Ticks and Tick-Borne Pathogens from Wild Pigs in Northern and Central Florida
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tick Collection
2.2. Pathogen Screening
2.3. Statistical Analyses
3. Results
3.1. Tick Collection
3.2. Pathogen Screening
3.3. Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halliday, J.E.B.; Meredith, A.L.; Knobel, D.L.; Shaw, D.J.; Bronsvoort, B.M.D.C.; Cleaveland, S. A framework for evaluating animals as sentinels for infectious disease surveillance. J. R. Soc. Interface 2007, 4, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Eidson, M.; Schmit, K.; Hagiwara, Y.; Anand, M.; Backenson, P.B.; Gotham, I.; Kramer, L. Dead crow density and West Nile virus monitoring, New York. Emerg. Infect. Dis. 2005, 11, 1370–1375. [Google Scholar] [CrossRef]
- Komar, N. West Nile virus surveillance using sentinel birds. Ann. N. Y. Acad. Sci. 2001, 951, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Falco, R.C.; Smith, H.A.; Fish, D.; Mojica, B.A.; Bellinger, M.A.; Hamis, H.L.; Hechemy, K.E. The distribution of canine exposure to Borrelia burgdorferi in a Lyme-disease endemic area. Am. J. Public Health 1993, 83, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Guerra, M.A.; Walker, E.; Kitron, U. Canine surveillance system for Lyme borreliosis in Wisconsin and northern Illinois: Geographic distribution and risk factor analysis. Am. J. Trop. Med. Hyg. 2001, 65, 546–552. [Google Scholar] [CrossRef] [Green Version]
- Smith, F.D.; Ballantyne, R.; Morgan, E.R.; Wall, R. Estimating Lyme disease risk using pet dogs as sentinels. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 163–167. [Google Scholar] [CrossRef]
- Hamer, S.A.; Tsao, J.I.; Walker, E.D.; Mansfield, L.S.; Foster, E.S.; Hickling, G.J. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am. J. Vet. Res. 2009, 70, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisen, R.J.; Eisen, L.; Beard, C.B. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J. Med. Entomol. 2016, 53, 349–386. [Google Scholar] [CrossRef] [Green Version]
- Ripoche, M.; Lindsay, L.R.; Ludwig, A.; Ogden, N.H.; Thivierge, K.; Leighton, P.A. Multi-scale clustering of Lyme disease risk at the expanding leading edge of the range of Ixodes scapularis in Canada. Int. J. Environ. Res. Public Health 2018, 15, 603. [Google Scholar] [CrossRef] [Green Version]
- Sonenshine, D.E. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [Green Version]
- Springer, Y.P.; Eisen, L.; Beati, L.; James, A.M.; Eisen, R.J. Spatial distribution of counties in the continental United States with records of occurrence of Amblyomma americanum (Ixodida: Ixodidae). J. Med. Entomol. 2014, 51, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Florin, D.A.; Brinkerhoff, R.J.; Gaff, H.; Jiang, J.; Robbins, R.G.; Eickmeyer, W.; Butler, J.; Nielsen, D.; Wright, C.; White, A.; et al. Additional U.S. collections of the Gulf Coast tick, Amblyomma maculatum (Acari: Ixodidae), from the state of Delaware, the first reported field collections of adult specimens from the state of Maryland, and data regarding this tick from surveillance of migratory songbirds in Maryland. Syst. Appl. Acarol. 2014, 19, 257. [Google Scholar] [CrossRef]
- Paddock, C.D.; Goddard, J. The evolving medical and veterinary importance of the Gulf Coast tick (Acari: Ixodidae). J. Med. Entomol. 2015, 52, 230–252. [Google Scholar] [CrossRef] [Green Version]
- Mays, S.E.; Houston, A.E.; Trout Fryxell, R.T. Specifying pathogen associations of Amblyomma maculatum (Acari: Ixodidae) in western Tennessee. J. Med. Entomol. 2016, 53, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef] [PubMed]
- USDA. APHIS National Haemaphysalis Longicornis (Asian Longhorned Tick) Situation Report; USDA: Washington, DC, USA, 2022. [Google Scholar]
- Graves, H.B. Behavior and ecology of wild and feral swine (Sus scrofa). J. Anim. Sci. 1984, 58, 482–492. [Google Scholar] [CrossRef]
- Bevins, S.N.; Pedersen, K.; Lutman, M.W.; Gidlewski, T.; Deliberto, T.J. Consequences associated with the recent range expansion of nonnative feral swine. Bioscience 2014, 64, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Seward, N.W.; Vercauteren, K.C.; Witmer, G.W.; Engeman, R.M. Feral swine impacts on agriculture and the environment. Sheep Goat Res. J. 2004, 19, 34–40. [Google Scholar]
- USDA. APHIS History of Feral Swine in the Americas. Available online: https://www.aphis.usda.gov/aphis/ourfocus/wildlifedamage/operational-activities/feral-swine/sa-fs-history (accessed on 2 November 2022).
