Morphological Characterstics of the Sensilla in a Monophagous Insect: Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae, Halticinae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection
2.2. Scanning Electron Microscopy (SEM) Procedures
2.3. Statistical Analysis
3. Results
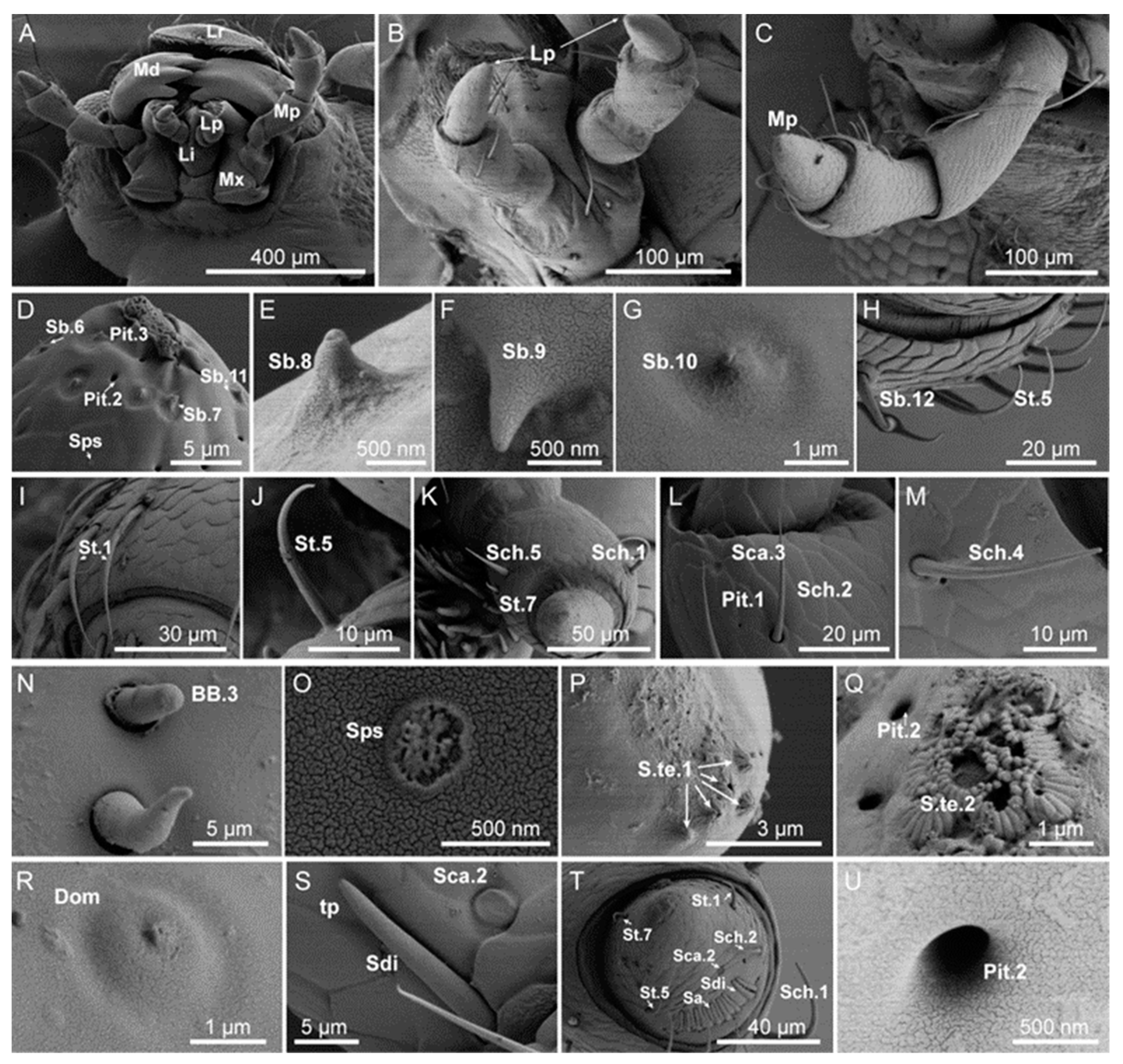
3.1. Typology, Characteristics, and Distribution of Sensilla in the Head Appendages
3.1.1. Sensilla Chaetica (Sch)
3.1.2. Sensilla Trichodea (St)
3.1.3. Sensilla Basiconica (Sb)
3.1.4. Sensilla Coeloconica (Sco)
3.1.5. Sensilla Styloconica (Sty)
3.1.6. Böhm Bristles (BB)
3.1.7. Sensilla Campaniformia (Sca)
3.1.8. Sensilla Terminal (S.te)
3.1.9. Sensilla Dome (Dom)
3.1.10. Sensilla Digit-like (Sdi)
3.1.11. Sensilla Aperture (Sa)
3.1.12. Sensilla Petal-Shaped (Sps)
3.2. Typology, Characteristics, and Distribution of Sensilla on the Tarsus
3.3. Typology, Characteristics, and Distribution of Sensilla in the External Genital Segments
4. Discussion
4.1. Types and Functions of Sensilla
4.2. Sexual Dimorphism of A. hygrophila Adults
4.3. Sensilla and Monophagy of A. hygrophila Adults
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Tarsus | Types | Sex | Max Length | Min Length | Max Basal Width | Min Basal Width | Coxal Cavity Width |
|---|---|---|---|---|---|---|---|
| Fore tarsus | Sch.1 (μm) | Female | 110.97 ± 0.63 * | 53.25 ± 0.48 * | 3.98 ± 0.11 | 3.83 ± 0.16 | 5.50 ± 0.08 |
| Male | 86.31 ± 0.43 * | 86.31 ± 0.43 * | 4.21 ± 0.09 | 4.21 ± 0.09 | 5.31 ± 0.19 | ||
| Sch.2 (μm) | Female | 117.99 ± 0.68 * | 52.04 ± 0.37 * | 2.99 ± 0.09 * | 2.13 ± 0.06 * | 5.19 ± 0.08 * | |
| Male | 61.98 ± 0.32 * | 35.51 ± 0.30 * | 2.59 ± 0.13 * | 3.02 ± 0.15 * | 4.08 ± 0.07 * | ||
| St.1 (μm) | Female | 60.78 ± 0.37 * | 33.57 ± 0.46 * | 3.63 ± 0.08 * | — | 4.57 ± 0.12 * | |
| Male | 52.19 ± 0.27 * | 37.99 ± 0.32 * | 1.83 ± 0.05 * | — | 3.41 ± 0.11 * | ||
| Sb.9 (nm) | Female | 1898.68 ± 91.81 * | 602.38 ± 7.60 * | 309.89 ± 2.61 * | 267.58 ± 10.74 * | — | |
| Male | 1197.83 ± 31.10 * | 476.93 ± 11.08 * | 435.51 ± 23.36 * | 219.40 ± 3.87 * | — | ||
| Pit.1 (μm) | Female | — | — | 1.11 ± 0.05 * | — | — | |
| Male | — | — | 0.80 ± 0.05 * | — | — | ||
| Mid-tarsus | Sch.1 (μm) | Female | 69.06 ± 0.46 * | 47.46 ± 0.65 * | 5.13 ± 0.07 * | 2.45 ± 0.09 * | 6.98 ± 0.06 |
| Male | 110.84 ± 0.60 * | 82.16 ± 0.32 * | 4.73 ± 0.08 * | 3.11 ± 0.17 * | 7.02 ± 0.09 | ||
| Sch.2 (μm) | Female | 66.92 ± 0.48 * | 53.45 ± 0.93 * | 2.83 ± 0.08 * | 2.83 ± 0.08 * | 3.23 ± 0.06 * | |
| Male | 64.70 ± 0.16 * | 38.55 ± 0.35 * | 3.29 ± 0.06 * | 2.36 ± 0.10 * | 4.12 ± 0.06 * | ||
| St.1 (μm) | Female | 74.22 ± 0.24 | 16.37 ± 0.26 * | 2.70 ± 0.09 * | — | 3.25 ± 0.09 * | |
| Male | 75.46 ± 0.47 | 14.52 ± 0.25 * | 3.14 ± 0.08 * | 2.33 ± 0.07 | 4.03 ± 0.13 * | ||
| Sb.9 (nm) | Female | 1467.82 ± 11.42 * | 890.08 ± 29.87 * | 587.50 ± 6.71 * | 395.50 ± 7.52 | — | |
| Male | 2750.86 ± 42.64 * | 288.66 ± 11.09 * | 1193.30 ± 35.90 * | 377.46 ± 7.72 | — | ||
| Pit.1 (μm) | Female | — | — | 0.98 ± 0.05 | — | — | |
| Male | — | — | 1.01 ± 0.05 | — | — | ||
| Hind tarsus | Sch.1 (μm) | Female | 121.92 ± 1.92 * | 68.13 ± 0.51 * | 4.37 ± 0.19 | 3.86 ± 0.09 * | 6.54 ± 0.09 * |
| Male | 129.17 ± 2.00 * | 86.31 ± 0.59 * | 3.94 ± 0.08 | 5.86 ± 0.15 * | 7.28 ± 0.06 * | ||
| Sch.2 (μm) | Female | 46.20 ± 0.58 * | — | 2.07 ± 0.08 * | — | 3.52 ± 0.11 * | |
| Male | 111.20 ± 1.80 * | 41.20 ± 0.99 | 4.21 ± 0.13 * | 2.56 ± 0.09 | 6.38 ± 0.27 * | ||
| St.1 (μm) | Female | 48.79 ± 2.14 * | 44.26 ± 0.30 * | 2.52 ± 0.08 | — | 3.69 ± 0.15 | |
