Effective Phytosanitary Treatment for Export of Oriental Melons (Cucumis melo var L.) Using Ethyl Formate and Modified Atmosphere Packaging to Control Trialeurodes vaporariorum (Hemiptera: Aleyrodidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Chemicals
2.2. Efficacy of EF on T. vaporariorum in Lab Conditions
2.3. Assessments of Sorption and Phytotoxicity of EF; Preliminary Tests with/without MAP
2.4. Commercial Trials
2.5. Statistical Analysis
3. Results
3.1. Efficacy of EF on T. vaporariorum under Lab Conditions
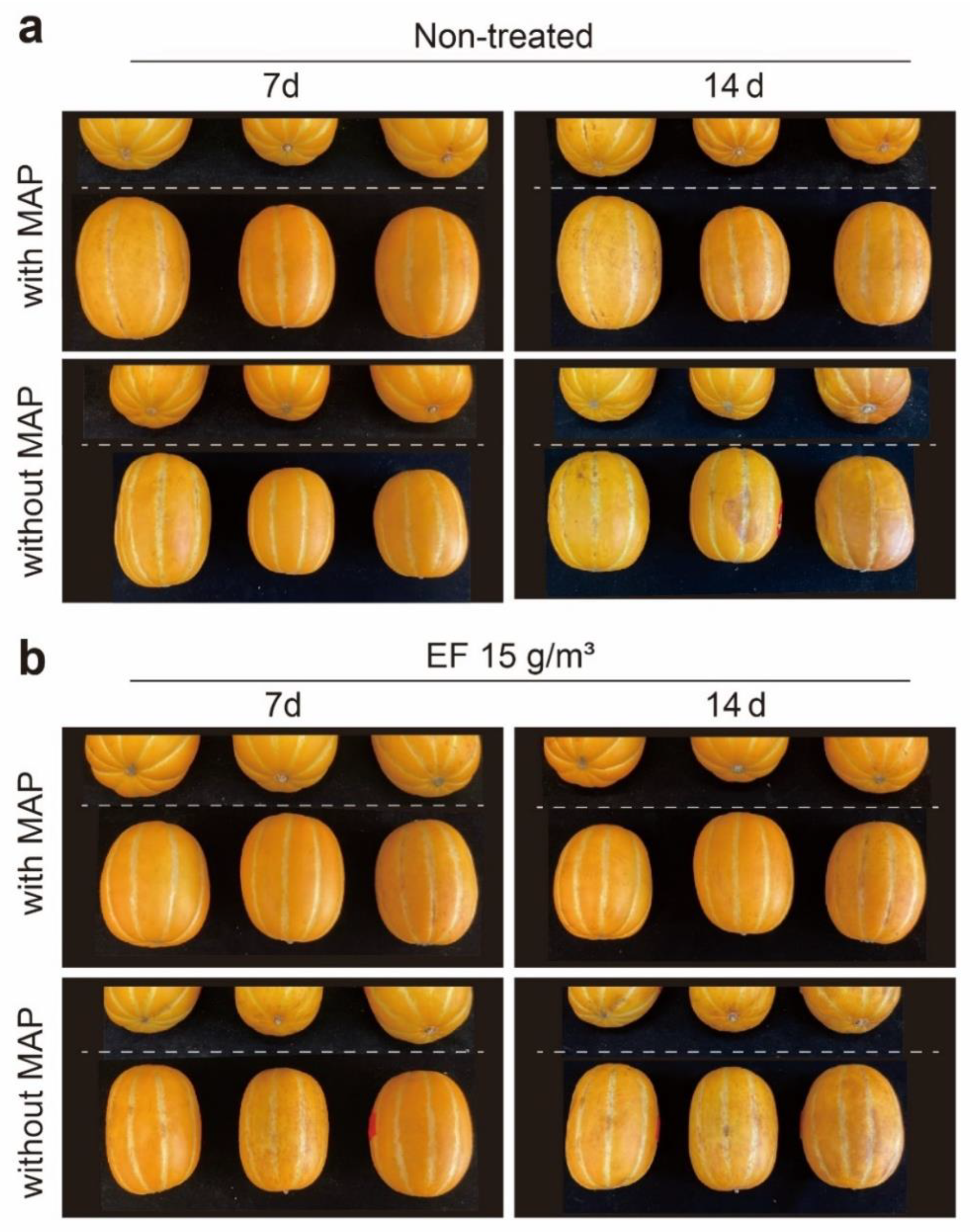
3.2. Assessments of Sorption and Phytotoxicity of EF Fumigation with/without MAP
3.3. Commercial Trials
4. Discussion
4.1. Fumigation of T. vaporariorum Using EF
4.2. Phytotoxicity of EF and Its Efficacy against Pests of Agricultural Fruits
4.3. Packaging of C. melo with or without EF Fumigation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UN Comtrade. UN Comtrade Database. 2019. Available online: https://comtrade.un.org/data (accessed on 5 March 2023).