- Forrester, D.J. Parasites and Diseases of Wild Mammals in Florida, 1st ed.; University Press of Florida: Gainesville, FL, USA, 1992. [Google Scholar]
- Ostfeld, R.S.; Cepeda, O.M.; Hazler, K.R.; Miller, M.C. Ecology of Lyme disease: Habitat associations of ticks (Ixodes scapularis) in a rural landscape. Ecol. Appl. 1995, 5, 353–361. [Google Scholar] [CrossRef]
- Nupp, T.E.; Swihart, R.K. Effect of forest patch area on population attributes of white-footed mice (Peromyscus leucopus) in fragmented landscapes. Can. J. Zool. 1996, 74, 467–472. [Google Scholar] [CrossRef]
- Nupp, T.E.; Swihart, R.K. Effects of forest fragmentation on population attributes of white-footed mice and eastern chipmunks. J. Mammal. 1998, 79, 1234–1243. [Google Scholar] [CrossRef] [Green Version]
- Paddock, C.D.; Yabsley, M.J. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. Curr. Top. Microbiol. Immunol. 2007, 315, 289–324. [Google Scholar]
- Allan, B.F.; Keesing, F.; Ostfeld, R.S. Effect of forest fragmentation on Lyme disease risk. Conserv. Biol. 2003, 17, 267–272. [Google Scholar] [CrossRef] [Green Version]
- Brownstein, J.S.; Skelly, D.K.; Holford, T.R.; Fish, D. Forest fragmentation predicts local scale heterogeneity of Lyme disease risk. Oecologia 2005, 146, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Cooley, R.A.; Kohls, G.M. The Genus Ixodes in North America; National Institute of Health Bulletin No. 184; National Institutes of Health: Bethesda, MD, USA, 1945. [Google Scholar]
- Keirans, J.E.; Litwak, T.R. Pictorial key to the adults of hard ticks, Family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J. Med. Entomol. 1989, 26, 435–448. [Google Scholar] [CrossRef]
- Keirans, J.E.; Durden, L.A. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 1998, 35, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ledger, K.; Beati, L.; Wisely, S. Survey of ticks and tick-borne rickettsial and protozoan pathogens in Eswatini. Pathogens 2021, 10, 1043. [Google Scholar] [CrossRef]
- Takano, A.; Ando, S.; Kishimoto, T.; Fujita, H.; Kadosaka, T.; Nitta, Y.; Kawabata, H.; Watanabe, H. Presence of a novel Ehrlichia sp. in Ixodes granulatus found in Okinawa, Japan. Microbiol. Immunol. 2009, 53, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Massung, R.F.; Slater, K.; Owens, J.H.; William, L.; Mather, T.N.; Solberg, V.B.; James, G.; Slater, K.I.M.; Nicholson, W.L.; Olson, J.G. Nested PCR sssay for detection of granulocytic Ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbour, A.G.; Maupin, G.O.; Teltow, G.J.; Carter, C.J.; Piesman, J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: Possible agent of a Lyme disease-like illness. J. Infect. Dis. 1996, 173, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Eremeeva, M.; Yu, X.; Raoult, D. Differentiation among spotted fever group Rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J. Clin. Microbiol. 1994, 32, 803–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshoo, M.W.; Carolan, H.E.; Massire, C.; Chou, D.M.; Crowder, C.D.; Rounds, M.A.; Phillipson, C.A.; Schutzer, S.E.; Ecker, D.J. Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread Anaplasmataceae species. PLoS ONE 2015, 10, e0135828. [Google Scholar] [CrossRef] [PubMed]
- Friebel, B.A.; Jodice, P.G.R. Home range and habitat use of feral hogs in Congaree National Park, South Carolina. Hum. Wildl. Confl. 2009, 3, 49–63. [Google Scholar]
- Franckowiak, G.A.; Poché, R.M.; Poché, P. Short-term home range and habitat selection by feral hogs in northern Texas. Am. Midl. Nat. 2018, 179, 28–37. [Google Scholar] [CrossRef]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. Landscapemetrics: An open-source R tool to calculate landscape metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Oksanen, J.; Guillaume Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 20 December 2021).
