Essential Oils and Their Components Control Behaviour of Yellow Mealworm (Tenebrio molitor) Larvae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Essential Oils and Chemicals of and Identification of Individual Components
2.3. GC-MS Analysis and Compound Identification
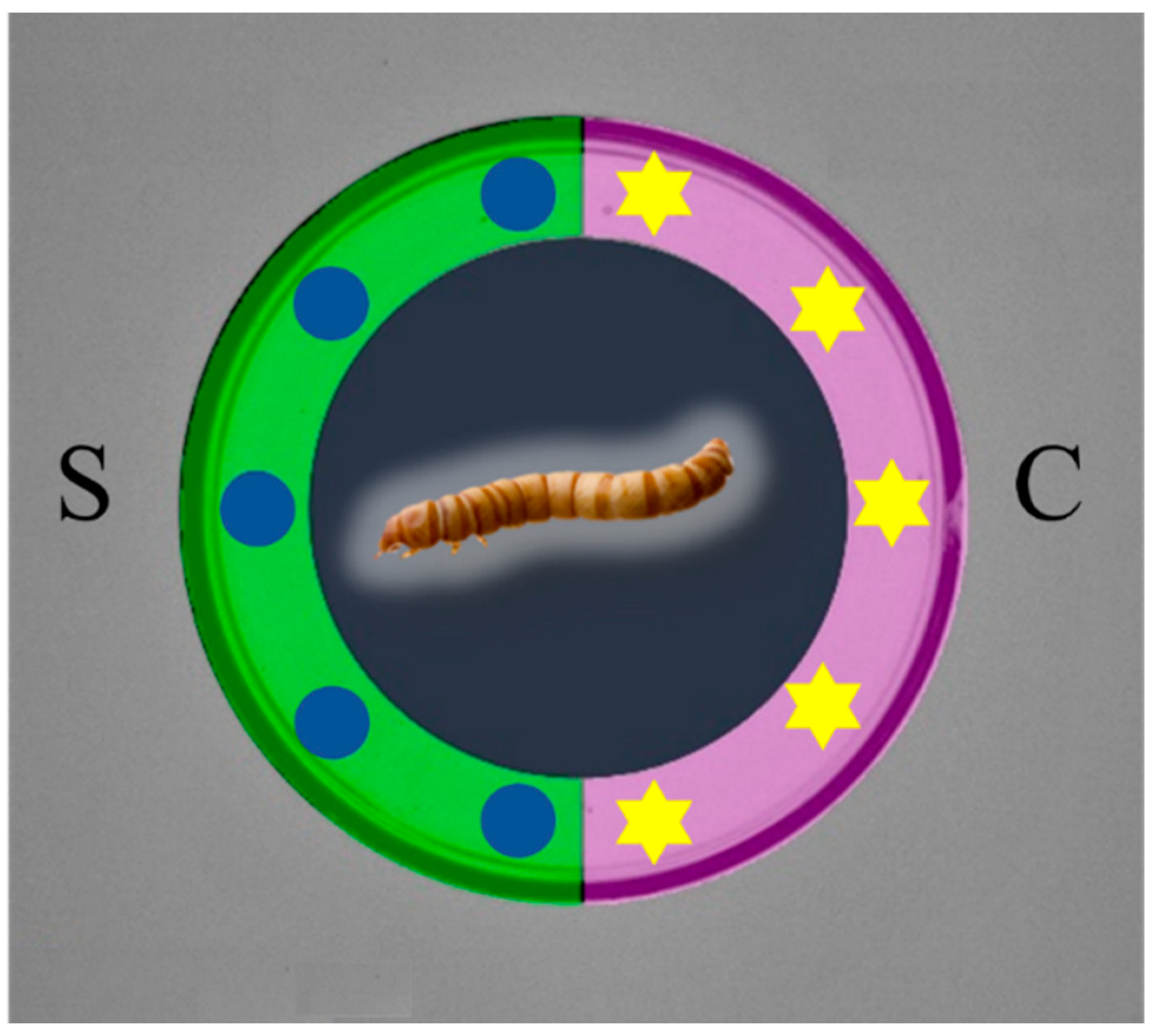
2.4. Behavioural Test
2.5. Data Analysis
3. Results
3.1. Movement of Mealworm Larva in the Arena
3.2. Effect of Essential Oils
3.3. Chemical Composition of EOs
3.4. Effect of Single Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in crop losses to insect pests in a warming climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Gunasekaran, N.; Shivanandappa, T. Insecticidal activity of the root extract of Decalepis hamiltonii against stored-product insect pests and its application in grain protection. J. Food Sci. 2010, 47, 310–314. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Quadrupole Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- George, D.R.; Sparagano, O.A.E.; Port, G.; Okello, E.; Shiel, R.S.; Guy, J.H. Repellence of plant essential oils to Dermanyssus gallinae and toxicity to the non-target invertebrate Tenebrio molitor. Vet. Parasitol. 2009, 162, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.S.; Campos-Soldini, M.P. Fumigant insecticidal activity of plant essential oils against pest blister beetle Epicauta atomaria (Germar) (Coleoptera: Meloidae). J. Plant Dis. 2022, 129, 783–789. [Google Scholar] [CrossRef]
- Martínez, L.C.; Plata-Rueda, A.; Colares, H.C.; Campos, J.M.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Toxic effects of two essential oils and their constituents on the mealworm beetle, Tenebrio molitor. Bull. Entomol. Res. 2018, 108, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Dason, J.S.; Cheung, A.; Anreiter, I.; Montemurri, V.A.; Allen, A.M.; Sokolowski, M.B. Drosophila melanogaster foraging regulates a nociceptive-like escape behaviour through a developmentally plastic sensory circuit. Proc. Natl. Acad. Sci. USA 2020, 117, 23286–23291. [Google Scholar] [CrossRef] [PubMed]
- Cosimi, S.; Rossi, E.; Cioni, P.L.; Canale, A. Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: Evaluation of repellency against Sitophilus zeamais Motschulsky, Cryptolestes ferrugineus Stephens, and Tenebrio molitor. J. Stored Prod. Res. 2009, 45, 125–132. [Google Scholar] [CrossRef]
- Wang, X.; Hao, Q.; Chen, Y.; Jiang, S.; Yang, Q.; Li, Q.; Kavallieratos, N. The effect of chemical composition and bioactivity of several essential oils on Tenebrio molitor Coleoptera: Tenebrionidae. J. Insect Sci. 2015, 15, 116. [Google Scholar] [CrossRef] [PubMed]
- Olivero-Verbel, J.; Tirado-Ballestas, I.; Caballero-Gallardo, K.; Stashenko, E.E. Essential oils applied to the food act as repellents toward Tribolium castaneum. J. Stored Prod. Res. 2013, 55, 145–147. [Google Scholar] [CrossRef]
- Kavallieratos, N.G.; Boukouvala, M.C.; Ntalaka, C.T.; Skourti, A.; Nika, E.P.; Maggi, F.; Spinozzi, E.; Mazzara, E.; Petrelli, R.; Lupidi, G.; et al. Efficacy of 12 commercial essential oils as wheat protectants against stored-product beetles, and their acetylcholinesterase inhibitory activity. Entomol. Gen. 2021, 41, 385–414. [Google Scholar] [CrossRef]