| Male | 74.75 ± 0.58 * | 51.75 ± 0.58 * | 2.65 ± 0.08 | — | 3.63 ± 0.11 | ||
| Sb.9 (nm) | Female | 1030.73 ± 21.15 * | 193.97 ± 4.84 * | 383.72 ± 5.61 * | — | 313.27 ± 5.13 * | |
| Male | 974.90 ± 13.64 * | 177.93 ± 4.61 * | 706.64 ± 17.89 * | — | 232.08 ± 12.80 * | ||
| Pit.1 (nm) | Female | — | — | 872.45 ± 25.72 * | — | — | |
| Male | — | — | 950.49 ± 26.55 * | — | — |
| Tarsus | Segment | Sex | Ch1 | Ch.2 | St.1 | Sb.9 | Pit.1 |
|---|---|---|---|---|---|---|---|
| Fore tarsus | 1 | Female | 4.00 ± 0 | 59.20 ± 1.07 * | 54.40 ± 0.71 * | 0 | 2.35 ± 0.13 |
| Male | 8.00 ± 0 | 47.70 ± 1.13 * | 88.50 ± 0.65 * | 0 | 1.73 ± 0.08 | ||
| 2 | Female | 4.00 ± 0 | 41.50 ± 0.86 * | 39.25 ± 0.63 | 0 | 1.9 ± 0.4 | |
| Male | 4.00 ± 0 | 35.60 ± 0.73 * | 42.00 ± 0.71 | 0 | 1.95 ± 0.06 | ||
| 3 | Female | 4.00 ± 0 | 11.60 ± 0.4 * | 49.17 ± 0.65 | 0 | 2.93 ± 0.05 * | |
| Male | 2.00 ± 0 | 30.20 ± 0.78 * | 44.25 ± 1.31 | 0 | 1.85 ± 0.06 * | ||
| 5 | Female | 6.00 ± 0 | 8.00 ± 0 | 10.00 ± 0 | 12.00 ± 0.81 | 1.02 ± 0.06 * | |
| Male | 8.00 ± 0 | 6.00 ± 0 | 8.00 ± 0 | 16.75 ± 0.48 | 0.75 ± 0.06 * | ||
| Pretarsus | Female | 0 | 0 | 0 | 74.25 ± 1.1 | 0 | |
| Male | 0 | 0 | 0 | 63.75 ± 0.48 | 0 | ||
| Mid-tarsus | 1 | Female | 10.00 ± 0 | 54.75 ± 0.48 * | 62 ± 0.63 * | 0 | 1.45 ± 0.06 * |
| Male | 14.00 ± 0 | 44.00 ± 0.41 * | 55.25 ± 0.48 * | 0 | 1.03 ± 0.06 * | ||
| 2 | Female | 8.00 ± 0 | 27.75 ± 0.48 * | 43.00 ± 0 * | 0 | 0.55 ± 0.06 * | |
| Male | 8.00 ± 0 | 42.25 ± 0.48 * | 52.25 ± 0.48 * | 0 | 2.00 ± 0.04 * | ||
| 3 | Female | 8.00 ± 0 | 61.25 ± 0.48 * | 30.50 ± 0 * | 0 | 0.90 ± 0.04 * | |
| Male | 6.00 ± 0 | 28.75 ± 0.48 * | 49.75 ± 0.48 * | 0 | 1.25 ± 0.03 * | ||
| 5 | Female | 6.00 ± 0 | 6.00 ± 0 | 6.00 ± 0 | 24.75 ± 0.48 * | 1.30 ± 0.04 * | |
| Male | 8.00 ± 0 | 6.00 ± 0 | 6.00 ± 0 | 18.75 ± 0.48 * | 0.53 ± 0.05 * | ||
| Pretarsus | Female | 0 | 0 | 0 | 136.00 ± 0.41 * | 0 | |
| Male | 0 | 0 | 0 | 157.75 ± 0.63 * | 0 | ||
| Hind tarsus | 1 | Female | 10.00 ± 0 | 39.25 ± 1.1 | 86.75 ± 0.85 | 0 | 1.02 ± 0.09 * |
| Male | 10.00 ± 0 | 45.5 ± 1.04 | 91.25 ± 0.85 | 0 | 1.39 ± 0.05 * | ||
| 2 | Female | 4.00 ± 0 | 34.50 ± 1.44 * | 50.50 ± 1.04 * | 0 | 1.99 ± 0.07 * | |
| Male | 4.00 ± 0 | 29.50 ± 0.65 * | 60.00 ± 0.91 * | 0 | 2.56 ± 0.07 * | ||
| 3 | Female | 6.00 ± 0 | 29.75 ± 1.11 * | 35.25 ± 0.85 * | 0 | 1.27 ± 0.05 | |
| Male | 6.00 ± 0 | 23.75 ± 1.31 * | 47.75 ± 1.03 * | 0 | 1.19 ± 0.05 | ||
| 5 | Female | 6.00 ± 0 | 4.00 ± 0 | 6.00 ± 0 | 26.25 ± 1.31 * | 0.56 ± 0.02 * | |
| Male | 6.00 ± 0 | 2.00 ± 0 | 8.00 ± 0 | 21.50 ± 1.32 * | 0.88 ± 0.05 * | ||
| Pretarsus | Female | 0 | 0 | 0 | 213.25 ± 1.89 * | 0 | |
| Male | 0 | 0 | 0 | 235.50 ± 2.72 * | 0 |
| Type | Sex | Ventral | Reverse | ||||
|---|---|---|---|---|---|---|---|
| Segment7 | Segment.8 | Segment.9 | Segment.7 | Segment.8 | Segment.9 | ||
| Sch.1 | Female | 0 | 3.03 ± 0.22 | 0 | 3.07 ± 0.21 | 0 | 0 |
| Male | 0 | 0 | 0 | 0 | 1.13 ± 0.12 | 0 | |
| St.1 | Female | 3.77 ± 0.74 * | 25.36 ± 0.36 * | 0 | 13.95 ± 0.35 * | 9.85 ± 0.24 * | 0 |
| Male | 10.67 ± 0.40 * | 13.09 ± 0.32 * | 0 | 17.05 ± 0.3 * | 11.95 ± 0.27 * | 0 | |
| St.5 | Female | 3.14 ± 0.20 | 9.35 ± 0.33 * | 0 | 0 | 0 | 0 |
| Male | 4.45 ± 0.32 * | 0 | 2.17 ± 0.14 | 7.60 ± 0.28 | 0 | ||
| St.6 | Female | 9.05 ± 0.29 | 0 | 0 | 5.31 ± 0.26 * | 1.02 ± 0.10 * | 0 |
| Male | 8.06 ± 0.31 | 2.15 ± 0.24 | 0 | 4.34 ± 0.31 * | 11.00 ± 0.38 * | 0 | |
| St.8 | Female | 0 | 0 | 0 | 0 | 0 | 0 |
