Flowering Coriander (Coriandrum sativum) Strips Do Not Enhance Ecosystem Services in Azorean Orchards
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Orchards
2.2. Experimental Design
2.3. Measuring Levels of Herbivory
2.4. Assessing the Levels of Seed Predation
2.5. Assessing Predation Pressure
2.6. Statistical Analysis
2.6.1. Analyzing Herbivory
2.6.2. Analyzing Seed Predation
2.6.3. Analyzing Predation
3. Results
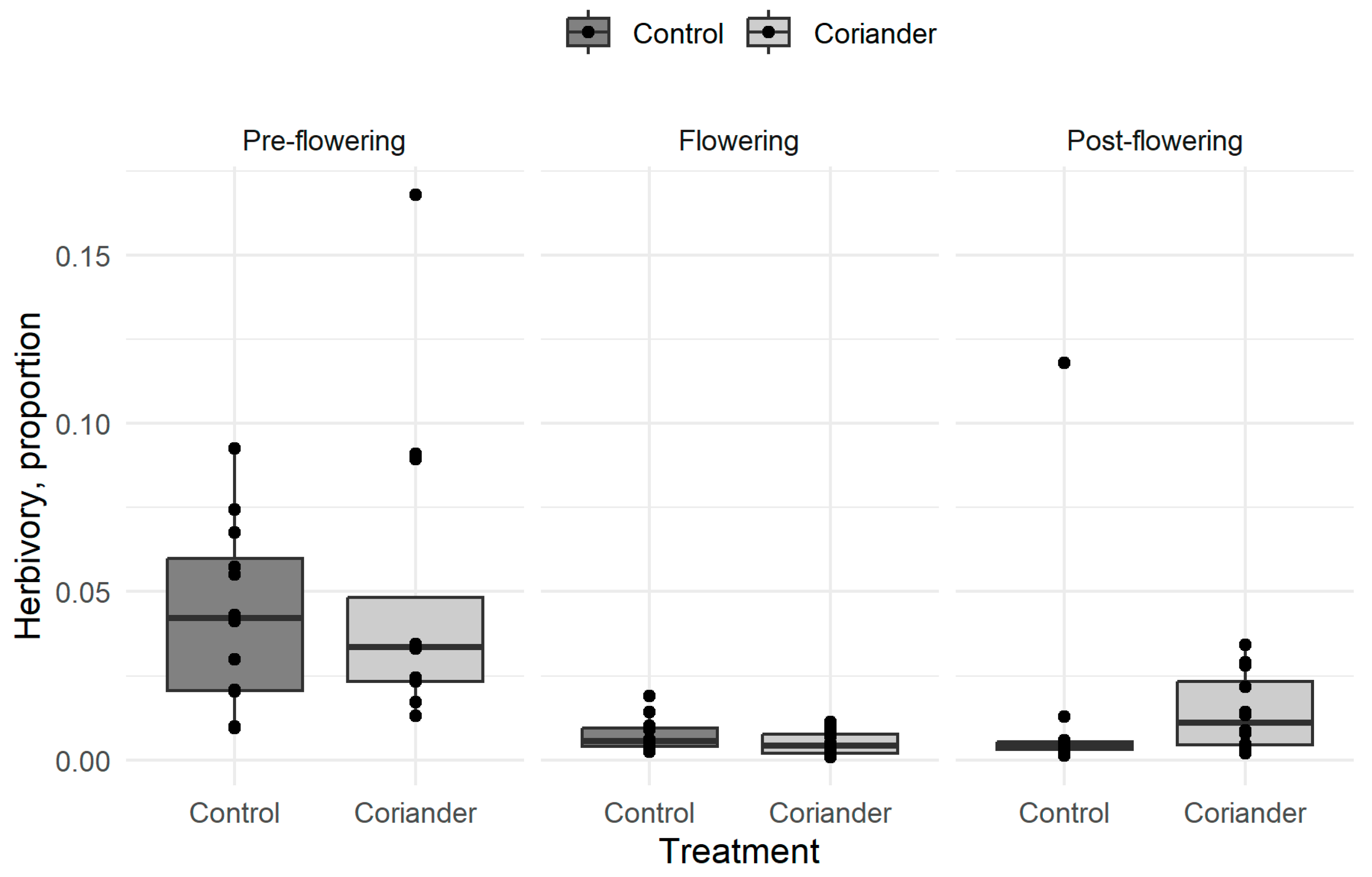
3.1. Herbivory
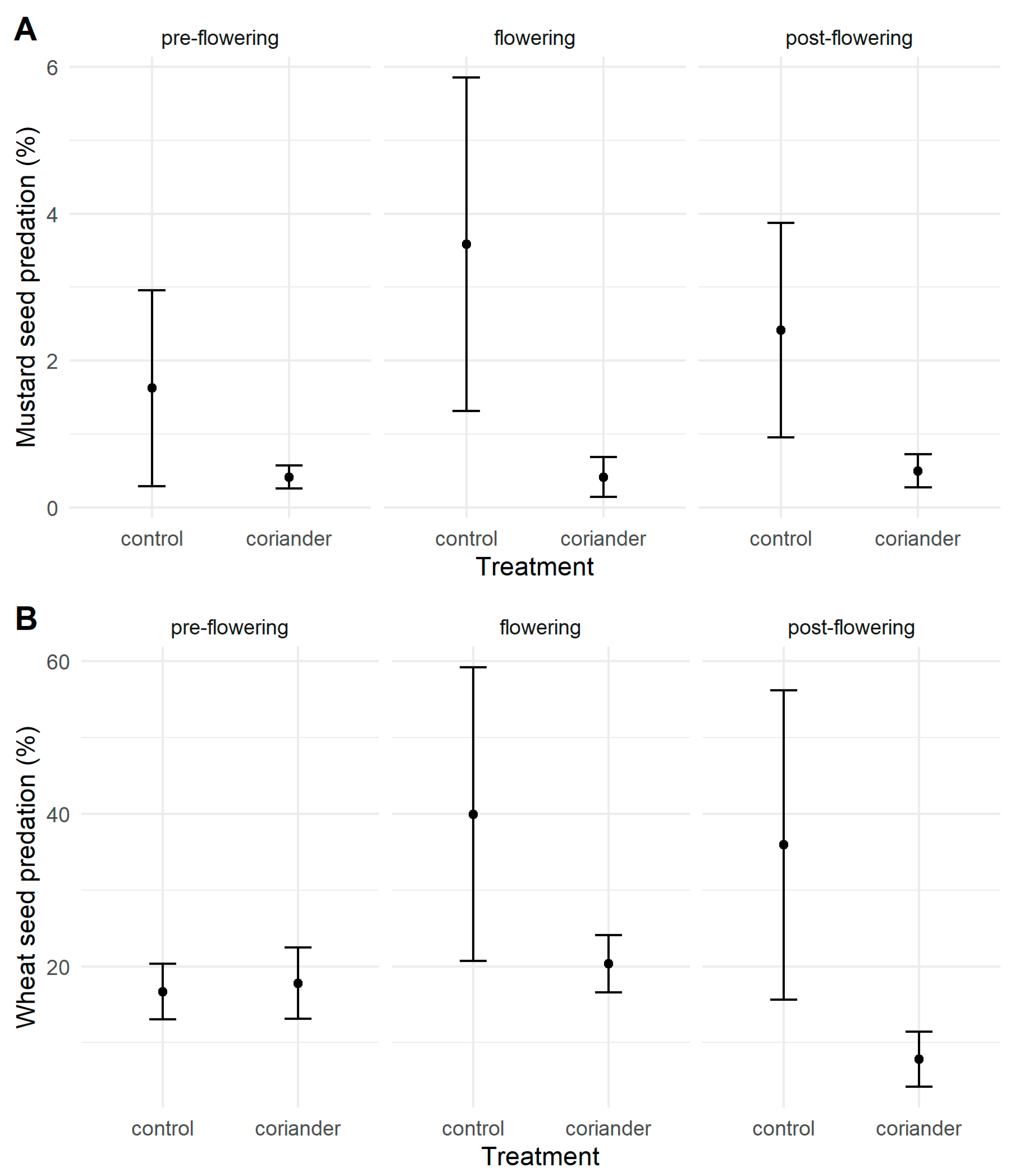
3.2. Seed Predation
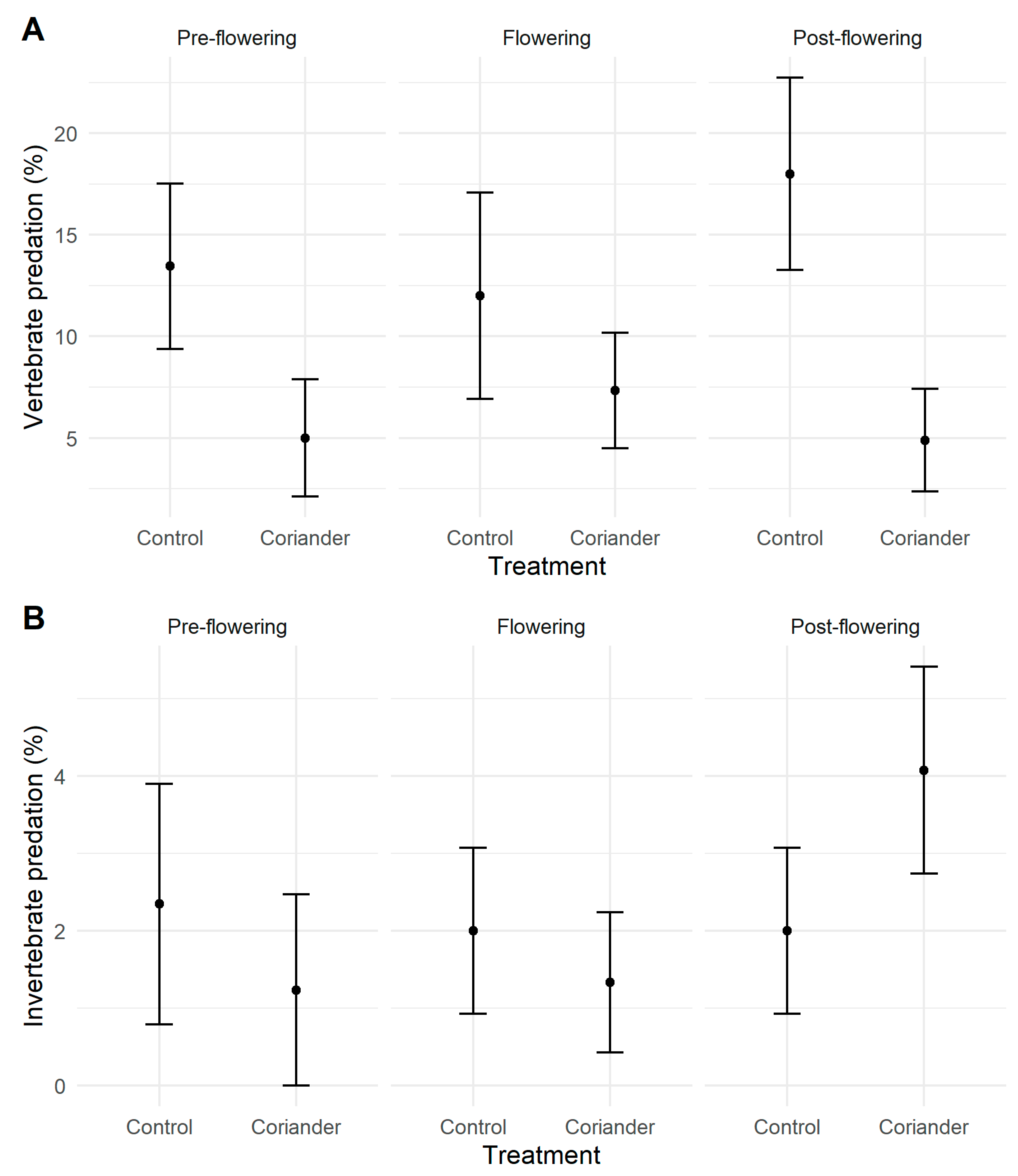
3.3. Predation
4. Discussion
4.1. ESs and EDs in Azorean Mixed Orchards
4.2. The Effectiveness of Coriander Strips
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Ricketts, T.H.; Kremen, C.; Carney, K.; Swinton, S.M. Ecosystem services and dis-services to agriculture. Ecol. Econ. 2007, 64, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Seibold, S.; Gossner, M.M.; Simons, N.K.; Blüthgen, N.; Müller, J.; Ambarlı, D.; Ammer, C.; Bauhus, J.; Fischer, M.; Habel, J.C.; et al. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 2019, 574, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Rusch, A.; Birkhofer, K.; Bommarco, R.; Smith, H.G.; Ekbom, B. Predator body sizes and habitat preferences predict predation rates in an agroecosystem. Basic Appl. Ecol. 2015, 16, 250–259. [Google Scholar] [CrossRef]
- Perović, D.J.; Gámez-Virués, S.; Landis, D.A.; Wäckers, F.; Gurr, G.M.; Wratten, S.D.; You, M.-S.; Desneux, N. Managing biological control services through multi-trophic trait interactions: Review and guidelines for implementation at local and landscape scales. Biol. Rev. 2018, 93, 306–321. [Google Scholar] [CrossRef]
- Shapiro, J.; Báldi, A. Accurate accounting: How to balance ecosystem services and disservices. Ecosyst. Serv. 2014, 7, 201–202. [Google Scholar] [CrossRef]
- Tuovinen, T.; Kikas, A.; Tolonen, T.; Kivijӓrvi, P. Organic mulches vs. black plastic in organic strawberry: Does it make a difference for ground beetles (Col., Carabidae)? J. Appl. Entomol. 2006, 130, 495–503. [Google Scholar] [CrossRef]