- Borcard, D.; Francois, G.; Legendre, P. Exploratory Data Analysis. In Numerical Ecology with R; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Shadish, W.R.; Zuur, A.F.; Sullivan, K.J. Using generalized (additive) mixed models to analyze single case designs. J. Sch. Psychol. 2014, 52, 149–178. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. Glmmtmb balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Barton, K. MuMIn: Multi-Model Inference; R Package Version 1.43.15. 2019. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 20 December 2021).
- Akaike, H. On the likelihood of a time series model. J. R. Stat. Soc. 1978, 27, 217–235. [Google Scholar] [CrossRef]
- Lüdecke, D. Ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Hartig, F. Dharma: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; R Package Version 0.3.3.0. 2020. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 20 December 2021).
- Bjornstad, O.N. ncf: Spatial Covariance Functions; R Package Version 1.2-9. 2020. Available online: https://CRAN.R-project.org/package=ncf (accessed on 20 December 2021).
- De Jesus, C.E.; Ganser, C.; Kessler, W.H.; White, Z.S.; Bhosale, C.R.; Glass, G.E.; Wisely, S.M. A survey of tick-borne bacterial pathogens in Florida. Insects 2019, 10, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, G.E.; Ganser, C.; Wisely, S.M.; Kessler, W.H. Standardized Ixodid tick survey in mainland Florida. Insects 2019, 10, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allan, S.A.; Simmons, L.A.; Burridge, M.J. Ixodid ticks on white-tailed deer and feral swine in Florida. J. Vector Ecol. 2001, 26, 93–102. [Google Scholar] [PubMed]