| Male | 0 | 0 | 0 | 0 | 0 | 40.03 ± 0.51 | |
| Sb.9 | Female | 0 | 0 | 32.00 ± 0.47 | 0 | 0 | 36.50 ± 0.50 * |
| Male | 0 | 0 | 28.60 ± 0.47 | 0 | 0 | 46.20 ± 0.55 * | |
| Pit.1 | Female | 2.93 ± 0.07 * | 1.56 ± 0.12 | 13.80 ± 0.53 | 0.94 ± 0.07 * | 1.66 ± 0.07 | 14.70 ± 0.45 |
| Male | 3.77 ± 0.11 * | 1.85 ± 0.07 | 18.20 ± 0.57 | 1.64 ± 0.07 * | 1.54 ± 0.06 | 16.00 ± 0.52 | |
References
- Julien, M.H.; Skarratt, B.; Maywald, G.F. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. J. Aquat. Plant Manag. 1995, 33, 55–60. [Google Scholar]
- Sainty, G.; McCorkelle, G.; Julien, M. Control and spread of Alligator Weed Alternanthera philoxeroides (Mart.) Griseb., in Australia: Lessons for other regions. Wetl. Ecol. Manag. 1997, 5, 195–201. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, L.; Ma, R.; Zhang, P.; Fan, R.; Zhang, J. Performance of the biological control agent flea beetle Agasicles hygrophila (Coleoptera: Chrysomelidae), on two plant species Alternanthera philoxeroides (alligatorweed) and A. sessilis (joyweed). Biol. Control 2010, 54, 9–13. [Google Scholar] [CrossRef]
- Lu, J.J.; Zhao, L.L.; Ma, R.Y.; Fan, R.J.; Zhang, J.T.; Wang, R. Non-target plant testing of the flea beetle Agasicles hygrophila, a biological control agent for Alternanthera philoxeroides (alligatorweed) in China. Biocontrol Sci. Technol. 2012, 22, 1093–1097. [Google Scholar] [CrossRef]
- Zhao, L.L.; Lu, J.J.; Hu, S.Q.; Li, N.; Ma, R.Y. The fitness of Agasicles hygrophila on several non-target plant species. J. Plant Prot. 2013, 40, 350–354. [Google Scholar]
- Pei, Y.H.; Kong, F.; Han, G.H.; Sun, L.G.; Sun, X.G. The research development on the feeding behavior of insects. J. Shandong For. Sci. Technol. 2007, 6, 97–101. [Google Scholar]
- Qin, J.D.; Wang, C.Z. The relation of interaction between insects and plants to evolution. Acta Entomol. Sin. 1987, 44, 360–365. [Google Scholar]
- Miller, J.R.; Stricker, K.L. Finding and accepting host plants. In The Chemical Ecology of Insects; Bell, W.J., Cardé, R.T., Eds.; Campman and Hall: London, UK, 1984; Chapter 6; pp. 125–157. [Google Scholar]
- Zacharuk, R.Y. Ultrastructure and Function of Insect Chemosensilla. Annu. Rev. Èntomol. 1980, 25, 27–47. [Google Scholar] [CrossRef]
- Silva, D.S.; Barp, E.; Kucharski, L.C.R.; Moreira, G.R.P. Sensing the Plant Surface Prior to Feeding and Oviposition: Differences in External Ultrastructure and Function among Tarsi of Heliconius erato. Neotrop. Èntomol. 2018, 47, 85–95. [Google Scholar] [CrossRef]
- Mitchell, B.K.; Low, R. The structure of feeding behavior in the colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). J. Insect Behav. 1994, 7, 707–729. [Google Scholar] [CrossRef]
- De Boer, G. The role of the antennae and maxillary palps in mediating food preference by larvae of the tobacco hornworm, Manduca sexta. Èntomol. Exp. Appl. 2006, 119, 29–38. [Google Scholar] [CrossRef]
- Maher, N.; Thiery, D. Distribution of chemo- and mechanoreceptors on the tarsi and ovipositor of female European grapevine moth, Lobesia botrana. Èntomol. Exp. Appl. 2004, 110, 135–143. [Google Scholar] [CrossRef]
- Acebes, A.; Cobb, M.; Ferveur, J.-F. Species-specific effects of single sensillum ablation on mating position in Drosophila. J. Exp. Biol. 2003, 206, 3095–3100. [Google Scholar] [CrossRef]
- Dweck, H.K.; Gadallah, N.S.; Darwish, E. Structure and sensory equipment of the ovipositor of Habrobracon hebetor (Say) (Hymenoptera: Braconidae). Micron 2008, 39, 1255–1261. [Google Scholar] [CrossRef]