- Nieto-Romero, M.; Oteros-Rozas, E.; González, J.A.; Martín-López, B. Exploring the knowledge landscape of ecosystem services assessments in Mediterranean agroecosystems: Insights for future research. Environ. Sci. Policy 2014, 37, 121–133. [Google Scholar] [CrossRef]
- Albrecht, M.; Kleijn, D.; Williams, N.M.; Tschumi, M.; Blaauw, B.R.; Bommarco, R.; Campbell, A.J.; Dainese, M.; Drummond, F.A.; Entling, M.H. The effectiveness of flower strips and hedgerows on pest control, pollination services and crop yield: A quantitative synthesis. Ecol. Lett. 2020, 23, 1488–1498. [Google Scholar] [CrossRef]
- Haaland, C.; Naisbit, R.E.; Bersier, L.F. Sown wildflower strips for insect conservation: A review. Ins. Cons. Div. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Campbell, A.J.; Wilby, A.; Sutton, P.; Wäckers, F. Getting more power from your flowers: Multi-functional flower strips enhance pollinators and pest control agents in apple orchards. Insects 2017, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Pellissier, M.E.; Jabbour, R. Herbivore and parasitoid insects respond differently to annual and perennial floral strips in an alfalfa ecosystem. Biol. Cont. 2018, 123, 28–35. [Google Scholar] [CrossRef]
- Mansion-Vaquié, A.; Ferrante, M.; Cook, S.M.; Pell, J.K.; Lövei, G.L. Manipulating field margins to increase predation intensity in fields of winter wheat (Triticum aestivum). J. Appl. Entomol. 2017, 141, 600–611. [Google Scholar] [CrossRef] [Green Version]
- Laha, S.; Chatterjee, S.; Das, A.; Smith, B.; Basu, P. Exploring the importance of floral resources and functional trait compatibility for maintaining bee fauna in tropical agricultural landscapes. J. Ins. Cons. 2020, 24, 431–443. [Google Scholar] [CrossRef]
- Balzan, M.V.; Bocci, G.; Moonen, A.C. Augmenting flower trait diversity in wildflower strips to optimise the conservation of arthropod functional groups for multiple agroecosystem services. J. Ins. Cons. 2014, 18, 713–728. [Google Scholar] [CrossRef]
