Effect of Temperature and Insect Infestation Levels on Oxygen Depletion in Hermetic Storage
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing and Grain Preparation
2.2. Experimental Design
2.3. Data Collection
2.3.1. Oxygen Measurement
2.3.2. Temperature and Relative Humidity
2.3.3. Grain Quality Assessments
2.4. Statistical Analysis
3. Results
3.1. Oxygen Measurement
3.2. Temperature and Relative Humidity
3.3. Grain Moisture Content and Percentage Germination
3.4. Insect Occurrence, Grain Damage, and Weight Loss
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baributsa, D.; Njoroge, A.W. The use and profitability of hermetic technologies for grain storage among smallholder farmers in eastern Kenya. J. Stored Prod. Res. 2020, 87, 101618. [Google Scholar] [CrossRef]
- Baoua, I.B.; Margam, V.; Amadou, L.; Murdock, L.L. Performance of triple bagging hermetic technology for postharvest storage of cowpea grain in Niger. J. Stored Prod. Res. 2012, 51, 81–85. [Google Scholar] [CrossRef]
- Kharel, K.; Mason, L.J.; Murdock, L.L.; Baributsa, D. Efficacy of hypoxia against Tribolium castaneum (Coleoptera: Tenebrionidae) throughout ontogeny. J. Econ. Entomol. 2019, 112, 1463–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, P.; Scharf, M.E.; Mason, L.J.; Baributsa, D. Effect of hypoxia on the lethal mortality time of adult Sitophilus oryzae L. Insects 2021, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Murdock, L.L.; Margam, V.; Baoua, I.; Balfe, S.; Shade, R.E. Death by desiccation: Effects of hermetic storage on cowpea bruchids. J. Stored Prod. Res. 2012, 49, 166–170. [Google Scholar] [CrossRef]
- Njoroge, A.W.; Mankin, R.W.; Smith, B.; Baributsa, D. Stored-product effects of hypoxia on acoustic activity of two stored-product pests, adult emergence, and grain quality. J. Econ. Entomol. 2019, 112, 1989–1996. [Google Scholar] [CrossRef]
