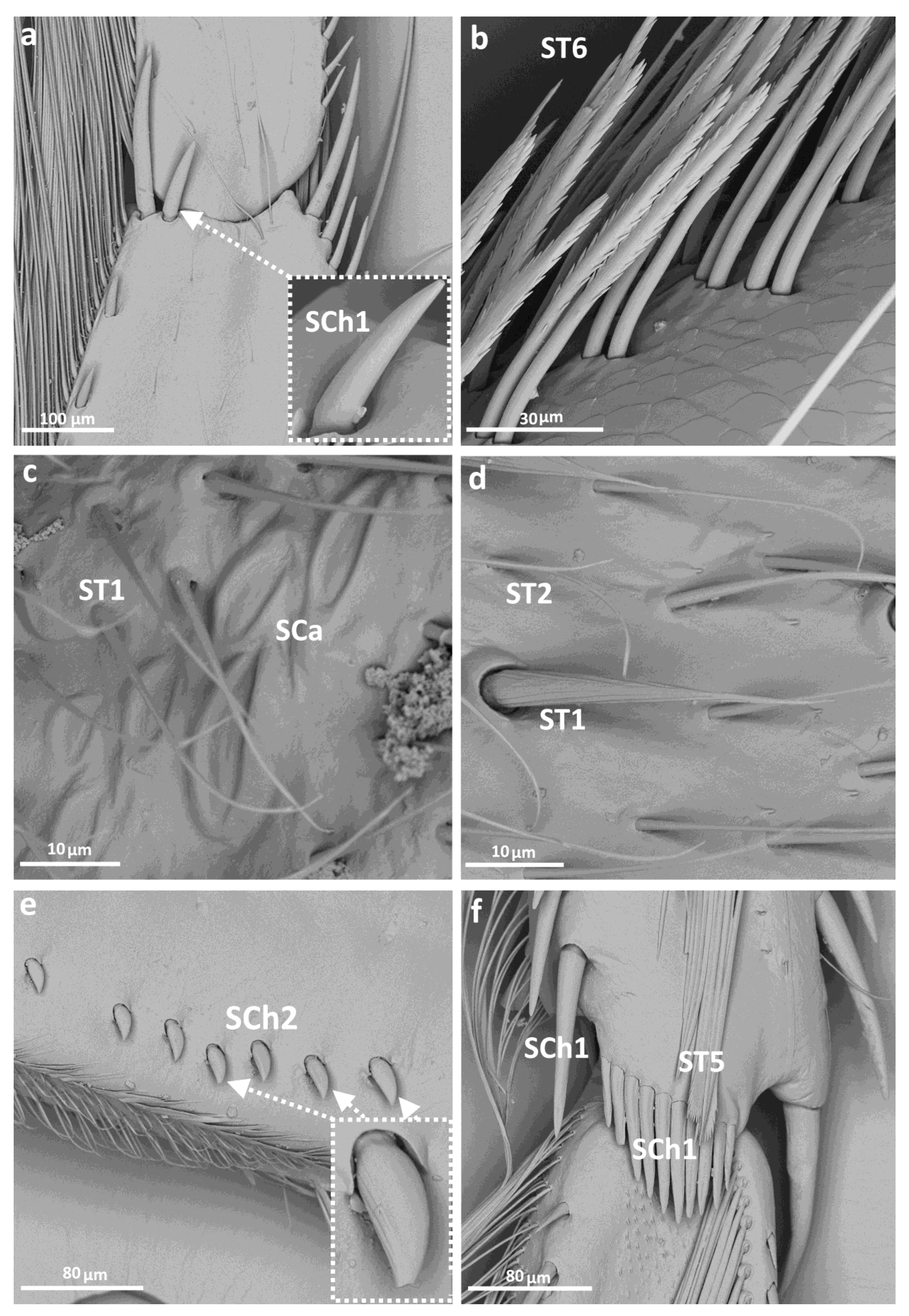
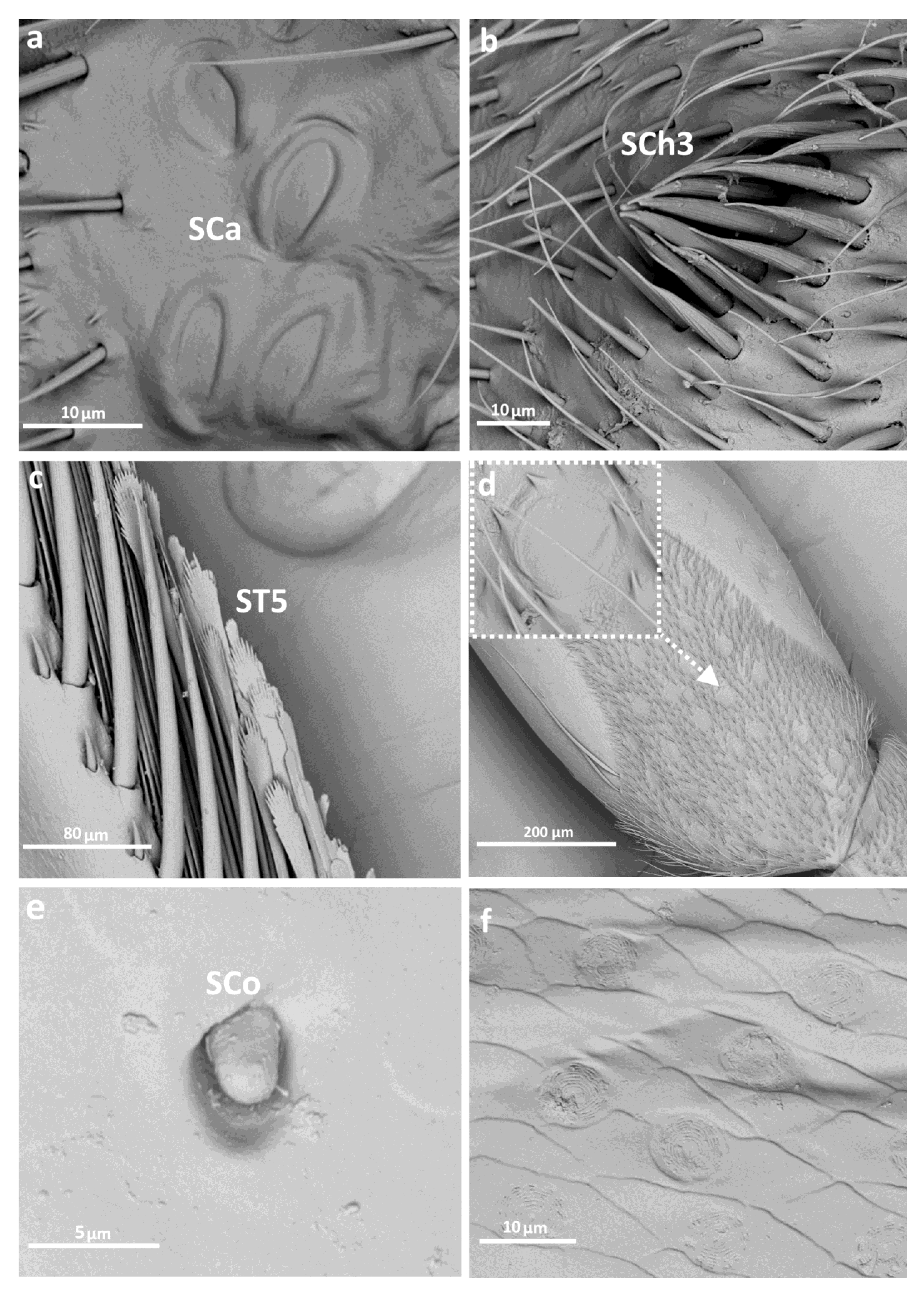
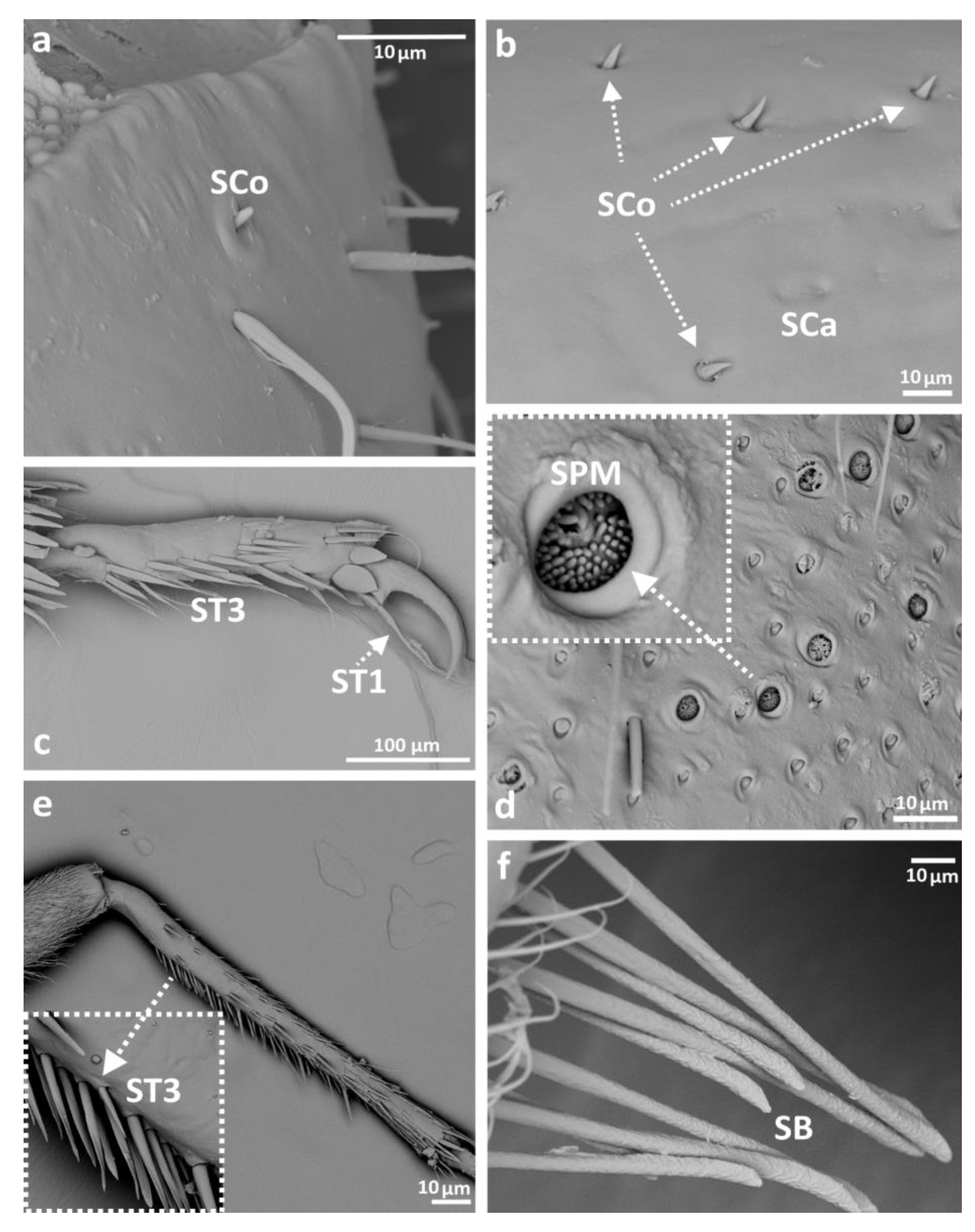
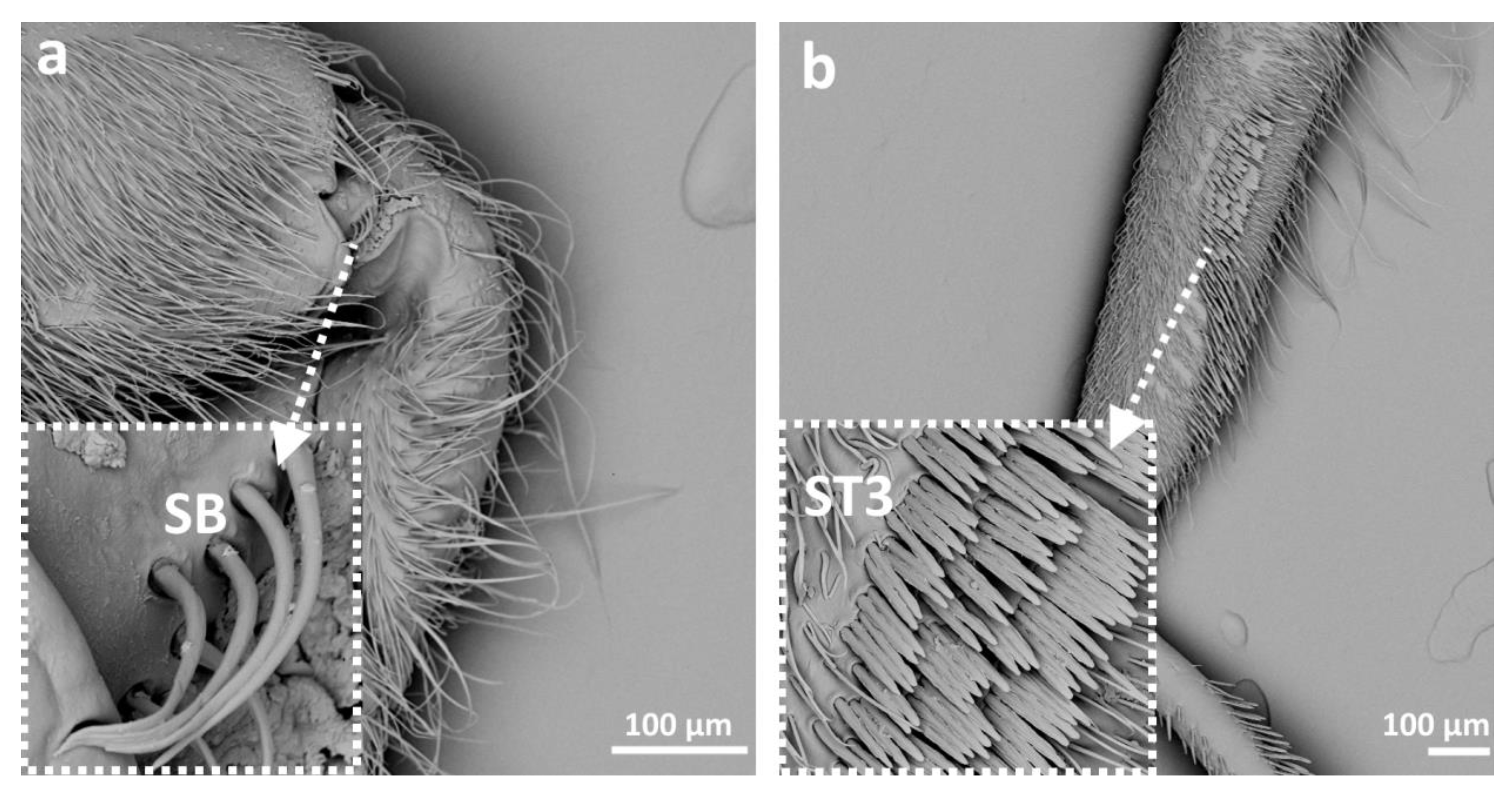
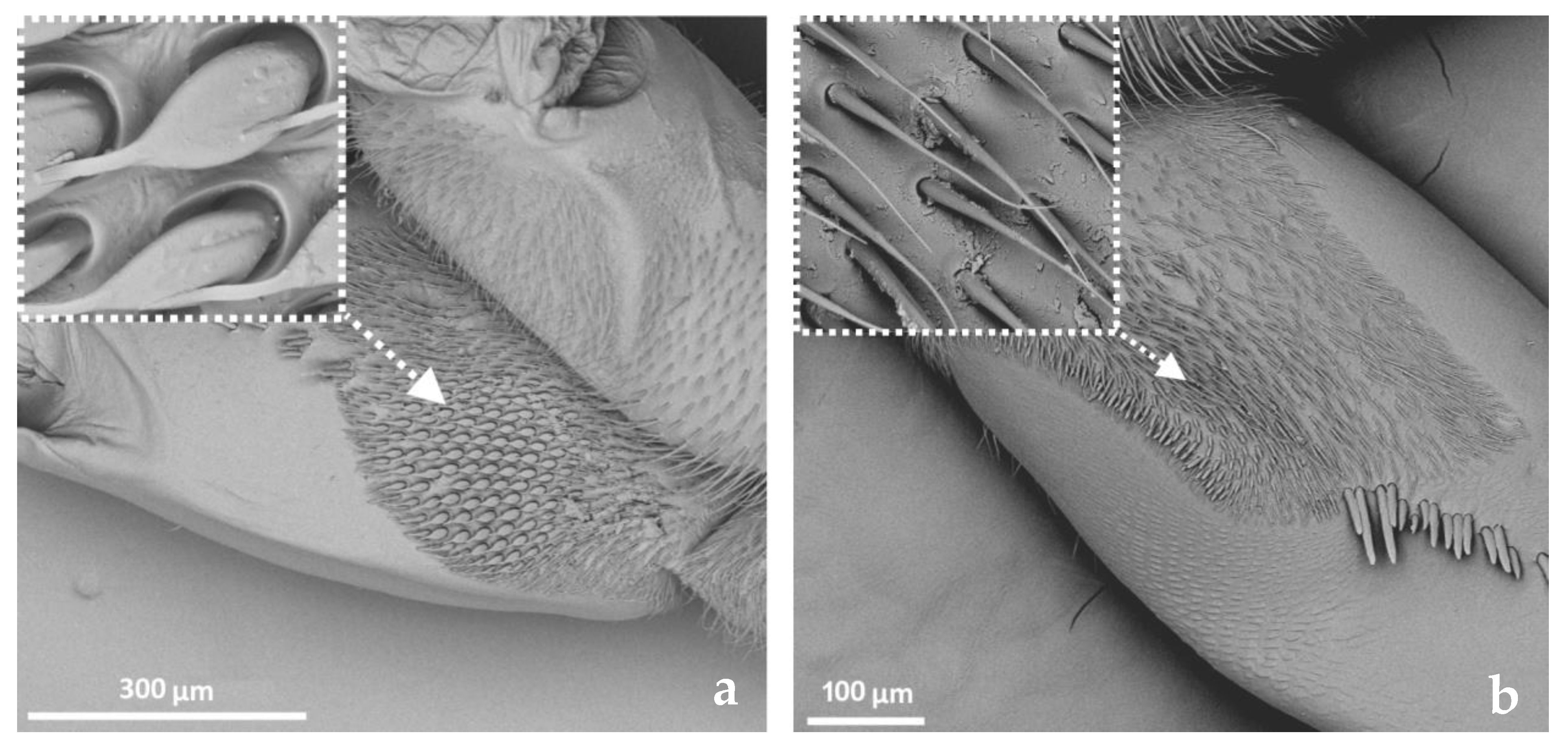
3.2. Categories and Morphology of Leg Sensilla
- Sensilla trichodea (ST)—long, thin, hairlike sensilla, with smooth or ribbed surfaces. They have a flexible socket (a thin membrane connects the cuticle of the leg with the cuticle of the sensillum, making it movable at the base), which gives them a putative mechanoreceptive function. The shape of this sensillum varies from tapered at the top to flattened at the top. The tip is either straight or bent. This type of sensilla appears in groups, covering large areas of the surface. The other function performed by sensilla trichodea is gustation. In this case, the sensillum occurs with a single pore on the tip.