- Noriega, J.A.; Hortal, J.; Azcárate, F.M.; Berg, M.P.; Bonada, N.; Briones, M.J.; Del Toro, I.; Goulson, D.; Ibanez, S.; Landis, D.A. Research trends in ecosystem services provided by insects. Basic. Appl. Ecol. 2018, 26, 8–23. [Google Scholar] [CrossRef] [Green Version]
- Amaral, D.S.; Venzon, M.; Duarte, M.V.; Sousa, F.F.; Pallini, A.; Harwood, J.D. Non-crop vegetation associated with chili pepper agroecosystems promote the abundance and survival of aphid predators. Biol. Cont. 2013, 64, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Tschumi, M.; Albrecht, M.; Bärtschi, C.; Collatz, J.; Entling, M.H.; Jacot, K. Perennial, species-rich wildflower strips enhance pest control and crop yield. Agric. Ecosyst. Env. 2016, 220, 97–103. [Google Scholar] [CrossRef]
- Amy, C.; Noël, G.; Hatt, S.; Uyttenbroeck, R.; Van de Meutter, F.; Genoud, D.; Francis, F. Flower strips in wheat intercropping systems: Effect on pollinator abundance and diversity in Belgium. Insects 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgkiss, D.; Brown, M.J.F.; Fountain, M.T. The effect of within-crop floral resources on pollination, aphid control and fruit quality in commercial strawberry. Agric. Ecosyst. Env. 2019, 275, 112–122. [Google Scholar] [CrossRef]
- Lövei, G.L.; Hodgson, D.J.; MacLeod, A.; Wratten, S.D. Attractiveness of some novel crops for flower-visiting hoverflies (Diptera: Syrphidae): Comparisons from two continents. In Pest Control and Sustainable Agriculture; Corey, S.A., Dall, D.J., Milne, W.M., Eds.; CSIRO: Canberra, Australia, 1993; pp. 368–370. [Google Scholar]
- Ferrante, M.; Lamelas-López, L.; Nunes, R.; Monjardino, P.; Lopes, D.J.H.; Soares, A.O.; Lövei, G.L.; Borges, P.A.V. A simultaneous assessment of multiple ecosystem services and disservices in vineyards and orchards on Terceira Island, Azores. Agric. Ecosyst. Env. 2022, 330, 107909. [Google Scholar] [CrossRef]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Baggen, L.R.; Gurr, G.M.; Meats, A. Flowers in tri-trophic systems: Mechanisms allowing selective exploitation by insect natural enemies for conservation biological control. In Proceedings of the 10th International Symposium on Insect-Plant Relationships; Simpson, S.J., Mordue, A.J., Hardie, J., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 155–161. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Arévalo, J.R.; Balguerías, E.; Barone, R.; De Nascimento, L.; Elias, R.B.; Delgado, J.D.; Fernández-Lugo, S.; Méndez, J.; Menezes de Sequeira, M.; et al. La Laurisilva. Canarias, Madeira y Azores; Macaronesia Editorial: Santa Cruz de Tenerife, Spain, 2017; 420p. [Google Scholar]
- Elias, R.B.; Gil, A.; Silva, L.; Fernández-Palacios, J.M.; Azevedo, E.B.; Reis, F. Natural zonal vegetation of the Azores Islands: Characterization and potential distribution. Phytocoenologia 2016, 46, 107–123. [Google Scholar] [CrossRef]
- Raposeiro, P.M.; Hernández, A.; Pla-Rabes, S.; Gonçalves, V.; Bao, R.; Sáez, A.; Shanahan, T.; Benavente, M.; de Boer, E.J.; Richter, N.; et al. Climate change facilitated the early colonization of the Azores Archipelago during medieval times. Proc. Nat. Acad. Sci. USA 2021, 118, e2108236118. [Google Scholar] [CrossRef]
- Calado, H.; Braga, A.; Moniz, F.; Gil, A.; Vergílio, M. Spatial planning and resource use in the Azores. Mitig. Adapt. Strat. Glob. Chang. 2015, 20, 1079–1095. [Google Scholar] [CrossRef]
- Karp, D.S.; Moses, R.; Gennet, S.; Jones, M.S.; Joseph, S.; M’Gonigle, L.K.; Ponisio, L.C.; Snyder, W.E.; Kremen, C. Agricultural practices for food safety threaten pest control services for fresh produce. J. Appl. Ecol. 2016, 53, 1402–1412. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.T.J.; Bertrand, J.A.; Turcotte, M.M. Precision and accuracy in quantifying herbivory. Ecol. Entomol. 2016, 41, 112–121. [Google Scholar] [CrossRef]
- Didon, U.M.E.; Boström, U. Growth and development of six barley (Hordeum vulgare ssp. vulgare L.) cultivars in response to a model weed (Sinapis alba L.). J. Agron. Crop Sci. 2003, 189, 409–417. [Google Scholar] [CrossRef]