- Kim, L.; Teiji, S. Sensilla on the External Genitalia of the Carabid Beetle, Carabus (Ohomopterus) dehaanii dehaanii Chaudoir (Coleoptera, Carabidae). Èntomol. Res. 2004, 34, 151–155. [Google Scholar] [CrossRef]
- Spänhoff, B.; Alecke, C.; Kaschek, N.; Lange, J.; Meyer, E.I. Morphological characteristics of sensilla on the female ovipositor of Lype phaeopa (Psychomyiidae; Trichoptera). J. Insect Sci. 2003, 3, 1–7. [Google Scholar] [CrossRef]
- Uhía, E.; Cordero Rivera, A. Male damselflies detect female mating status: Importance for postcopulatory sexual selection. Anim. Behav. 2005, 69, 797–804. [Google Scholar] [CrossRef]
- Li, N.; Li, S.; Ge, J.; Schuman, M.C.; Wei, J.-N.; Ma, R.-Y. Manipulating two olfactory cues causes a biological control beetle to shift to non-target plant species. J. Ecol. 2017, 105, 1534–1546. [Google Scholar] [CrossRef]
- Fu, S.H. Gene Cloning and Preliminary Functional Study of the Odor Binding Protein in Agasicles hygrophila (Coleoptera: Chrysomelidae). Master’s Thesis, Shanxi Agricultural University, Jinzhong, China, 2016. [Google Scholar]
- Hu, J.; Fu, L.; Zhang, B.; Liu, Y.H.; Jia, D.; Ma, R.Y. Identification of odorant-binding proteins and their expression in the antennae of alligator weed flea beetle Agasicles hygrophila. J. Plant Prot. 2019, 46, 849–859. [Google Scholar]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Antennae and sensilla. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 6, pp. 1–69. [Google Scholar]
- Dyer, L.J.; Seabrook, W.D. Sensilla on the antennal flagellum of the sawyer beetlesMonochamus notatus (Drury) and Monochamus scutellatus (Say) (Coleoptera: Cerambycidae). J. Morphol. 1975, 146, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Crook, D.J.; Higgins, R.A.; Ramaswamy, S.B. Antennal morphology of the soybean stemborer Dectes texanus texanus LeConte (Coleoptera: Cerambycidae). J. Kans. Entomol. Soc. 2003, 76, 397–405. [Google Scholar]
- Tarumingkeng, R.; Coppel, H.; Matsumura, F. Morphology and ultrastructure of the antennal chemoreceptors and mechanoreceptors of worker Coptotermes formosanus Shiraki. Cell Tissue Res. 1976, 173, 173–178. [Google Scholar] [CrossRef]
- Shanbhag, S.R.; Müller, B.; Steinbrecht, R.A. Atlas of olfactory organs of Drosophila melanogaster 1. Types, external organization, innervation, and distribution of olfactory sensilla. Int. J. Insect Morphol. Embryol. 1999, 28, 377–397. [Google Scholar] [CrossRef]
- Lopes, O.; Barata, E.N.; Mustaparta, H.; Araújo, J. Fine structure of antennal sensilla basiconica and their detection of plant volatiles in the eucalyptus woodborer, Phoracantha semipunctata Fabricius (Coleoptera: Cerambycidae). Arthropod Struct. Dev. 2002, 31, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stacconi, M.V.R.; Romani, R. Antennal sensory structures in Scaphoideus titanus ball (Hemiptera: Cicadellidae). Microsc. Res. Tech. 2012, 75, 458–466. [Google Scholar] [CrossRef]
- Guo, X.; Yu, Q.; Chen, D.; Wei, J.; Yang, P.; Yu, J.; Wang, X.; Kang, L. 4-Vinylanisole is an aggregation pheromone in locusts. Nature 2020, 584, 584–588. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Zhang, L.; Xu, X.; Cao, Z.; Zhang, L. Expressions of Olfactory Proteins in Locust Olfactory Organs and a Palp Odorant Receptor Involved in Plant Aldehydes Detection. Front. Physiol. 2018, 9, 663. [Google Scholar] [CrossRef]
- Inoue, T.A.; Asaoka, K.; Seta, K.; Imaeda, D.; Ozaki, M. Sugar receptor response of the food-canal taste sensilla in a nectar-feeding swallowtail butterfly, Papilio xuthus. Nat. Sci. Nat.-Heidelberg. 2009, 96, 355–363. [Google Scholar] [CrossRef]