- Yan, Y.; Williams, S.B.; Baributsa, D.; Murdock, L.L. Hypoxia treatment of Callosobruchus maculatus females and its effects on reproductive output and development of progeny following exposure. Insects 2016, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Quellhorst, H.E. Oxgen Consumption by Grain Storage Pests in Relation to Hermetic Storage Systems and Evaluation of Postharvest Management Practices by Smallholder Farmers in Haiti. Doctoral Dissertation, Purdue University, West Lafayette, IN, USA, 2018. [Google Scholar]
- Donahaye, E.J.; Navarro, S.; Rindner, M.; Azrieli, A. The combined influence of temperature and modified atmospheres on Tribolium castaneum (Herbst) (coleoptera: Tenebrionidae). J. Stored Prod. Res. 1996, 32, 225–232. [Google Scholar] [CrossRef]
- Njoroge, A.W.; Affognon, H.D.; Mutungi, C.M.; Manono, J.; Lamuka, P.O.; Murdock, L.L. Triple bag hermetic storage delivers a lethal punch to Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in stored maize. J. Stored Prod. Res. 2014, 58, 12–19. [Google Scholar] [CrossRef]
- Mutungi, C.M.; Affognon, H.; Njoroge, A.W.; Baributsa, D.; Murdock, L.L. Storage of mung bean (Vigna radiata [L.] Wilczek) and pigeonpea grains (Cajanus cajan [L.] Millsp) in hermetic triple-layer bags stops losses caused by Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J. Stored Prod. Res. 2014, 58, 39–47. [Google Scholar] [CrossRef]
- Baoua, I.B.; Bakoye, O.; Amadou, L.; Murdock, L.L.; Baributsa, D. Performance of PICS bags under extreme conditions in the sahel zone of Niger. J. Stored Prod. Res. 2018, 76, 96–101. [Google Scholar] [CrossRef]
- Neven, L.G. Physiological responses of insects to heat. Postharvest Biol. Technol. 2000, 21, 103–111. [Google Scholar] [CrossRef]
- Renault, D.; Hervant, F.; Vernon, P. Effect of food shortage and temperature on oxygen consumption in the lesser mealworm, Alphitobius diaperinus (Panzer) (Coleoptera: Tenebrionidae). Physiol. Entomol. 2003, 28, 261–267. [Google Scholar] [CrossRef]