- Tschumi, M.; Ekroos, J.; Hjort, C.; Smith, H.G.; Birkhofer, K. Rodents, not birds, dominate predation-related ecosystem services and disservices in vertebrate communities of agricultural landscapes. Oecologia 2018, 188, 863–873. [Google Scholar] [CrossRef]
- Gallandt, E.R.; Molloy, T.; Lynch, R.P.; Drummond, F.A. Effect of cover-cropping systems on invertebrate seed predation. Weed Sci. 2005, 53, 69–76. [Google Scholar] [CrossRef]
- Linabury, M.C.; Turley, N.E.; Brudvig, L.A. Insects remove more seeds than mammals in first-year prairie restorations. Restor. Ecol. 2019, 27, 1300–1306. [Google Scholar] [CrossRef]
- Howe, A.; Lövei, G.L.; Nachman, G. Dummy caterpillars as a simple method to assess predation rates on invertebrates in a tropical agroecosystem. Entomol. Exp. Appl. 2009, 131, 325–329. [Google Scholar] [CrossRef]
- Novotny, V.; Basset, Y. Body size and host plant specialization: A relationship from a community of herbivorous insects on Ficus from Papua New Guinea. J. Trop. Ecol. 1999, 15, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Ferrante, M. Using Artificial Sentinel Prey to Quantify Predation Intensity under Field Conditions. Ph.D. Thesis, Aarhus University, Aarhus, Denmark, 2017. [Google Scholar]
- Low, P.A.; Sam, K.; McArthur, C.; Posa, M.R.C.; Hochuli, D.F. Determining predator identity from attack marks left in model caterpillars: Guidelines for best practice. Entomol. Exp. Appl. 2014, 152, 120–126. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio Team: Boston, MA, USA, 2021. [Google Scholar]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models, Version 0.4.6; R Package: Madison, WI, USA, 2022. [Google Scholar]
- Lüdecke, D. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Daedlow, D.; Westerman, P.R.; Baraibar, B.; Rouphael, S.; Gerowitt, B. Weed seed predation rate in cereals as a function of seed density and patch size, under high predation pressure by rodents. Weed Res. 2014, 54, 186–195. [Google Scholar] [CrossRef]
- Ferrante, M.; González, E.; Lövei, G.L. Predators do not spill over from forest fragments to maize fields in a landscape mosaic in central Argentina. Ecol. Evol. 2017, 7, 7699–7707. [Google Scholar] [CrossRef]