- Wang, X.M.; Wang, H.P.; Lu, S.X.; Yang, F.Y.; Zhang, Z. Study on the host selection process of Carposina niponensis oviposition. Hubei Agric. Sci. 2011, 50, 2236–2239. [Google Scholar]
- Xu, Y.C.; Li, D.X.; Wang, H.W.; Wang, T.T. Effects of temperature and humidity on the pupae and adults of peach fruit moth, Carposina sasakii. Plant Prot. 2015, 41, 50–53+67. [Google Scholar]
- Hu, F.; Zhang, G.-N.; Wang, J.-J. Scanning electron microscopy studies of antennal sensilla of bruchid beetles, Callosobruchus chinensis (L.) and Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Micron 2008, 40, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.F.; Wang, G.L.; Liu, J.W.; Wu, Q.Y.; Zhu, Y.P. Antennal Morphology and Sensilla of Asian Multicolored Ladybird Beetles, Harmonia axyridis Pallas (Coleoptera: Coccinellidae). Èntomol. News 2009, 120, 137–152. [Google Scholar] [CrossRef]
- Ren, L.; Shi, J.; Zhang, Y.; Luo, Y. Antennal morphology and sensillar ultrastructure of Dastarcus helophoroides (Fairmaire) (Coleoptera: Bothrideridae). Micron 2012, 43, 921–928. [Google Scholar] [CrossRef]
- Li, R.T.; Huang, L.Q.; Dong, J.F.; Wang, C.Z. A moth odorant receptor highly expressed in the ovipositor is involved in detecting host-plant volatiles. eLife 2020, 9, e53706. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liang, Q.C.; Wu, W.J.; Huang, J. Ultrastructural studies on sensilla of Quadrastichus erythrinak Kim (Hymenoptera: Eulophidae) adult. J. South China Agric. Univ. 2007, 28, 52–55. [Google Scholar]
- Isidoro, N.; Solinas, M. Functional morphology of the antennal chemosensilla of Ceutorhynchus assimilis Payk. (Coleoptera: Curculionidae). Entomologica 1992, 27, 69–84. [Google Scholar]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Radek, R.; Adler, C. Structure and distribution of antennal sensilla in the Indianmeal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2014, 59, 66–75. [Google Scholar] [CrossRef]
- Ma, L.; Bian, L.; Li, Z.Q.; Cai, X.M.; Luo, Z.X.; Chen, Z.M. Ultrastructure of chemosensilla on antennae and tarsi of Ectropis obliqu (Lepidoptera: Geometridae). Ann. Entomol. Soc. Am. 2016, 109, 574–584. [Google Scholar] [CrossRef]
- Jiang, X.-J.; Ning, C.; Guo, H.; Jia, Y.-Y.; Huang, L.-Q.; Qu, M.-J.; Wang, C.-Z. A gustatory receptor tuned to d-fructose in antennal sensilla chaetica of Helicoverpa armigera. Insect Biochem. Mol. Biol. 2015, 60, 39–46. [Google Scholar] [CrossRef]
- Bartlet, E.; Romani, R.; Williams, I.H.; Isidoro, N. Functional anatomy of sensory structures on the antennae of Psylliodes chrysocephala L. (Coleoptera: Chrysomelidae). Int. J. Insect Morphol. Embryol. 1999, 28, 291–300. [Google Scholar] [CrossRef]
- Merivee, E.; Ploomi, A.; Rahi, M.; Bresciani, J.; Ravn, H.P.; Luik, A.; Sammelselg, V. Antennal sensilla of the ground beetle Bembidion properans Steph. (Coleoptera, Carabidae). Micron 2002, 33, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Prabhakar, S.; Sudarsan, S.; Sane, S.P. The neural mechanisms of antennal positioning in flying moths. J. Exp. Biol. 2012, 215, 3096–3105. [Google Scholar] [CrossRef]
- Merivee, E.; Ploomi, A.; Rahi, M.; Luik, A.; Sammelselg, V. Antennal sensilla of the ground beetle Bembidion lampros Hbst (Coleoptera, Carabidae). Acta Zool. 2000, 81, 339–350. [Google Scholar] [CrossRef]