- Choi, J.-W.; Chang, M.-S.; Lee, J.H.; Hong, Y.; Kim, J.G. Changes in Quality of Oriental Melon ‘Smartkkul’ During Vessel Transportation. Korean J. Hortic. Sci. Technol. 2018, 36, 560–568. [Google Scholar]
- Kim, J.S.; Choi, H.R.; Chung, D.S.; Lee, Y.S. Current research status of postharvest and packaging technology of oriental melon (Cucumis melo var. makuwa) in Korea. Korean J. Hortic. Sci. Technol. 2010, 28, 902–911. [Google Scholar]
- Gayasan Wild Flower Botanical Garden. 2020. Available online: https://www.sj.go.kr/gayasan/page.do?mnu_uid=1098& (accessed on 9 March 2023).
- Soria, C.; Sese, A.I.L.; Gomez-Guillamon, M.L. Resistance mechanism of Cucumis melo var. agrestis against Trialeurodes vaporariorum and their use to control a closterovirus that causes a yellowing disease of melon. Plant Pathol. 1996, 45, 761–766. [Google Scholar] [CrossRef]
- Choi, W.I.; Lee, E.H.; Choi, B.R.; Park, H.M.; Ahn, Y.J. Toxicity of plant essential oils to Trialeurodes vapaorariorum (Homoptera: Aleyrodidae). J. Econ. Enotomol. 2003, 96, 1479–1484. [Google Scholar] [CrossRef]
- Qasim, M.; Akutse, K.S.; Hussain, D.; Al-Zoubi, O.M.; Mustafa, T.; Aguila, L.C.R.; Alamri, S.; Hashem, M.; Wang, L. Powdery Mildew Fungus Oidium lycopersici Infected-Tomato Plants Attracts the Non-Vector Greenhouse Whitefly, Trialeurodes vaporariorum, but Seems Impair Their Development. Agronomy 2022, 12, 2791. [Google Scholar] [CrossRef]
- Huang, J.; Qasim, M.; Khan, K.A.; Noman, A.; Islam, W.; Haider, I.; Jamal, Z.A.; Ghramh, H.A.; Wang, L. Role of powdery mildew in the behavior of parasitoid: A case study using whiteflies and Encarsia formosa on tomato plants. Physiol. Mol. Plant Pathol. 2022, 122, 101901. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; López-Moya, J.J.; Aranda, M.A. Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann. Appl. Biol. 2014, 165, 155–171. [Google Scholar] [CrossRef]
- Wainaina, J.M.; De Barro, P.; Kubatko, L.; Kehoe, M.A.; Harvey, J.; Karanja, D.; Boykin, D. Global phylogenetic relationships, population structure and gene flow estimation of Trialeurodes vaporariorum (greenhouse whitefly). Bull. Entomol. Res. 2018, 108, 5–13. [Google Scholar] [CrossRef]
- Gamarra, H.; Carhuapoma, P.; Mujica, N.; Jreuze, J.; Kroschel, J. Green Whitefly, Trialeurodes Vaporariorum. 2016. Available online: https://cipotato.org/riskatlasforafrica/trialeurodes-vaporariorum/ (accessed on 3 January 2023).
- Animal & Plant Quarantine Agency (APQA). Available online: http://www.qia.go.kr/bbs/lawAnn/listLawWebAction.do (accessed on 4 August 2022).