- Lale, N.E.; Vidal, S. Effect of constant temperature and humidity on oviposition and development of Callosobruchus maculatus (F.) and Callosobruchus subinnotatus (Pic) on bambara groundnut, Vigna subterranea (L.) Verdcourt. J. Stored Prod. Res. 2003, 39, 459–470. [Google Scholar] [CrossRef]
- Lalouette, L.; Williams, C.M.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef]
- Baoua, I.B.; Amadou, L.; Baributsa, D.; Murdock, L.L. Triple bag hermetic technology for post-harvest preservation of Bambara groundnut (Vigna subterranea (L.) Verdc.). J. Stored Prod. Res. 2014, 58, 48–52. [Google Scholar] [CrossRef]
- Boxall, R. A critical review of the methodology for assessing farm level grain losses after harvest. Int. Biodeterior. 1986, 25, 318–319. [Google Scholar] [CrossRef]
- Williams, S.B.; Murdock, L.L.; Baributsa, D. Sorghum seed storage in Purdue Improved Crop Storage (PICS) bags and improvised containers. J. Stored Prod. Res. 2017, 72, 138–142. [Google Scholar] [CrossRef]
- Osman, M.; Mahmoud, M.; Mohamed, K. Susceptibility of Certain Pulse Grains to Callosobruchus maculatus (F.) (Bruchidae: Coleoptera), and Influence of Temperature on Its Biological Attributes. J. Appl. Plant Prot. 2015, 3, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Chandrakantha, J.; Mathavan, S. Changes in developmental rates and biomass energy in Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) reared on different foods and temperatures. J. Stored Prod. Res. 1986, 22, 71–75. [Google Scholar] [CrossRef]
- Loganathan, M.; Jayas, D.S.; Fields, P.G.; White, N.D.G. Low and high temperatures for the control of cowpea beetle, Callosobruchus maculatus (F.) (coleoptera: Bruchidae) in chickpeas. J. Stored Prod. Res. 2011, 47, 244–248. [Google Scholar] [CrossRef]
- Njoroge, A.W.W.; Mankin, R.W.W.; Smith, B.W.W.; Baributsa, D. Effects of Hermetic Storage on Adult Sitophilus oryzae L. (Coleoptera: Curculionidae) Acoustic Activity Patterns and Mortality. J. Econ. Entomol. 2017, 110, 2707–2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singano, C.D.; Mvumi, B.M.; Stathers, T.E. Effectiveness of grain storage facilities and protectants in controlling stored-maize insect pests in a climate-risk prone area of Shire Valley, Southern Malawi. J. Stored Prod. Res. 2019, 83, 130–147. [Google Scholar] [CrossRef]