- Burroughs, J.E.; Thomasson, J.A.; Marsella, R.; Greiner, E.C.; Allan, S.A. Ticks associated with domestic dogs and cats in Florida, USA. Exp. Appl. Acarol. 2016, 69, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Hertz, J.C.; Ferree Clemons, B.C.; Lord, C.C.; Allan, S.A.; Kaufman, P.E. Distribution and host associations of Ixodid ticks collected from wildlife in Florida, USA. Exp. Appl. Acarol. 2017, 73, 223–236. [Google Scholar] [CrossRef]
- Merrill, M.M.; Boughton, R.K.; Lord, C.C.; Sayler, K.A.; Wight, B.; Anderson, W.M.; Wisely, S.M. Wild pigs as sentinels for hard ticks: A case study from south-central Florida. Int. J. Parasitol. Parasites Wildl. 2018, 7, 161–170. [Google Scholar] [CrossRef]
- Mixson, T.R.; Campbell, S.R.; Gill, J.S.; Ginsberg, H.S.; Reichard, M.V.; Schulze, T.L.; Dasch, G.A. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J. Med. Entomol. 2006, 43, 1261–1268. [Google Scholar] [CrossRef]
- Hertz, J.C. Ehrlichia and Rickettsia Tick-Borne Infections Associated with Lone Star Ticks and Under-Sampled Wildlife Hosts in Florida. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2016. [Google Scholar]
- Sayler, K.A.; Loftis, A.D.; Beatty, S.K.; Boyce, C.L.; Garrison, E.; Clemons, B.; Cunningham, M.; Alleman, A.R.; Barbet, A.F. Prevalence of tick-borne pathogens in host-seeking Amblyomma americanum (Acari: Ixodidae) and Odocoileus virginianus (Artiodactyla: Cervidae) in Florida. J. Med. Entomol. 2016, 53, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Trout Fryxell, R.T.; Moore, J.E.; Collins, M.D.; Kwon, Y.; Jean-Philippe, S.R.; Schaeffer, S.M.; Odoi, A.; Kennedy, M.; Houston, A.E. Habitat and vegetation variables are not enough when predicting tick populations in the southeastern United States. PLoS ONE 2015, 10, e0144092. [Google Scholar] [CrossRef]