- Food and Agriculture Organization (FAO) of the United Nations and International Plant Protection Convention (IPPC). Recommendation on: Replacement or Reduction of the Use of Methyl Bromide as a Phytosanitary Measure; FAO: Rome, Italy, 2017. [Google Scholar]
- Park, M.H.; Chang, E.H.; Yang, H.J.; Lee, J.S.; Do, G.R.; Song, H.J.; Chang, M.S.; Ku, K.M. Modified atmosphere and humidity film reduces browning susceptibility of oriental melon suture tissue during cold storage. Foods 2020, 9, 1329. [Google Scholar] [CrossRef]
- Bai, J.H.; Saftner, R.A.; Watada, A.E.; Lee, Y.S. Modified atmosphere maintains quality of fresh-cut cantaloupe (Cucumis melo L.). J. Food Sci. 2001, 66, 1207–1211. [Google Scholar] [CrossRef]
- Park, M.G.; Park, C.G.; Yang, J.O.; Kim, K.H.; Ren, Y.L.; Lee, B.H.; Cha, D.H. Ethyl formate as a methyl bromide alternative for phytosanitary disinfestation of imported banana in Korea with logistical considerations. J. Econ. Entomol. 2020, 113, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Park, M.G.; Lee, B.H.; Yang, J.O.; Kim, B.S.; Roh, G.H.; Kendra, P.E.; Cha, D.H. Ethyl formate as a methyl bromide alternative for fumigation of citrus: Efficacy, fruit quality, and workplace safety. J. Econ. Entomol. 2021, 114, 2290–2296. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lim, E.; Park, M.G.; Cha, W. Assessing the Retest Reliability of Prefrontal EEG Markers of Brain Rhythm Slowing in the Eyes-Closed Resting State. Clin. EEG Neurosci. 2020, 51, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kyung, Y.J.; Kim, H.K.; Cho, S.W.; Kim, B.S.; Yang, J.O.; Koo, H.N.; Kim, G.H. Comparison of the efficacy and phytotoxicity of phosphine and ethyl formate for controlling Pseudococcus longispinus (Hemiptera: Pseudococcidae) and Pseudococcus orchidicola in imported foliage nursery plants. J. Econ. Entomol. 2019, 112, 2149–2156. [Google Scholar] [CrossRef]
- Simpson, T.; Bikoba, V.; Mitcham, E.J. Effects of ethyl formate on fruit quality and target pest mortality for harvested strawberries. Postharvest Biol. Technol. 2004, 34, 313–319. [Google Scholar] [CrossRef]
- Simpson, T.; Bikoba, V.; Tipping, C.; Mitcham, E.J. Ethyl formate as a postharvest fumigant for selected pests of table grapes. J. Econ. Entomol. 2007, 100, 1084–1090. [Google Scholar] [CrossRef]
- Agarwal, M.; Ren, Y.L.; Newman, J.; Learmonth, S. Ethyl formate: A potential disinfestation treatment for Eucalyptus weevil (Gonipterus platensis) (Coleoptera: Curculionidae) in apples. J. Econ. Entomol. 2015, 108, 2566–2571. [Google Scholar] [CrossRef]
- Kwon, T.H.; Park, C.G.; Lee, B.H.; Zarders, D.R.; Roh, G.H.; Kendra, P.E.; Cha, D.H. Ethyl formate fumigation and ethyl formate plus cold treatment combination as potential phytosanitary quarantine treatments of Dsorophila suzukii in blueberries. J. Asia-Pac. Entomol. 2021, 24, 129–135. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.K.; Kyung, Y.J.; Park, G.H.; Lee, B.H.; Yang, J.O.; Koo, H.N.; Kim, G.H. Fumigation activity of ethyl formate and phosphine against Tetranychus urticae (Acari: Tetranychidae) on imported sweet pumpkin. J. Econ. Entomol. 2018, 114, 1625–1632. [Google Scholar] [CrossRef]