- Lowman, M.D. An assessment of techniques for measuring herbivory: Is rainforest defoliation more intense than we thought? Biotropica 1984, 16, 264–268. [Google Scholar] [CrossRef]
- Brown, B.J.; Allen, T.F.H. The importance of scale in evaluating herbivory impacts. Oikos 1989, 54, 189–194. [Google Scholar] [CrossRef]
- Tschumi, M.; Ekroos, J.; Hjort, C.; Smith, H.G.; Birkhofer, K. Predation-mediated ecosystem services and disservices in agricultural landscapes. Ecol. Appl. 2018, 28, 2109–2118. [Google Scholar] [CrossRef] [Green Version]
- Lamelas-López, L.; Salgado, I. Applying camera traps to detect and monitor introduced mammals on oceanic islands. Oryx 2021, 55, 181–188. [Google Scholar] [CrossRef]
- Honek, A.; Martinkova, Z.; Saska, P.; Pekar, S. Size and taxonomic constraints determine the seed preferences of Carabidae (Coleoptera). Basic. Appl. Ecol. 2007, 8, 343–353. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Nunes, R.; Lamelas-López, L.; Pereira, E.; Costa, R.; Monjardino, P.; Lopes, D.H.; Soares, A.O.; Gil, A.; Rigal, F.; et al. Monitoring Arthropods in Azorean Agroecosystems: The project AGROECOSERVICES. Biodiv Data J. 2021, 9, e77548. [Google Scholar] [CrossRef]
- Youngerman, C.Z.; DiTommaso, A.; Losey, J.E.; Ryan, M.R. Cover crop seed preference of four common weed seed predators. Renew. Agric. Food Syst. 2020, 35, 522–532. [Google Scholar] [CrossRef] [Green Version]
- Hariraveendra, M.; Rajesh, T.P.; Unni, A.P.; Sinu, P.A. Prey–predator interaction suggests sacred groves are not functionally different from neighbouring used lands. J. Trop. Ecol. 2020, 36, 220–224. [Google Scholar] [CrossRef]
- Imboma, T.S.; Gao, D.-P.; You, M.-S.; You, S.-J.; Lövei, G.L. Predation pressure in tea (Camellia sinensis) plantations in southeastern China measured by the sentinel prey method. Insects 2020, 11, 212. [Google Scholar] [CrossRef] [Green Version]