- Mays, S.E.; Houston, A.E.; Trout Fryxell, R.T. Comparison of novel and conventional methods of trapping Ixodid ticks in the southeastern U.S.A. Med. Vet. Entomol. 2016, 30, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Kessler, W.H.; Blackburn, J.K.; Sayler, K.A.; Glass, G.E. Estimating the geographic distribution of host-seeking adult Amblyomma americanum (Acari: Ixodidae) in Florida. J. Med. Entomol. 2018, 56, 55–64. [Google Scholar] [CrossRef]
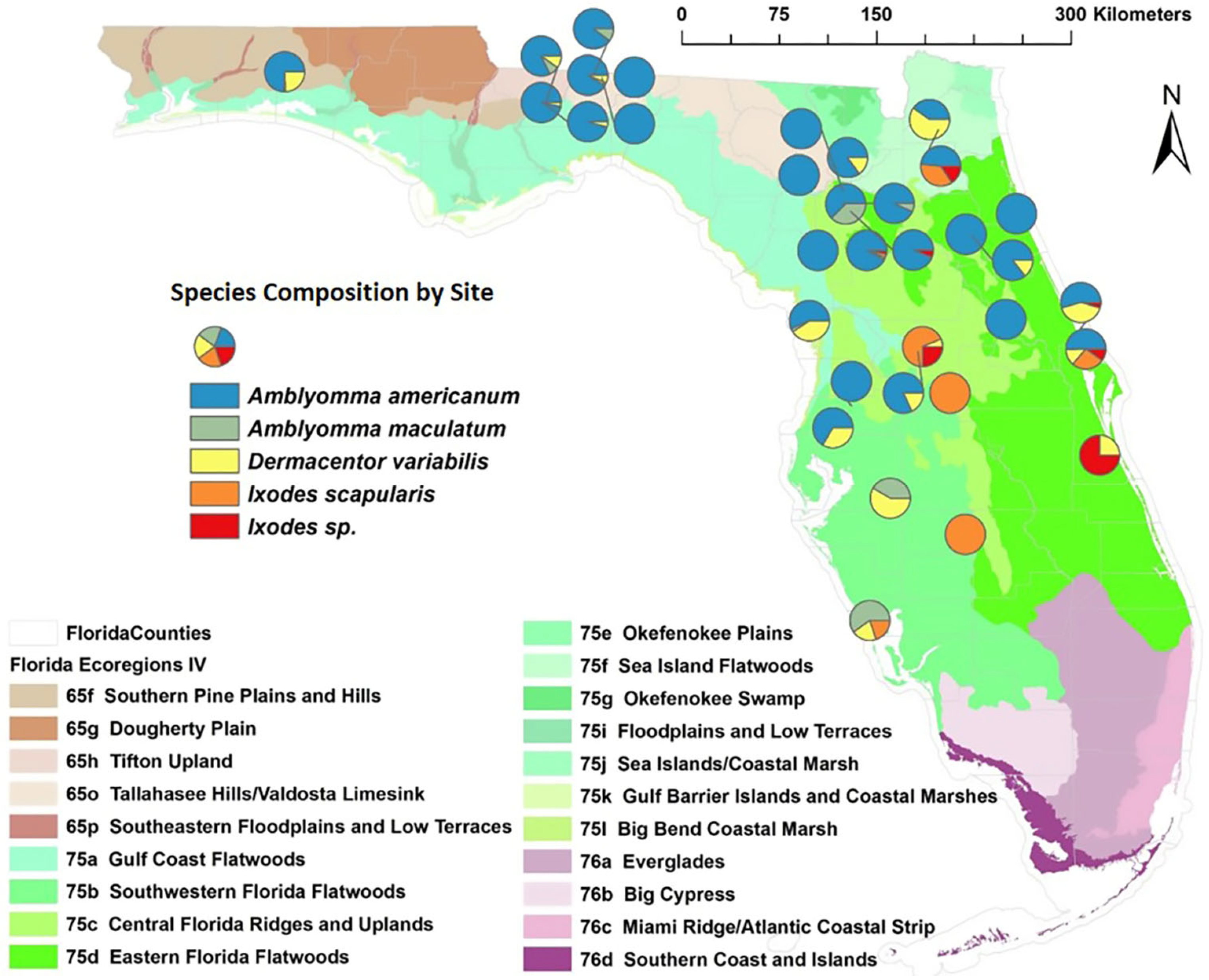


| Species | Total | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|---|
| Amblyomma americanum | 1117 | 436 | 453 | 60 | 168 |
| Amblyomma maculatum | 32 | 2 | 5 | 25 | 0 |
| Dermacentor variabilis | 149 | 80 | 41 | 19 | 9 |
| Ixodes scapularis | 79 | 0 | 0 | 64 | 15 |
| Ixodes sp. | 38 | 1 | 0 | 28 | 9 |
| Total ticks | 1415 | 519 | 499 | 196 | 201 |
| Total pigs sampled | 157 | 49 | 37 | 41 | 30 |
| Partial RDA—Partitioning of Variance | ||||||
|---|---|---|---|---|---|---|
| Edge Density 1 km | Number of Patches 1 km | Percent Landcover 1 km | ||||
| Inertia | Proportion | Inertia | Proportion | Inertia | Proportion | |
| Conditioned | 0.056 | 0.128 | 0.056 | 0.128 | 0.056 | 0.128 |
| Constrained | 0.025 | 0.056 | 0.022 | 0.052 | 0.035 | 0.081 |
| Unconstrained | 0.354 | 0.815 | 0.356 | 0.819 | 0.343 | 0.791 |
| F = 2.06, df = 5149, p = 0.014 Adjusted R2 = 0.029 | F = 1.90, df = 5149, p = 0.021 Adjusted R2 = 0.025 | F = 3.05, df = 5149, p < 0.001 Adjusted R2 = 0.055 | ||||
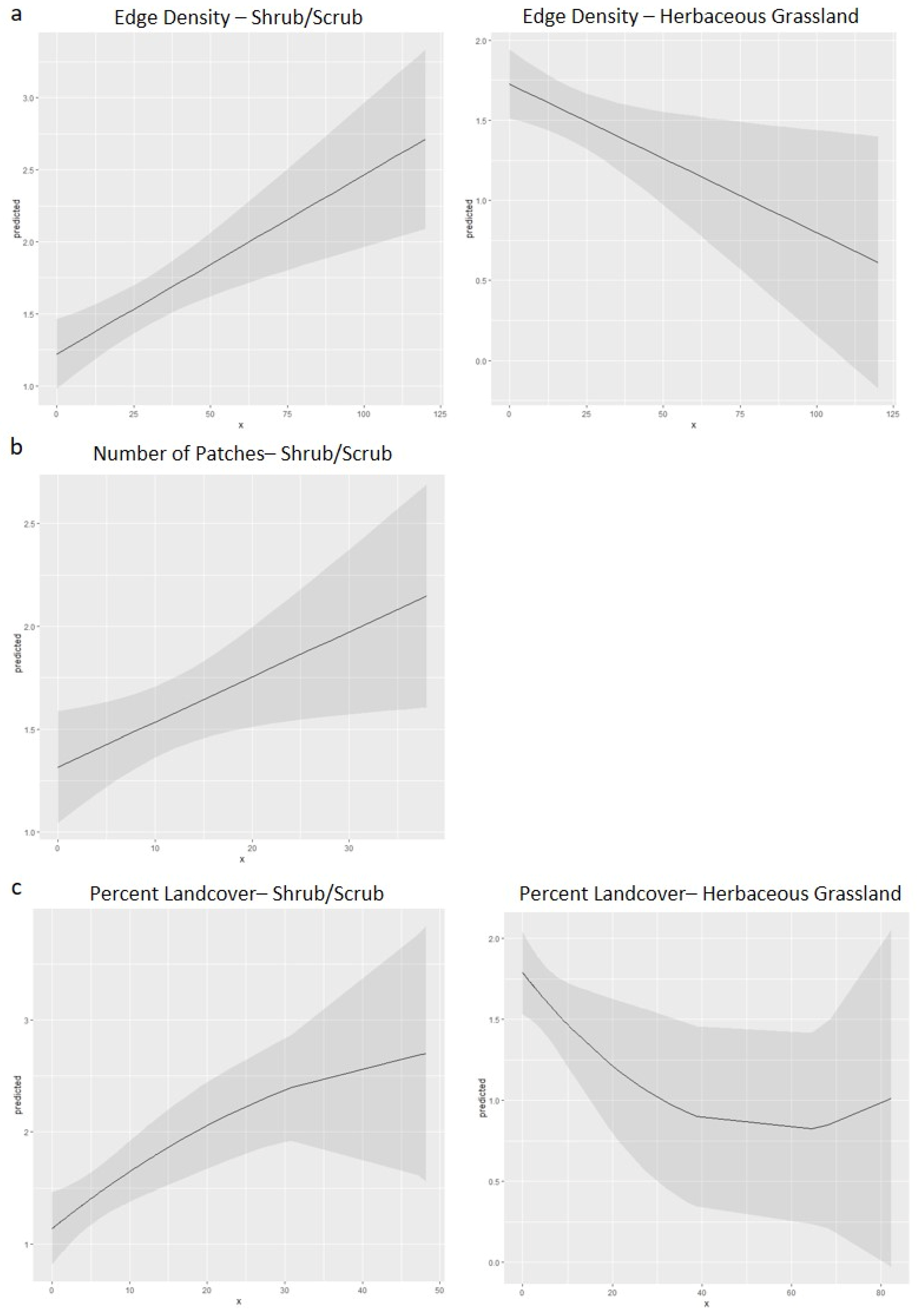
| Landscape Metric | Intercept | Dev. Open Space | Decid. Forest | Mixed Forest | Shrub/Scrub | Grassland | df | Log(Lᵢ) * | AIC | ∆AIC † | AICw ‡ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Edge Density | 1.391 | X § | X | X | 0.012437 | −0.00929 | 6 | −231.695 | 475.389 | 0.000 | 0.205 |