- Merivee, E.; Vanatoa, A.; Luik, A.; Rahi, M.; Sammelselg, V.; Ploomi, A. Electrophysiological identification of cold receptors on the antennae of the ground beetle Pterostichus aethiops. Physiol. Èntomol. 2003, 28, 88–96. [Google Scholar] [CrossRef]
- Must, A.; Merivee, E.; Luik, A.; Williams, I.; Ploomi, A.; Heidemaa, M. Spike bursts generated by the thermosensitive (cold) neuron from the antennal campaniform sensilla of the ground beetle Platynus assimilis. J. Insect Physiol. 2010, 56, 412–421. [Google Scholar] [CrossRef]
- Hallberg, E. Sensory organs in Ips typographus (Insecta: Coleoptera) ? Fine structure of antennal sensilla. Protoplasma 1982, 111, 206–214. [Google Scholar] [CrossRef]
- Blum, M.S. “Alarm pheromone” in Comparative Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Britain: Oxford, UK, 1985; Volume 9, pp. 193–224. [Google Scholar]
- Ma, R.Y.; Du, J.W. Antennal sensilla in insects. Chin. J. Appl. Entomol. 2000, 37, 179–183. [Google Scholar]
- Hull, C.D.; Cribb, B.W. Ultrastructure of the antennal sensilla of Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Int. J. Insect Morphol. Embryol. 1997, 26, 27–34. [Google Scholar] [CrossRef]
- Nemeth, D.C.; Ammagarahalli, B.; Layne, J.E.; Rollmann, S.M. Evolution of coeloconic sensilla in the peripheral olfactory system of Drosophila mojavensis. J. Insect Physiol. 2018, 110, 13–22. [Google Scholar] [CrossRef]
- Yang, K.; Gong, X.L.; Li, G.C.; Huang, L.Q.; Ning, C.; Wang, C.Z. A gustatory receptor tuned to the steroid plant hormone brassinolide in Plutella xylostella (Lepidoptera: Plutellidae). eLife 2020, 9, e64114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, P.-C.; Zhang, S.-S.; Yang, J.; Li, G.-C.; Huang, L.-Q.; Wang, C.-Z. Functional analysis of a bitter gustatory receptor highly expressed in the larval maxillary galea of Helicoverpa armigera. PLoS Genet. 2022, 18, e1010455. [Google Scholar] [CrossRef] [PubMed]
- Schoonhoven, L.M.; Van Loon, J.A. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. Hung. 2002, 48, 215–263. [Google Scholar]
- Xiao, F.S.; Liu, Q. The type and characteristic of olfaction sensilla in Asias halodendri. Chin. J. Appl. Entomol. 2011, 48, 680–687. [Google Scholar]
- Ning, T.; Liu, Y.J.; Sun, J.H. The ultrastructure of sensilla on head of Monochamus alternatus adult. Chin. J. Appl. Entomol. 2004, 41, 566–571. [Google Scholar]
- Baaren, J.V.; Boivin, G.; Bourdais, D.; Roux, O. Antennal sensilla of hymenopteran parasitic wasps: Variations linked to host exploitation behavior. In Modern Research and Educational Topics in Microscopy; Mendez-Vilas, A., Dias, J., Eds.; Formatex: Badajoz, Spain, 2008; pp. 345–352. [Google Scholar]
- Merivee, E.; Ploomi, A.; Luik, A.; Rahi, M.; Sammelselg, V. Antennal sensilla of the ground beetle Platynus dorsalis (Pontoppidan, 1763) (Coleoptera, Carabidae). Microsc. Res. Tech. 2001, 55, 339–349. [Google Scholar] [CrossRef]
- Eilers, E.J.; Talarico, G.; Hansson, B.; Hilker, M.; Reinecke, A. Sensing the Underground—Ultrastructure and Function of Sensory Organs in Root-Feeding Melolontha melolontha (Coleoptera: Scarabaeinae) Larvae. PLoS ONE 2012, 7, e41357. [Google Scholar] [CrossRef]
- Honomichl, K.; Guse, G.-W. Digitiform Sensilla on the Maxillar Palp of Coleoptera. Acta Zool. 1981, 62, 17–25. [Google Scholar] [CrossRef]
- Devitt, B.; Smith, J. Morphology and fine structure of mouthpart sensilla in the dark-sided cutworm Euxoa messoria (Harris) (Lepidoptera: Noctuidae). Int. J. Insect Morphol. Embryol. 1982, 11, 255–270. [Google Scholar] [CrossRef]