- Ramadan, M.M.; Abdel-Hady, A.A.A.; Guedes, R.N.C.; Hashem, A.S. Low temperature shock and chill-coma consequences for the red flour beetle (Tribolium castaneum) and the rice weevil (Sitophilus oryzae). J. Therm. Biol. 2020, 94, 102774. [Google Scholar] [CrossRef] [PubMed]
- Hutfilz, C. Endocrine Regulation of Lifespan in Insect Diapause. Front. Physiol. 2022, 13, 825057. [Google Scholar] [CrossRef]
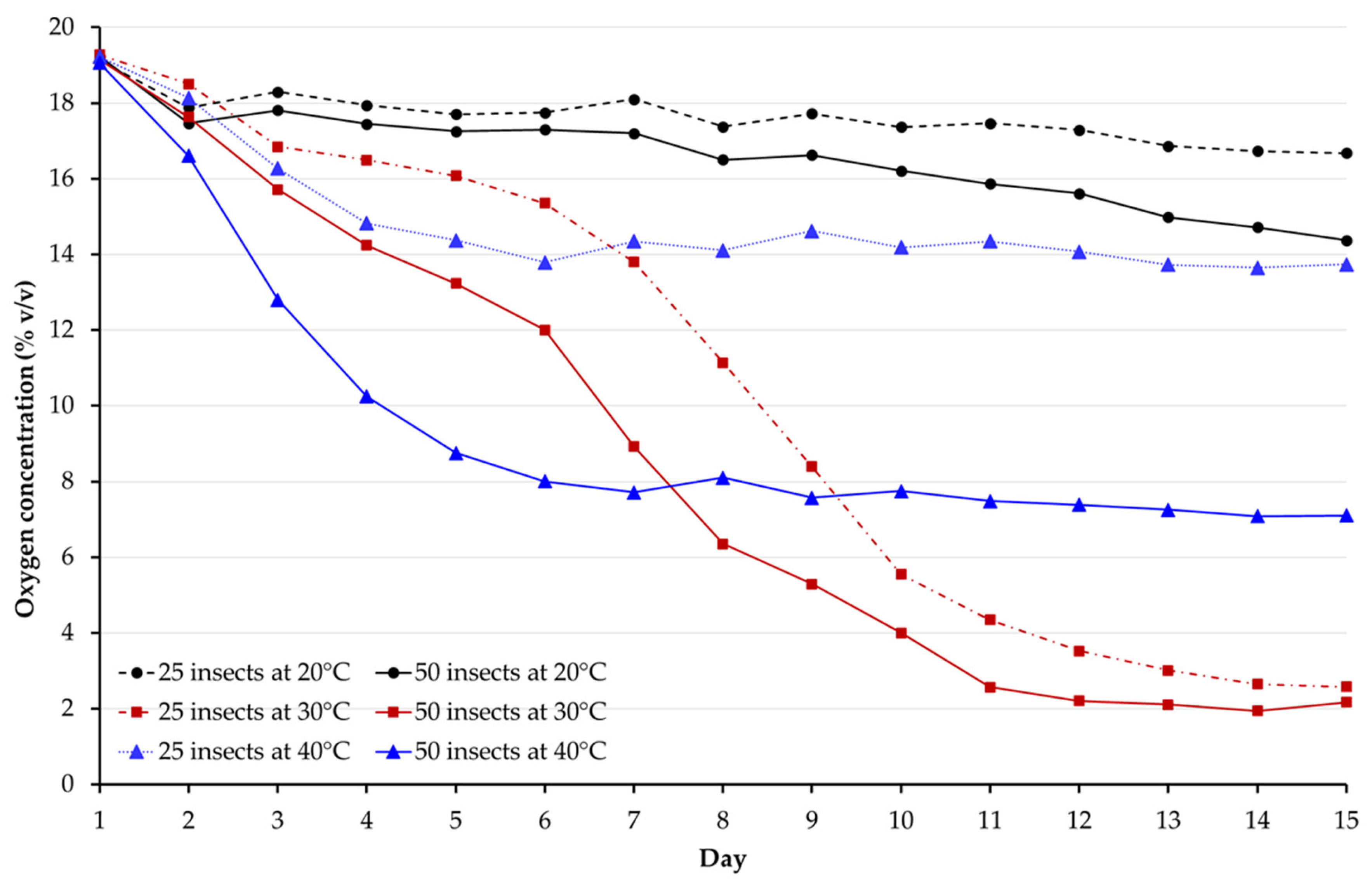
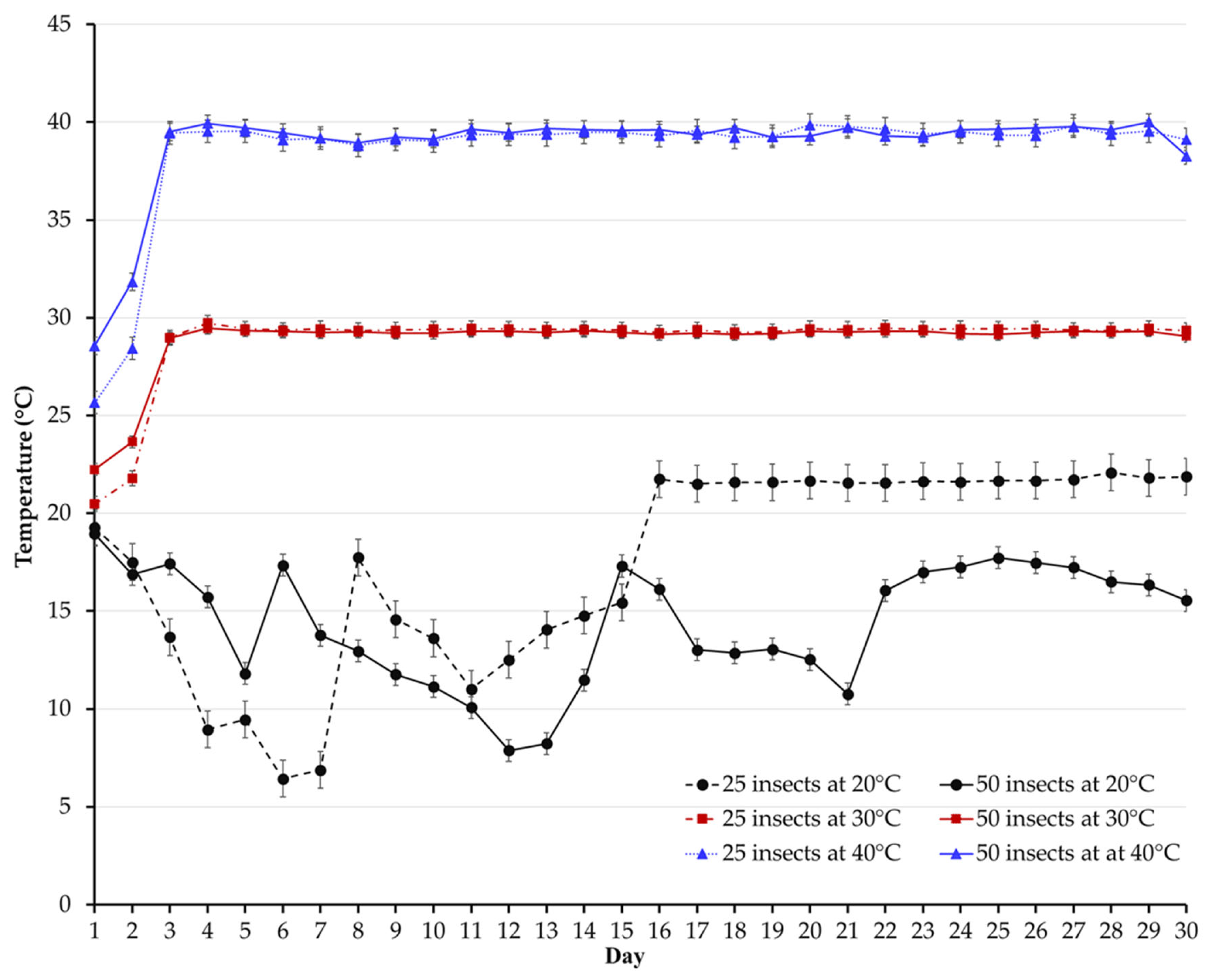
| Treatment | Residual Oxygen Level (Percentage, Mean ± SEM) | |||
|---|---|---|---|---|
| Day 5 | Day 10 | Day 15 | Day 30 | |
| 25 insects at 20 °C | 18 ± 0.200a * | 17 ± 0.149a | 16 ± 0.354a | 7 ± 1.091b |
| 50 insects at 20 °C | 17 ± 0.302a | 16 ± 0.575a | 14 ± 1.002a | 7 ± 1.060b |
| 25 insects at 30 °C | 16 ± 0.402a | 5 ± 0.806c | 3 ± 0.766d | 1 ± 0.469c |
| 50 insects at 30 °C | 12 ± 1.096b | 3 ± 0.752c | 2 ± 0.532d | 3 ± 1.966c |
| 25 insects at 40 °C | 13 ± 0.291b | 12 ± 2.169b | 11 ± 2.538b | 9 ± 1.396a |
| 50 insects at 40 °C | 8 ± 1.037c | 7 ± 1.186c | 7 ± 1.089c | 6 ± 1.475ab |
| F (p-value) | 38.58 (<0.001) | 69.61 (<0.001) | 88.58 (<0.001) | 4.06 (0.004) |
| Parameter * | Estimate | SE a | t | p-Value | LCI b | UCI c |
|---|---|---|---|---|---|---|
| Intercept | 6.84 | 0.66 | 10.34 | <0.001 | 5.54 | 8.13 |
| 25 insects | 4.34 | 1.07 | 4.07 | <0.001 | 2.25 | 6.43 |
| 50 insects | 0 | 0 | . | . | . | . |
| 20 °C | 7.46 | 0.97 | 7.71 | <0.001 | 5.56 | 9.36 |
| 30 °C | −4.91 | 0.94 | −5.26 | <0.001 | −6.75 | −3.08 |
| 40 °C | 0 | 0 | . | . | . | . |
| 25 insects at 30 °C | −3.92 | 1.42 | −2.77 | 0.006 | −6.70 | −1.14 |
| 25 insects at 40 °C | 0 | 0 | . | . | . | . |
| Initially (time = 0) | 12.23 | 0.94 | 13.09 | <0.001 | 10.40 | 14.07 |
| 25 insects initially | −4.18 | 1.42 | −2.95 | 0.003 | −6.97 | −1.40 |