- Ren, Y.; Lee, B.H.; Padovan, B. Penetration of methyl bromide, sulfuryl fluoride, ethanedinitrile and phosphine into timber blocks and the sorption rate of the fumigants. J. Stored Prod. Res. 2011, 47, 63–68. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Kim, K.; Yang, J.O.; Sung, J.Y.; Lee, J.Y.; Park, J.S.; Lee, H.S.; Lee, B.H.; Ren, Y.; Lee, D.W.; Lee, S.E. Minimization of energy transduction confers resistance to phosphine in the rice weevil, Sitophilus oryzae. Sci. Rep. 2019, 9, 14605. [Google Scholar] [CrossRef] [PubMed]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yang, J.O.; Roh, G.H.; Ren, Y.L.; Lee, B.H.; Lee, S.E. Reciprocal effect of ethyl formate and phosphine gas on two quarantine pests, Tetranychus urticae (Acari: Tetranychidae) and Myzus persicae (Hemiptera: Aphididae). Korean J. Environ. Biol. 2021, 39, 336–343. [Google Scholar] [CrossRef]
- Desmarchelier, J.M. Ethyl formate and formic acid: Occurrence and environmental fate. Postharvest News Inf. 1999, 10, 7–12. [Google Scholar]
- Ren, Y.L.; Desmarchelier, J.M. Natural occurrence of carbonyl sulfide and ethyl formate in grains. In Proceedings of the International Conference on Controlled Atmosphere and Fumigation in Stored Products, Fresno, CA, USA, 29 October–3 November 2000; pp. 639–649. [Google Scholar]
- Yao, X.; Liu, Y.; Li, T.; Zhang, T.; Li, H.; Wang, W.; Shen, X.; Qian, F.; Yao, Z. Adsorption behavior of multicomponent volatile organic compounds on a citric acid residue waste-based activated carbon: Experiment and molecular simulation. J. Hazard. Mater. 2020, 392, 122323. [Google Scholar] [CrossRef]
- Kwon, T.H.; Park, C.G.; Lee, B.H.; Jeong, I.H.; Lee, S.E. A New Approach: Ethyl Formate Fumigation to Control Bemisia tabaci (Hemiptera: Aleyrodidae) in a Yellow Melon Vinyl House. Appl. Sci. 2022, 12, 5173. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.M.; Kim, B.S.; Yang, J.O.; Moon, Y.M.; Ren, Y.L. Evaluation of the synergistic effect between ethyl formate and phosphine for control of Ahpis gosypii (Homoptera: Aphididae). J. Econ. Entomol. 2015, 109, 143–147. [Google Scholar] [CrossRef]
- Jeon, J.C.; Kim, H.K.; Koo, H.N.; Kim, B.S.; Yang, J.O.; Kim, G.H. Synergistic Effect of Cold Treatment Combined with Ethyl Formate Fumigation against Drosophila suzukii (Diptera: Drosophilidae). Insects 2022, 13, 664. [Google Scholar] [CrossRef]
- Choi, J.W.; Lee, J.H.; Hong, Y.P.; Do, K.R.; Han, K.S.; Bae, Y.S. Survey on disorders of oriental melon during simulated marine container transport export. Korean J. Hortic. Sci. Technol. 2016, 34, 108–109. [Google Scholar]



| Temp. (°C) | Stage | 1 L(Ct)50 (g·h/m3, 95% CL 2) | L(Ct)99 (g·h/m3, 95% CL) | Probit-9 (g·h/m3) | Slope ± SE | df | χ2 |
|---|---|---|---|---|---|---|---|
| 5 | Adult | 0.79 (0.73–0.84) | 2.32 (2.01–2.82) | 3.02 (2.84–3.23) | 4.95 ± 0.4 | 10 | 19.22 |
| EF Dosage (g/m3) with or without MAP 1 | Fumigation Time (h) | Ct Products (g·h/m3) | Storage Period (d) | Firmness (kgf, Mean ± SE) | Sugar Content (Brix, %, Mean ± SE) | Weight Loss (Mean ± SE) | Color Change (Mean ± SE) | External Damage 2 |
|---|---|---|---|---|---|---|---|---|
| Untreated with MAP | - | - | 14 | 23.97 ± 0.15 a3 | 12.86 ± 1.57 a | 5.08 ± 0.56 a | 119.45 ± 2.51 a | 0 |