- Liu, Z.P.; Li, Y.F.; Wang, J.J. Comparision of sensilla on the maxillary and labial palpus of three species of Staphylinidae (Coleoptera: Staphylinoidea). Entomotaxonomia 2008, 30, 25–30. [Google Scholar]
- Boer, G.; Dethier, V.G.; Schoonhoven, L.M. Chemoreceptors in the preoral cavity of the tobacco hornworm, Manduca sexta, and their possible function in feeding behaviour. Èntomol. Exp. Appl. 1977, 21, 287–298. [Google Scholar] [CrossRef]
- Albert, P.J. Morphology and innervation of mouthpart sensilla in larvae of the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). Can. J. Zool. 1980, 58, 842–851. [Google Scholar] [CrossRef]
- Faucheux, M.J. Sensilla on the larval antennae and mouthparts of the european sunflower moth, Homoeosoma nebulella Den. and Schiff. (Lepidoptera: Pyralidae). Int. J. Insect Morphol. Embryol. 1995, 24, 391–403. [Google Scholar] [CrossRef]
- Wang, M.Q.; Yang, D. Sexual dimorphism in insects. Chin. J. Appl. Entomol. 2005, 42, 721–725. [Google Scholar]
- Anderson, A.R.; Wanner, K.W.; Trowell, S.C.; Warr, C.; Jaquin-Joly, E.; Zagatti, P.; Robertson, H.; Newcomb, R.D. Molecular basis of female-specific odorant responses in Bombyx mori. Insect Biochem. Mol. Biol. 2009, 39, 189–197. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, Y.; Zhang, J.; Wang, G.R.; Dong, S.L. Cloning and localization of an odorant receptor gene OR18 in the antenna of Spodoptera exigua. Sci. Agric. Sin. 2013, 46, 4263–4271. [Google Scholar]
- Zhang, S.; Zhang, Y.J.; Su, H.H.; Gao, X.W.; Guo, Y.Y. Cloning and tissue-specific expression of olfactory receptors in Helicoverpa armigera (Hübner). Acta Entomol. Sin. 2009, 52, 728–735. [Google Scholar]
- Na, J.; Yu, W.X.; Li, Y.P.; Dong, X.; Jiao, J. Types and physiological ecology significance of insect antenna sensilla. J. Shenyang Norm. Univ. Nat. Sci. Ed. 2008, 26, 213–216. [Google Scholar]
- Städler, E. Behavioral responses of insects to plant secondary compounds. In Herbivores and Their Interactions with Secondary Plant Metabolites; Academic Press: Cambridge, MA, USA, 1992; Volume 2, pp. 45–88. [Google Scholar]
- Rajapakse, C.N.K.; Walter, G.H.; Moore, C.J.; Hull, C.D.; Cribb, B.W. Host recognition by a polyphagous lepidopteran (Helicoverpa armigera): Primary host plants, host produced volatiles and neurosensory stimulation. Physiol. Èntomol. 2010, 31, 270–277. [Google Scholar] [CrossRef]
- Del Campo, M.; Renwick, J.A.A. Induction of host specificity in larvae of Manduca sexta: Chemical dependence controlling host recognition and developmental rate. Chemoecology 2000, 10, 115–121. [Google Scholar] [CrossRef]
- Del Campo, M.L. Chemosensory tuning to a host recognition cue in the facultative specialist larvae of the moth Manduca sexta. J. Exp. Biol. 2003, 206, 3979–3990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, F.; Zhang, C.N.; Dai, W. Morphological characters of antenna and types of antennal sensilla of leaf beetle Luperomorpha suturalis Chen (Coleoptera:Chrysomeloidea). Acta Agric. Boreali-Occident. Sin. 2016, 25, 1567–1574. [Google Scholar]
- Zhang, F.; Hong, B.; Wang, Y.Z.; Ren, P.; Li, Y.M.; Chen, Z.J. Observation of antennal sensilla from Scythropus yasumatsui (Coleoptera: Curculionidae) with scanning electron microscope. Acta Agric. Boreali-Occident. Sin. 2019, 28, 1373–1379. [Google Scholar]
- Niklas, J.; Sören, N. The Oscillation Hypothesis of Host-Plant Range and Speciation; University of California Press: Berkeley, CA, USA, 2008; pp. 203–215. [Google Scholar]


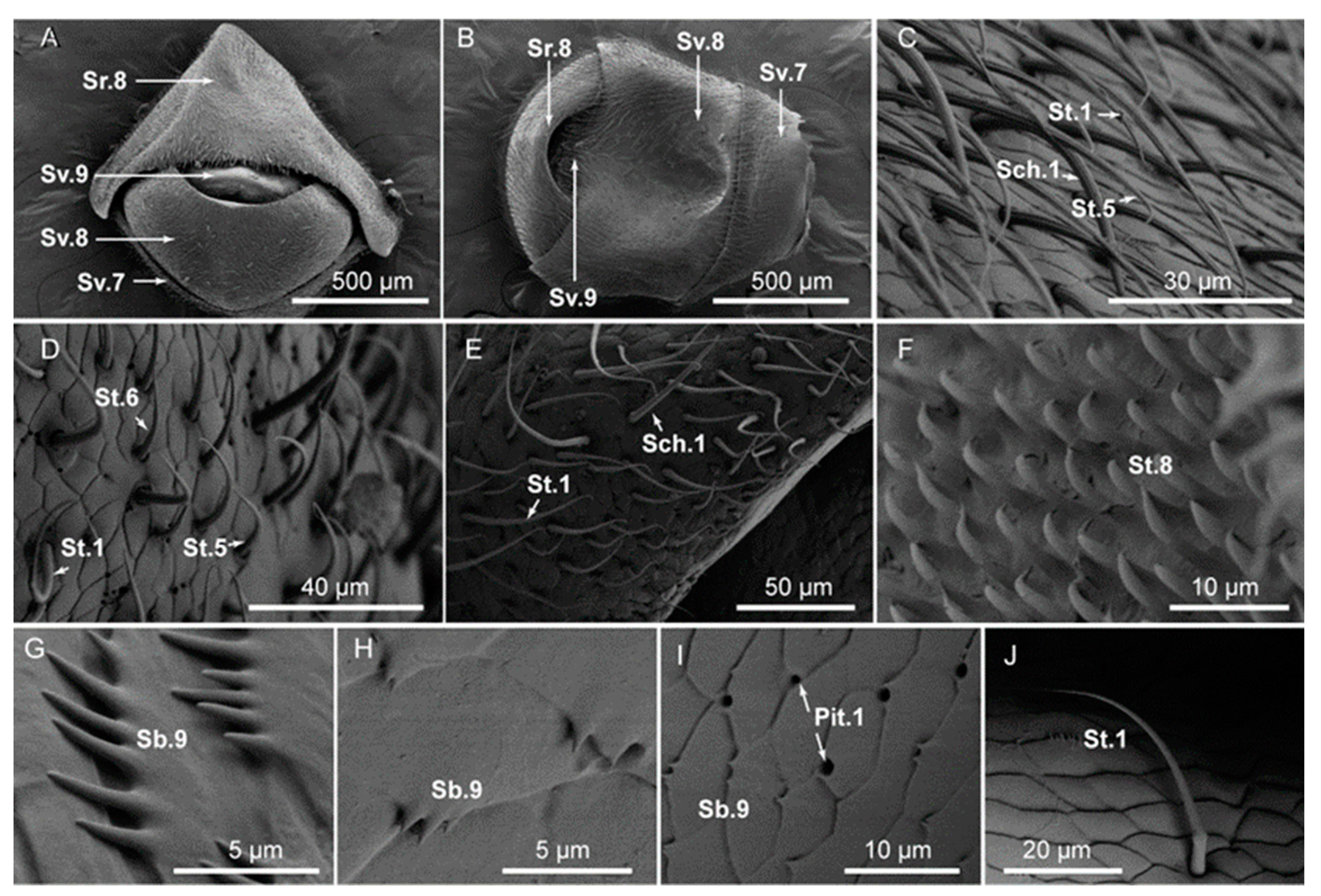