| Percent Landcover | 1.424 | X | X | X | 0.034478 | −0.01278 | 6 | −231.714 | 475.428 | 0.000 | 0.325 |
| Edge Density | 1.289 | X | X | 0.007797 | 0.013215 | −0.00883 | 7 | −230.919 | 475.839 | 0.450 | 0.164 |
| Edge Density | 1.181 | 0.003159 | X | 0.009587 | 0.013057 | −0.00833 | 8 | −230.223 | 476.446 | 1.057 | 0.121 |
| Edge Density | 1.331 | 0.002213 | X | X | 0.012201 | −0.00901 | 7 | −231.337 | 476.674 | 1.285 | 0.108 |
| Percent Landcover | 1.388 | 0.007351 | X | X | 0.034619 | −0.01269 | 7 | −231.526 | 477.052 | 1.624 | 0.144 |
| Edge Density | 1.392 | X | −0.00218 | X | 0.012857 | −0.00935 | 7 | −231.661 | 477.321 | 1.932 | 0.078 |
| Percent Landcover | 1.406 | X | X | 0.010425 | 0.034777 | −0.01258 | 7 | −231.678 | 477.357 | 1.928 | 0.124 |
| Patch Number | 1.316 | NA | NA | NA | 0.021892 | NA | 5 | −236.504 | 483.008 | 0.000 | 0.106 |
| Patch Number | 1.465 | X | 0.019994 | X | X | X | 5 | −236.650 | 483.301 | 0.293 | 0.092 |
| Landscape Metric | Intercept | Dev. Open Space | Decid. Forest | Mixed Forest | Shrub/Scrub | Grassland | df | Log(Lᵢ) | AIC | ∆AIC | AICw |
| Patch Number | 1.111 | 0.009235 | X | 0.023128 | 0.025415 | −0.00999 | 8 | −233.907 | 483.815 | 0.807 | 0.071 |
| Patch Number | 1.247 | 0.005662 | X | X | 0.021306 | X | 6 | −235.920 | 483.840 | 0.831 | 0.070 |
| Patch Number | 1.114 | 0.009175 | 0.008612 | 0.018955 | 0.016897 | X | 8 | −233.990 | 483.979 | 0.971 | 0.065 |
| Patch Number | 1.279 | 0.0095 | 0.017761 | 0.014107 | X | X | 7 | −235.089 | 484.179 | 1.170 | 0.059 |
| Patch Number | 1.346 | X | X | X | 0.026004 | −0.01029 | 6 | −236.131 | 484.262 | 1.254 | 0.057 |