- Barros, F.A.; Radünz, M.; Scariot, M.A.; Camargo, T.M.; Nunes, C.F.; de Souza, R.R.; Gilson, I.K.; Hackbart, H.C.; Radünz, L.L.; Oliveira, J.V.; et al. Efficacy of encapsulated and non-encapsulated thyme essential oil Thymus vulgaris L. in the control of Sitophilus zeamais and its effects on the quality of corn grains throughout storage. Crop Prot. 2022, 153, 105885. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal and repellent activity of selected essential oils against of the pollen beetle, Meligethes aeneus Fabricius adults. Ind. Crops Prod. 2011, 34, 888–892. [Google Scholar] [CrossRef]
- Mudrončeková, S.; Ferenčík, J.; Gruľová, D.; Barta, M. Insecticidal and repellent effects of plant essential oils against Ips typographus. J. Pest Sci. 2019, 92, 595–608. [Google Scholar] [CrossRef]
- Wang, Y.C.; Li, P.; Chi, D. Electrophysiological and Behavioural Responses of Tenebrio molitor L. to Fourteen Kinds of Plant Volatiles. J. Asia Pac. Entomol. 2016, 19, 261–267. [Google Scholar] [CrossRef]
- Martynov, V.O.; Hladkyi, O.Y.; Kolombar, T.M.; Brygadyrenko, V.V. Impact of essential oil from plants on migratory activity of Sitophilus granarius and Tenebrio molitor. Regul. Mech. 2019, 10, 359–371. [Google Scholar] [CrossRef]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Groendahl, E.; Ehlers, B.K.; Keefover-Ring, E. A new cis-sabinene hydrate chemotype detected in large thyme Thymus pulegioides L. growing wild in Denmark. J. Essent. Oil Res. 2008, 20, 40–41. [Google Scholar] [CrossRef]
- Salgueiro, L.; Vila, R.; Tomas, X.; Tomi, F.; Cañigueral, S.; Casanova, J.; Proença da Cunha, A.; Adzet, T. Chemical polymorphism of the essential oil of Thymus carnosus from Portugal. Phytochemistry 1995, 38, 391–396. [Google Scholar] [CrossRef]
- Wu, Z.; Ta, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Kaul, M.K.; Shahi, A.K.; Kumar, A.; Ram, G.; Tawa, A. Chemical composition of essential oils in Mentha spicata L. accession IIIMJ26 from North-West Himalayan region, India. Ind. Crops Prod. 2009, 29, 654–656. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Shahid, M.; Ashraf, M.; Przybylski, R. Chemical composition, and antioxidant and antimicrobial activities of essential oil of spearmint Mentha spicata L. from Pakistan. J. Essent. Oil Res. 2010, 22, 78–84. [Google Scholar] [CrossRef]
- MacTavish, H.; Harris, D. A case study comparing non-UK lavender/lavandin production and peppermint/spearmint with UK production techniques and costs. In ADAS R & D contract report (M137/62) to the Government-Industry Forum on Non Food Uses of Crops; ADAS: Nottingham, UK, 2002. [Google Scholar]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil Syzygium aromaticum L. Myrtaceae: Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Čmiková, N.; Galovičová, L.; Schwarzová, M.; Vukic, M.D.; Vukovic, N.L.; Kowalczewski, P.Ł.; Bakay, L.; Kluz, M.I.; Puchal-ski, C.; Kačániová, M. Chemical Composition and Biological Activities of Eucalyptus globulus Essential Oil. Plants 2023, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef]