- ST1—long, thin, hairlike sensilla with a ribbed surface, without pores—they perform a mechanoreceptive function
- ST2—long, thin, hairlike sensilla with a smooth surface, without pores—they perform a mechanoreceptive function
- ST3—long, flattened, sometimes curved inwards sensilla, more/less ribbed surface, without pores—they perform a mechanoreceptive function
- ST4—short flattened sensilla resembling a leaf with a frayed end, without pores—they perform a mechanoreceptive function
- ST5—long ribbed sensilla, flattening and widening along the length, with a ribbed frayed end resembling a brush, without pores—they perform a mechanoreceptive function
- ST6—long ribbed sensilla with frayed edges, without pores—they perform a mechanoreceptive function
- Sensilla chaetica (SCh)—thick sensilla with pronounced ribs on the surface. The length varies. The tip is either pointed or rounded. It has a flexible socket, like the sensilla trichodea, but is easy to distinguish from this other type because it is visibly thicker and more rigid. This type is also described in the literature as mechanoreceptive sensilla.
- SCh1—thick and rigid sensilla with pronounced ribs on the surface. The sensillum is long, thicker at the base and tapers to the tip. The tip is sharpened or slightly rounded.
- SCh2—thick and rigid sensilla with pronounced ribs on the surface. The sensillum is short and slightly bent. Observed on the “pala”, arranged in rows.
- SCh3—thick and rigid sensilla with pronounced ribs on the surface. The sensillum narrows strongly towards the tip and ends with a thin, long tip.