| 20 °C initially | −7.30 | 1.35 | −5.43 | <.001 | −9.94 | −4.66 |
| 25 insects at 30 °C initially | 3.89 | 1.94 | 2.01 | 0.045 | 0.085 | 7.70 |
| Treatment | Equation * | R² Value |
|---|---|---|
| 25 insects at 20 °C | y = −0.148x + 18.77 | 0.897 |
| 50 insects at 20 °C | y = −0.3137x + 19.024 | 0.961 |
| 25 insects at 30 °C | y = 0.009x3 − 0.2204x2 + 0.1119x + 19.271 | 0.979 |
| 50 insects at 30 °C | y = 0.0029x3 − 0.0055x2 − 1.9252x + 21.602 | 0.986 |
| 25 insects at 40 °C | y = −0.0045x3 + 0.1611x2 − 1.8512x + 20.687 | 0.923 |
| 50 insects at 40 °C | y = −0.0087x3 + 0.3296x2 − 3.9746x + 22.408 | 0.968 |
| Grain Infestation Levels after 30 Days | Adult Emergence 45 Days Post-Treatment | |||
|---|---|---|---|---|
| Treatments | Eggs | Larvae | Adults | |
| 25 insects at 20 °C | 13.5 ± 5.98c | 0 ± 0.47a | 1.4 ± 0.72ab | 1.25 ± 0.75b |
| 50 insects at 20 °C | 17.6 ± 6.93c | 0 ± 0.54a | 4.7 ± 0.83a | 5.86 ± 1.78a |
| 25 insects at 30 °C | 30.8 ± 5.99b | 0 ± 0.47a | 0 ± 0.00b | 0 ± 0.00c |
| 50 insects at 30 °C | 48.0 ± 5.99a | 1.1 ± 0.47a | 0 ± 0.00b | 0 ± 0.00c |
| 25 insects at 40 °C | 14.0 ± 5.99c | 0 ± 0.47a | 0 ± 0.00b | 0 ± 0.00c |
| 50 insects at 40 °C | 30.5 ± 5.99b | 0 ± 0.47a | 0 ± 0.00b | 0 ± 0.00c |
| F (p-value) | 9.53 (0.007) | 1.00 (0.332) | 11.97 (0.003) | 11.16 (0.004) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donga, T.K.; Baributsa, D. Effect of Temperature and Insect Infestation Levels on Oxygen Depletion in Hermetic Storage. Insects 2023, 14, 621. https://doi.org/10.3390/insects14070621
Donga TK, Baributsa D. Effect of Temperature and Insect Infestation Levels on Oxygen Depletion in Hermetic Storage. Insects. 2023; 14(7):621. https://doi.org/10.3390/insects14070621
Chicago/Turabian StyleDonga, Trust Kasambala, and Dieudonné Baributsa. 2023. "Effect of Temperature and Insect Infestation Levels on Oxygen Depletion in Hermetic Storage" Insects 14, no. 7: 621. https://doi.org/10.3390/insects14070621