| Patch Number | 1.262 | X | X | 0.015632 | 0.026143 | −0.00981 | 7 | −235.300 | 484.599 | 1.591 | 0.048 |
| Patch Number | 1.265 | X | 0.008696 | 0.011459 | 0.017647 | X | 7 | −235.363 | 484.725 | 1.717 | 0.045 |
| Model Type and Variables | Estimate | Std. Error | Z-Value | p-Value |
|---|---|---|---|---|
| Edge Density | ||||
| Intercept | 1.391 | 0.123 | 11.308 | <0.001 |
| Shrub/Scrub | 0.012 | 0.003 | 3.779 | <0.001 |
| Herbaceous grassland | −0.009 | 0.004 | −2.403 | 0.016 |
| Number of Patches | ||||
| Intercept | 1.316 | 0.138 | 9.526 | <0.001 |
| Shrub/Scrub | 0.022 | 0.010 | 2.248 | 0.025 |
| Percent Landcover | ||||
| Intercept | 1.417 | 0.126 | 11.223 | <0.001 |
| Shrub/Scrub | 0.056 | 0.025 | 2.225 | 0.024 |
| I Shrub/Scrub2 | −0.000 | 0.000 | −0.786 | 0.432 |
| Herbaceous grassland | −0.035 | 0.019 | −1.952 | 0.051 |
| I Herbaceous grassland2 | 0.000 | 0.000 | 1.230 | 0.219 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mays Maestas, S.E.; Campbell, L.P.; Milleson, M.P.; Reeves, L.E.; Kaufman, P.E.; Wisely, S.M. Ticks and Tick-Borne Pathogens from Wild Pigs in Northern and Central Florida. Insects 2023, 14, 612. https://doi.org/10.3390/insects14070612
Mays Maestas SE, Campbell LP, Milleson MP, Reeves LE, Kaufman PE, Wisely SM. Ticks and Tick-Borne Pathogens from Wild Pigs in Northern and Central Florida. Insects. 2023; 14(7):612. https://doi.org/10.3390/insects14070612
Chicago/Turabian StyleMays Maestas, Sarah E., Lindsay P. Campbell, Michael P. Milleson, Lawrence E. Reeves, Phillip E. Kaufman, and Samantha M. Wisely. 2023. "Ticks and Tick-Borne Pathogens from Wild Pigs in Northern and Central Florida" Insects 14, no. 7: 612. https://doi.org/10.3390/insects14070612