- SCh4—thick and rigid sensilla with pronounced ribs on the surface with a clearly rounded tip and a well-developed socket.
- Sensilla campaniformia (SCa)—oval or elongated disks lying flat on the surface, usually with a visible pore in the middle. This type also has a flexible socket. These sensilla belong to mechanoreceptors and are described as pressure sensilla.
- Sensilla basiconica (SB)—cone-like structures, usually smaller than trichoid sensilla, with a porous or non-porous surface and inflexible socket (no membrane connecting the cuticle of the leg with the cuticle of the sensillum). In our studies, only the non-porous sensilla basiconica were observed. They are long, have a wrinkled surface, a round end, and occur in groups between the segments of the legs. They are believed to perform a proprioceptive function.
- Sensilla placodea multilobated (SPM)—round cavities with small, fingerlike protuberances. As they were observed before on the antennae of studied species, the name was given according to these other studies. The probable function is olfaction; however, olfactory structures are not specific for leg sensilla. Therefore, they might play another role.
- Sensilla coeloconica (SCo)—small cones with an inflexible socket and smooth surface. They have been observed as either single or covering a bigger part of the leg surface. They are believed to perform a thermo-hygroreceptive function.
- Sensilla ampullacea (SA)—peg in pit sensilla, with the opening being the only part visible on the surface. The body of the sensillum is hidden in a cavity and rises from an inflexible socket. The sensillum is believed to perform a thermo-hygroreceptive function.