- Denan, N.; Wan Zaki, W.M.; Norhisham, A.R.; Sanusi, R.; Nasir, D.M.; Nobilly, F.; Ashton-Butt, A.; Lechner, A.M.; Azhar, B. Predation of potential insect pests in oil palm plantations, rubber tree plantations, and fruit orchards. Ecol. Evol. 2020, 10, 654–661. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Hortal, J. Time, area and isolation: Factors driving the diversification of Azorean arthropods. J. Biogeogr. 2009, 36, 178–191. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, D.J.; Lövei, G.L. Novel crops in cereal fields: Habitat refuges for arthropod natural enemies. In Proceedings of the Forty Sixth New Zealand Plant Protection Conference, Christchurch, New Zealand, 10–12 August 1993; Volume 46, pp. 329–333. [Google Scholar]
- Colley, M.; Luna, J. Relative attractiveness of potential beneficial insectary plants to aphidophagous hoverflies (Diptera: Syrphidae). Environ. Entomol. 2000, 29, 1054–1059. [Google Scholar] [CrossRef]
- Mészáros, Z. (Ed.) Results of faunistical and floristical studies in Hungarian apple orchards. Acta Phytopathol. Entomol. Hung. 1984, 19, 91–176. [Google Scholar]
- Andow, D.A. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 1991, 36, 561–586. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.M.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity–ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Mooney, H.A.; Agard, J.; Capistrano, D.; DeFries, R.S.; Díaz, S.; Dietz, T.; Duraiappah, A.K.; Oteng-Yeboah, A.; Pereira, H.M.; et al. Science for managing ecosystem services: Beyond the Millennium Ecosystem Assessment. Proc. Nat. Acad. Sci. USA 2009, 106, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Retsa, A.; Schelske, O.; Wilke, B.; Rutherford-Liske, G.; de Jong, R. Biodiversity and Ecosystem Services: A Business Case for Re/Insurance; Swiss Reinsurance Company Ltd.: Zurich, Switzerland, 2020. [Google Scholar]
| Rate | ||||
|---|---|---|---|---|
| Ecosystem Function | Phenology | Coriander | Control | n |
| Herbivory | Pre-flowering | 4.35 ± 2.66 | 4.88 ± 4.54 | 12 |
| Flowering | 0.76 ± 0.5 | 0.52 ± 0.38 | 12 | |