| Untreated without MAP | 23.53 ± 0.73 a | 13.63 ± 1.36 a | 6.73 ± 0.61 a | 119.69 ± 4.49 a | 2 | |||
| EF 15 g/m3 with MAP | 2 | 11.88 | 25.40 ± 0.60 a | 15.76 ± 1.18 a | 5.16 ± 0.44 a | 117.00 ± 1.52 a | 0 | |
| EF 15 g/m3 without MAP | 25.03 ± 0.58 a | 14.20 ± 1.07 a | 6.94 ± 0.50 a | 115.08 ± 3.04 a | 2 | |||
| Untreated with MAP | - | - | 28 | 20.05 ± 0.05 a | 16.33 ± 3.28 a | 11.41 ± 1.19 a | 113.65 ± 1.35 a | 0 |
| Untreated without MAP | 7.7 ± 2.28 b | 15.33 ± 2.13 a | 15.77 ± 1.53 a | 108.62 ± 6.48 a | 2 | |||
| EF 15 g/m3 with MAP | 2 | 11.88 | 22.89 ± 1.84 a | 16.43 ± 4.86 a | 13.60 ± 0.71 a | 112.64 ± 1.74 a | 0 | |
| EF 15 g/m3 without MAP | 8.52 ± 0.95 b | 16.73 ± 1.20 a | 14.03 ± 1.04 a | 99.13 ± 3.00 a | 2 |
| EF Dosage (g/m3) with or without MAP 1 | Fumigation Time (h) | Ct Products (g·h/m3) | Storage Period (d) | Firmness (kgf, Mean ± SE) | Sugar Content (Brix, %, Mean ± SE) | Weight Loss (Mean ± SE) | Color Change (Mean ± SE) | External Damage 2 |
|---|---|---|---|---|---|---|---|---|
| Untreated with MAP | - | - | 14 | 24.11 ± 0.14 a3 | 13.87 ± 1.44 a | 4.79 ± 0.15 a | 121.40 ± 1.41 a | 0 |
| Untreated without MAP | 24.53 ± 0.66 a | 13.15 ± 0.36 a | 5.73 ± 0.71 a | 119.23 ± 3.40 a | 2 | |||
| EF 8 g/m3 with MAP | 2 | 8.77 | 23.40 ± 0.31 a | 14.76 ± 1.09 a | 5.36 ± 0.54 a | 117.46 ± 2.51 a | 0 | |
| EF 8 g/m3 without MAP | 24.03 ± 0.19 a | 13.20 ± 2.07 a | 6.51 ± 0.40 a | 116.18 ± 2.84 a | 2 | |||
| Untreated with MAP | - | - | 28 | 21.15 ± 0.25 a | 15.33 ± 3.10 a | 12.44 ± 1.40 a | 114.60 ± 1.90 a | 0 |
| Untreated without MAP | 7.1 ± 1.22 b b | 15.89 ± 1.13 a | 13.70 ± 1.61 a | 118.60 ± 3.40 a | 2 | |||
| EF 8 g/m3 with MAP | 2 | 8.77 | 24.49 ± 0.94 a | 15.87 ± 3.61 a | 13.13 ± 1.70 a | 113.61 ± 2.70 a | 0 | |
| EF 8 g/m3 without MAP | 8.12 ± 1.91 b | 16.12 ± 0.91 a | 14.24 ± 1.21 a | 110.33 ± 2.08 a | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Kim, D.; Kwon, T.H.; Lee, B.-H.; Lee, S.-E. Effective Phytosanitary Treatment for Export of Oriental Melons (Cucumis melo var L.) Using Ethyl Formate and Modified Atmosphere Packaging to Control Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Insects 2023, 14, 442. https://doi.org/10.3390/insects14050442
Kim K, Kim D, Kwon TH, Lee B-H, Lee S-E. Effective Phytosanitary Treatment for Export of Oriental Melons (Cucumis melo var L.) Using Ethyl Formate and Modified Atmosphere Packaging to Control Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Insects. 2023; 14(5):442. https://doi.org/10.3390/insects14050442
Chicago/Turabian StyleKim, Kyeongnam, Dongbin Kim, Tae Hyung Kwon, Byung-Ho Lee, and Sung-Eun Lee. 2023. "Effective Phytosanitary Treatment for Export of Oriental Melons (Cucumis melo var L.) Using Ethyl Formate and Modified Atmosphere Packaging to Control Trialeurodes vaporariorum (Hemiptera: Aleyrodidae)" Insects 14, no. 5: 442. https://doi.org/10.3390/insects14050442
APA StyleKim, K., Kim, D., Kwon, T. H., Lee, B.-H., & Lee, S.-E. (2023). Effective Phytosanitary Treatment for Export of Oriental Melons (Cucumis melo var L.) Using Ethyl Formate and Modified Atmosphere Packaging to Control Trialeurodes vaporariorum (Hemiptera: Aleyrodidae). Insects, 14(5), 442. https://doi.org/10.3390/insects14050442