- Sensilla styloconica (SS)—pegs arising from a bulge of cuticle, with a flexible socket and a ribbed surface. The sensillum is believed to perform mechanoreceptive or gustatory function.
3.3. Sensilla Observed among Studied Families
3.3.1. Corixidae
3.3.2. Gelastocoridae
3.3.3. Ochteridae
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Z.; Damgaard, J.; Yaang, H.; Habsgaard, M.B.; Weir, T.; Bu, W. Phylogeny and diversification of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha). Cladistics 2020, 36, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Štys, P.; Jansson, A. Check-list of recent family-group and genus-group names of Nepomorpha (Heteroptera) of the world. Entomol. Fenn. 1988, 50, 1–44. [Google Scholar]
- Nieser, N. Guide to aquatic Heteroptera of Singapore and Peninsular Malaysia. IV. Corixoidea. Raffles Bull. Zool. 2002, 50, 263–274. [Google Scholar]
- Chen, P.-P.; Nieser, N.; Zettel, H. The Aquatic and Semi-Aquatic Bugs (Heteroptera: Nepomorpha & Gerromorpha) of Malesia; Fauna Malesiana Handbook 5; Brill: Leiden, The Netherlands; Boston, MA, USA, 2005. [Google Scholar]
- Nieser, N.; Chen, P.-P. Two new genera and a new subfamily of Micronectidae (Heteroptera, Nepomorpha) from Brazil. In Hug the Bug—For Love of True Bugs; Rabitsch, W., Ed.; Festschrift zum 70. Geburtstag vonErnst Heiss. Series; Denisia, Biologiezentrum der Oberösterreichische Landesmuseen: Linz, Austria, 2006; Volume 19, pp. 523–534. [Google Scholar]
- Tinerella, P.P. Taxonomic revision and systematics of New Guinea and Oceania pygmy water boatmen (Hemiptera: Heteroptera: Corixoidea: Micronectidae). Zootaxa 2008, 1797, 1–66. [Google Scholar] [CrossRef]
- Fent, M.; Kment, P.; Çamur-Elipek, B.; Kirgiz, T. Annotated catalogue of Enicocephalomorpha, Dipsocoromorpha, Nepomorpha, Gerromorpha, and Leptopodomorpha (Hemiptera: Heteroptera) of Turkey, with new records. Zootaxa 2011, 2856, 1–84. [Google Scholar] [CrossRef]
- Weirauch, C.; Schuh, R.T. Systematics and evolution of Heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef]
- China, W.E. The evolution of the water bugs. Symposium on organic evolution. Bull. Nat. Inst. Sci. India. 1955, 7, 91–103. [Google Scholar]
- Rieger, C. Skelett und Muskulatur des Kopfes und Prothorax von Ochterus marginatus Latreille. Zoomorphologie 1976, 83, 109–191. [Google Scholar] [CrossRef]
- Popov, Y.A. Historical development of the hemipterous infraorder Nepomorpha. Tr. Paleontol. Inst. Acad. Sci. Nauk USSR 1971, 129, 1–228. (In Russian) [Google Scholar]
- Mahner, M. Systema Cryptoceratorum Phylogeneticum (Insecta, Heteroptera); Zoologica; Schweizerbart Science Publishers: Stuttgart, Germany, 1993. [Google Scholar]
- Hebsgaard, M.B.; Andersen, N.M.; Damgaard, J. Phylogeny of the true water bugs (Nepomorpha: Hemiptera-Heteroptera) based on 16S and 28S rDNA and morphology. Syst. Entomol. 2004, 29, 488–508. [Google Scholar] [CrossRef]
- Hua, J.; Li, M.; Dong, P.; Cui, Y.; Xie, Q.; Bu, W. Phylogenetic analysis of the true water bugs (Insecta: Hemiptera: Heteroptera: Nepomorpha): Evidence from mitochondrial genomes. BMC Evol. Biol. 2009, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J. Phylogenetic signals from Nepomorpha (Insecta: Hemiptera: Heteroptera) mouthparts: Styletsbundle, sense organs, and labial segments. Sci. World J. 2014, 2014, 237854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Rédei, D.; Baňař, P.; Xie, Q.; Štys, P.; Damgaard, J.; Chen, P.-P.; Yi, W.-B.; Wang, Y.; et al. Phylogenetic divergences of the true bugs (Insecta: Hemiptera: Heteroptera), with emphasis on the aquatic lineages: The last piece of the aquatic insect jigsaw originated in the Late Permian/Early Triassic. Cladistics 2015, 34, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, L.; Oscarson, H.G. Corixids (Hemiptera-Heteroptera), the new top predators in acidified lakes. Verh. Internat. Verein. Limnol. 1981, 21, 1616–1620. [Google Scholar] [CrossRef]