| Type of Sensillum | Gender | Number | Length (μm) | Basal Width (μm) | Subtype | Location |
|---|---|---|---|---|---|---|
| Sensilla chaeticum | Female | 803.36 * | 90.49–39.45 * | 5.65–2.37 * | 4 | S,P,F1-F9 |
| Male | 761.33 * | 80.11–17.70 * | 4.15–1.62 * | 4 | S,P,F1-F9 | |
| Sensilla trichodeum | Female | 913.07 * | 46.33–8.96 * | 3.76–2.04 * | 6 | P,F1-F9 |
| Male | 1221.67 * | 37.20–12.26 * | 3.07–2.07 * | 6 | P,F1-F9 | |
| Sensilla basiconicum | Female | 859.67 * | 14.2–6.6 * | 5.97–1.56 * | 5 | F2-F9 |
| Male | 776.33 * | 11.33–4.25 * | 2.86–1.69 | 4 (no Sb.4) | F1-F9 | |
| Sensilla coeloconica | Female | 103.33 | 4.90–3.29 * | 1.84–1.39 * | 4 (no Sb.3) | F2-F9 |
| Male | 100.33 | 2.97–2.93 * | 1.54–1.50 * | 1 (no Sco.1, Sco.2) | F4-F8 | |
| Sensilla styloconica | Female | 255.35 * | 5.44–1.18 * | 2.78–0.97 * | 5 (no Sty.3) | F2-F9 |
| Male | 181.00 * | 4.73–1.49 | 1.97–0.95 * | 5 (no Sty.2) | F2-F9 | |
| Sensilla campaniform | Female | 4.00 * | — | 2.26–1.45 * | 1 | F9 |
| Male | — | — | — | — | — |
| Type of Sensillum | Gender | Number | Length (μm) | Basal Width (μm) |
|---|---|---|---|---|
| Sensilla chaeticum | Female | 3 | 45.28–36.96 * | 5.63–1.90 * |
| Male | 4 | 53.14–27.80 * | 3.55–2.45 * | |
| Sensilla trichodeum | Female | 7 | 16.63–12.48 * | 2.01–2.00 |
| Male | 3 | 23.35–10.40 * | 2.20–2.06 | |
| Sensilla basiconicum | Female | 9 | 0.81–0.37 | 0.68–0.55 * |
| Male | 12 | 0.94–0.37 | 0.77–0.34 * | |
| Sensilla terminal | Female | 10 | 0.39–0.43 | 0.69–0.65 * |
| Male | 12 | 0.43–0.45 | 0.61–0.59 * | |
| Sensilla campaniform | Female | 2 | — | 3.06–2.92 * |
| Male | 3 | — | 3.22–3.13 * | |
| Sensilla dome | Female | — | — | — |
| Male | 2 | 0.28–0.27 | 0.31–0.30 |
| Type of Sensillum | Gender | Number | Length (μm) | Basal Width (μm) | Subtype |
|---|---|---|---|---|---|
| Sensilla chaeticum | Female | 7 | 66.20–17.04 * | 5.60–1.56 * | 3 |
| Male | 8 | 101.54–20.60 * | 3.93–1.12 * | 3 | |
| Sensilla trichodeum | Female | 42 | 52.05–9.14 * | 4.13–1.27 * | 3 |
| Male | 34 | 23.59–6.61 * | 3.23–1.39 * | 3 | |
| Sensilla basiconicum | Female | 59.23 | 0.86–0.68 | 1.16–0.41 * | 6 (no Sb.8) |
| Male | 63.47 | 0.97–0.73 | 0.90–0.59 * | 6 (no Sb.7) | |
| Sensilla terminal | Female | 14 | — | 0.51–0.38 * | 1 |
| Male | 18 | — | 0.78–0.73 * | 1 | |
| Sensilla petal-shaped | Female | 3 | — | 0.37–0.36 * | 1 |
| Male | 4 | — | 1.05–1.03 * | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, S.; Wang, Y.; Jia, D.; Wang, Y.; Ma, R. Morphological Characterstics of the Sensilla in a Monophagous Insect: Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae, Halticinae). Insects 2023, 14, 501. https://doi.org/10.3390/insects14060501
Chen Q, Li S, Wang Y, Jia D, Wang Y, Ma R. Morphological Characterstics of the Sensilla in a Monophagous Insect: Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae, Halticinae). Insects. 2023; 14(6):501. https://doi.org/10.3390/insects14060501
Chicago/Turabian StyleChen, Qianhui, Shuang Li, Yingying Wang, Dong Jia, Yuanxin Wang, and Ruiyan Ma. 2023. "Morphological Characterstics of the Sensilla in a Monophagous Insect: Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae, Halticinae)" Insects 14, no. 6: 501. https://doi.org/10.3390/insects14060501
APA StyleChen, Q., Li, S., Wang, Y., Jia, D., Wang, Y., & Ma, R. (2023). Morphological Characterstics of the Sensilla in a Monophagous Insect: Agasicles hygrophila Selman and Vogt (Coleoptera: Chrysomelidae, Halticinae). Insects, 14(6), 501. https://doi.org/10.3390/insects14060501