| Post-flowering | 1.4 ± 3.29 | 1.42 ± 1.13 | 12 | |
| Seed predation, open, wheat | Pre-flowering | 20.43 ± 14.67 | 16.5 ± 5.89 | 3 |
| Flowering | 17.67 ± 12.39 | 43 ± 50.09 | 3 | |
| Post-flowering | 8.83 ± 12.29 | 35.17 ± 56.15 | 3 | |
| Seed predation, open, mustard | Pre-flowering | 0.5 ± 0.5 | 2.73 ± 4.72 | 3 |
| Flowering | 0.33 ± 0.58 | 3.67 ± 6.35 | 3 | |
| Post-flowering | 0.83 ± 0.58 | 3.83 ± 5.01 | 3 | |
| Seed predation, exclusion, wheat | Pre-flowering | 15.17 ± 9.44 | 16.83 ± 12.86 | 3 |
| Flowering | 23 ± 6.06 | 36.83 ± 54.87 | 3 | |
| Post-flowering | 6.83 ± 6.53 | 36.67 ± 54.85 | 3 | |
| Seed predation, exclusion, mustard | Pre-flowering | 0.33 ± 0.29 | 0.5 ± 0.5 | 3 |
| Flowering | 0.5 ± 0.87 | 3.5 ± 6.06 | 3 | |
| Post-flowering | 0.17 ± 2.89 | 1 ± 0.87 | 3 | |
| Vertebrate predation | Pre-flowering | 5 ± 8.57 | 12.46 ± 12.22 | 9 |
| Flowering | 7.3 ± 11 | 12 ± 19.71 | 15 | |
| Post-flowering | 4.89 ± 9.83 | 18 ± 18.33 | 15 | |
| Invertebrate predation | Pre-flowering | 1.23 ± 3.7 | 2.35 ± 4.67 | 9 |
| Flowering | 1.33 ± 3.52 | 2 ± 4.14 | 15 | |
| Post-flowering | 4.07 ± 5.17 | 2 ± 4.14 | 15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrante, M.; Lövei, G.L.; Lavigne, L.; Vicente, M.C.; Tarantino, E.; Lopes, D.H.; Monjardino, P.; Borges, P.A.V. Flowering Coriander (Coriandrum sativum) Strips Do Not Enhance Ecosystem Services in Azorean Orchards. Insects 2023, 14, 634. https://doi.org/10.3390/insects14070634
Ferrante M, Lövei GL, Lavigne L, Vicente MC, Tarantino E, Lopes DH, Monjardino P, Borges PAV. Flowering Coriander (Coriandrum sativum) Strips Do Not Enhance Ecosystem Services in Azorean Orchards. Insects. 2023; 14(7):634. https://doi.org/10.3390/insects14070634
Chicago/Turabian StyleFerrante, Marco, Gabor L. Lövei, Lambert Lavigne, Mario Caballero Vicente, Elisa Tarantino, David Horta Lopes, Paulo Monjardino, and Paulo A. V. Borges. 2023. "Flowering Coriander (Coriandrum sativum) Strips Do Not Enhance Ecosystem Services in Azorean Orchards" Insects 14, no. 7: 634. https://doi.org/10.3390/insects14070634