- Papáček, M. Small aquatic and ripicolous bugs (Heteroptera: Nepomorpha) as predators and prey: The question of economic importance. Eur. J. Entomol. 2001, 98, 1–12. [Google Scholar] [CrossRef]
- Polhemus, J.T.; Polhemus, D.A. Global diversity of the true bugs (Heteroptera; Insecta). Hydrobiologia 2008, 595, 379–391. [Google Scholar] [CrossRef]
- Kment, P.; Carapezza, A.; Jindra, Z. Taxonomic catalogue of the family Ochteridae with description of Ochterus papaceki sp. nov. from Socotra Island and Tanzania (Hemiptera: Heteroptera). Acta Entomol. Musei Natl. Pragae 2020, 60, 23–64. [Google Scholar] [CrossRef]
- Schuh, R.T.; Weirauch, C. Nepomorpha in True bugs of the world (Hemiptera: Heteroptera). In Classification and Natural History; Cornell University Press: New York, NY, USA, 2020; pp. 201–244. [Google Scholar]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- McIver, S.B. Structure of cuticular mechanoreceptors of arthropods. Annu. Rev. Entomol. 1975, 20, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F. The chemoreceptive organs: Structural aspects. In Insect Chemoreception Fundamental and Applied; Ryan, M.F., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 111–139. [Google Scholar]
- Slifer, E.H. The structure of arthropod chemoreceptors. Annu. Rev. Entomol. 1970, 15, 121–142. [Google Scholar] [CrossRef]
- Altner, H.; Prillinger, L. Ultrastructure of invertebrate chemo- thermo- and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980, 67, 69–139. [Google Scholar]
- Hallberg, E.; Hansson, B.S. Arthropod sensilla: Morphology and phylogenetic consideration. Microsc. Res. Tech. 1999, 47, 428–439. [Google Scholar] [CrossRef]
- Shields, V.D.C. Ultrastructure of insect sensilla. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2004; pp. 2408–2420. [Google Scholar]
- Shields, V.D.C. High resolution ultrastructural investigation of insect sensory organs using field emission scanning electron microscopy. In Microscopy: Science, Technology, Applications and Education; Mendez, V.A., Diaz, J., Eds.; Formatex: Badajoz, Spain, 2010; pp. 321–328. [Google Scholar]
- Zacharuk, R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Smith, J.J.B. The External Sensory Morphology of the Legs and Hairplate System of Female Trichogramma minutum Riley (Hymenoptera: Trichogrammatidae). Proc. Royal Soc. B 1987, 232, 323–366. [Google Scholar]
- Dinges, G.F.; Chockley, A.S.; Bockemühl, T.; Ito, K.; Blanke, A.; Büschges, A. Location and arrangement of campaniform sensilla in Drosophila melanogaster. J. Comp. Neurol. 2021, 529, 905–925. [Google Scholar] [CrossRef] [PubMed]
- McIver, S.; Siemicki, R. Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae). J. Morphol. 1978, 155, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Wülker, W.; Eisele, H.; Rossler, R. Tarsal sensilla of Chironomus (Diptera: Chironomidae): Number, parasitogenic changes, ultrastructure, and function. Int. J. Insect Morphol Embryol. 1982, 11, 137–146. [Google Scholar] [CrossRef]
- Yosano, S.; Kutsuwada, Y.; Akatsu, M.; Masuda, S.; Kakazu, R.; Masuoka, N.; Matsuda, K.; Hori, M. Taste recognition through tarsal gustatory sensilla potentially important for host selection in leaf beetles (Coleoptera: Chrysomelidae). Sci. Rep. 2020, 10, 4931. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Morphological study of the antennal sensilla in Gerromorpha (Insecta: Hemiptera: Heteroptera). Zoomorphology 2017, 136, 327–347. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Antennal sensory structures in water bugs of Nepoidea (Insecta: Hemiptera: Nepomorpha), their morphology and function. Zoomorphology 2019, 136, 327–347. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Insect evolution toward aquatic habitats; reassessment of antennal sensilla in the water bug families Ochteridae, Gelastocoridae and Aphelocheiridae (Hemiptera: Heteroptera: Nepomorpha). Contrib. Zool. 2020, 89, 412–433. [Google Scholar] [CrossRef]
- Nowińska, A.; Chen, P.-P.; Brożek, J. Comparative study of antennal sensilla of Corixidae and Micronectidae (Hemiptera: Heteroptera: Nepomorpha: Corixoidea). Insects 2020, 11, 734. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. The variability of antennal sensilla in Naucoridae (Heteroptera: Nepomorpha). Sci. Rep. 2021, 11, 19651. [Google Scholar] [CrossRef]
- Nowińska, A.; Brożek, J. Morphology of the Antennal Sensilla of Notonectoidea and Comparison of Evolutionary Changes in Sensilla Types and Distribution in Infraorder Nepomorpha (Insecta: Heteroptera). Insects 2021, 12, 1121. [Google Scholar] [CrossRef]
- Brożek, J. Comparative analysis and systematic mapping of the labial sensilla in the Nepomorpha (Heteroptera: Insecta). Sci. World J. 2013, 2013, 518034. [Google Scholar] [CrossRef] [PubMed]
- Polhemus, J.T.; Lindskog, P. The stridulatory mechanism of Nerthra Say, a new species, and synonymy (Heteroptera: Gelastocoridae). J. New York Entomol. Soc. 1994, 102, 242–248. [Google Scholar]
- Ball, R. On noises produced by one of the Notonetidae. Rep. Br. Assoc. Adv. Sci. 1846, 15, 64–65. [Google Scholar]
- Jansson, A. Mechanisms of sound production and morphology of the stridulatory apparatus in the genus Cenocorixa (Hemiptera, Corixidae). Ann. Zool. Fenn. 1972, 9, 120–129. [Google Scholar]
- Polhemus, J.T. Stridulatory mechanisms in aquatic and semiaquatic Heteroptera. J. New York Entomol. Soc. 1994, 102, 270–274. [Google Scholar]



| Family | Genus | Species | Nr of Studied Specimens |
|---|---|---|---|
| Corixidae | Callicorixa | Callicorixa preaeusta (Fieber, 1848) | 3 |
| Heliocorisa | Heliocorisa vermiculata (Puton, 1874) | 2 | |
| Sigara | Sigara falleni (Fieber, 1848) Sigara striata (Linnaeus, 1758) | 3 4 | |
| Gelastocoridae | Gelastocoris | Gelastocoris flavus (Guérin-Méneville, 1835) Gelastocoris oculatus (Fabricius, 1798) | 6 6 |
| Nerthra | Nerthra colaticollis (Todd, 1959) Nerthra grandicollis (Germar, 1837) Nerthra mixta (Montandon, 1929) Nerthra ranina (Herrich-Schäffer, 1853) | 5 6 4 4 | |
| Ochteridae | Ochterus | Ochterus marginatus (Latreille, 1804) Ochterus perbosci (Guérin-Méneville, 1843) | 8 5 |
| Sensillum Type | Corixidae | Gelastocoridae | Ochteridae |
|---|---|---|---|
| ST1 | + | + | + |
| ST2 | + | + | + |
| ST3 | + | ||
| ST4 | + | ||
| ST5 | + | ||
| ST6 | + | ||
| SCh1 | + | + | |
| SCh2 | + | ||
| SCh3 | + | ||
| SCh4 | + | ||
| SCa | + | + | + |
| SCo | + | + | + |
| SA | + | ||
| SB | + | + | |
| SPM | + | + | |
| SS | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowińska, A.; Franielczyk-Pietyra, B.; Polhemus, D.A. The Leg Sensilla of Insects from Different Habitats—Comparison of Strictly Aquatic and Riparian Bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera). Insects 2023, 14, 441. https://doi.org/10.3390/insects14050441
Nowińska A, Franielczyk-Pietyra B, Polhemus DA. The Leg Sensilla of Insects from Different Habitats—Comparison of Strictly Aquatic and Riparian Bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera). Insects. 2023; 14(5):441. https://doi.org/10.3390/insects14050441
Chicago/Turabian StyleNowińska, Agnieszka, Barbara Franielczyk-Pietyra, and Dan A. Polhemus. 2023. "The Leg Sensilla of Insects from Different Habitats—Comparison of Strictly Aquatic and Riparian Bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera)" Insects 14, no. 5: 441. https://doi.org/10.3390/insects14050441
APA StyleNowińska, A., Franielczyk-Pietyra, B., & Polhemus, D. A. (2023). The Leg Sensilla of Insects from Different Habitats—Comparison of Strictly Aquatic and Riparian Bugs (Corixidae, Ochteridae, Gelastocoridae: Nepomorpha: Insecta: Heteroptera). Insects, 14(5), 441. https://doi.org/10.3390/insects14050441