- Fang, R.; Jiang, C.H.; Wang, X.Y.; Zhang, H.M.; Liu, Z.L.; Zhou, L.; Du, S.S.; Deng, Z.W. Insecticidal activity of essential oil of Carum carvi fruits from China and its main components against two grain storage insects. Molecules 2010, 15, 9391–9402. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Prajapati, V.; Kumar, S. Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J. Econ. Entomol. 2003, 96, 1594–1601. [Google Scholar] [CrossRef]
- Petrović, M.; Popović, A.; Kojić, D.; Šućur, J.; Bursić, V.; Aćimović, M.; Malenčić, Đ.; Stojanović, T.; Vuković, G. Assessment of toxicity and biochemical response of Tenebrio molitor and Tribolium confusum exposed to Carum carvi essential oil. Entomol. Gen. 2019, 38, 333–348. [Google Scholar] [CrossRef]
- Shaaya, E.; Ravid, U.; Paster, N.; Juven, B.; Zisman, U.; Pissarev, V. Fumigant toxicity of essential oils against four major stored-product insects. J. Chem. Ecol. 1991, 17, 499–504. [Google Scholar] [CrossRef]
- Alkan, M. Chemical composition and insecticidal potential of different Origanum spp. essential oils against four stored product pests. Turk. J. Entomol. 2020, 44, 149–163. [Google Scholar] [CrossRef]
- Sadeghi, A.; Pourya, M.; Smagghe, G. Insecticidal activity and composition of essential oils from Pistacia atlantica subsp. kurdica against the model and stored product pest beetle Tribolium castaneum. Phytoparasitica 2016, 44, 601–607. [Google Scholar] [CrossRef]






| Distance Moved (d) | Time Spent (t) | (d + t)/2 | ||||
|---|---|---|---|---|---|---|
| EO | Sd/Cd | Effect | St/Ct | Effect | (Sd/Cd + St/Ct)/2 | Effect |
| Cymbopogon flexuosus | 6.47 * | Average | 4.87 * | Low | 5.67 | Low |
| Thymus vulgaris | 5.29 * | Low | 4.99 * | Low | 5.14 | Low |
| Levandula hybrida | 5.15 * | Low | 6.35 * | Average | 5.75 | Low |
| Eugenia caryophyllus | 12.76 * | High | 10.85 * | Average | 11.80 | Average |
| Mentha spicata | 17.24 * | High | 15.47 * | High | 16.36 | High |
| Eucalyptus globulus | 2.23 * | Low | 1.84 | No | 2.04 | Low |
| No | Compound Name | RIExp/RILit | Thymus EO % | Spearmint EO % | Lavandin EO % | Clove EO % | Eucalyptus EO % | Lemongrass EO % |
|---|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | 939/939 | 0.95 | 0.26 | 0.23 | 5.22 | 0.12 | |
| 2 | Sabinene | 975/975 | 2.84 | 0.11 | 0.06 | 0.09 | ||
| 3 | Myrcene | 990/990 | 3.75 | 0.83 | 0.32 | 0.38 | 0.04 | |
| 4 | α-Terpinene | 1017/1018 | 2.89 | 0.16 | ||||
| 5 | para-Cymene | 1024/1024 | 6.00 | 0.19 | 0.10 | 0.72 | 0.01 | |
| 6 | Limonene | 1030/1029 | 1.95 | 11.31 | 0.50 | 0.16 | tr. | 0.24 |
| 7 | 1.8-Cineole | 1031/1031 | 0.28 | 0.56 | 4.15 | 86.56 | ||
| 8 | γ-Terpinene | 1059/1059 | 6.52 | 0.04 | 0.02 | 3.97 | ||
| 9 | cis-Sabinene hydrate | 1071/1070 | 36.55 | 0.40 | ||||
| 10 | Allyl hexanoate | 1080/1079 | 1.28 | |||||
| 11 | Terpinolene | 1088/1088 | 1.02 | 0.02 | 0.04 | 0.04 | 0.05 | |
| 12 | Linalool | 1096 | tr. | 0.07 | 27.88 | 0.02 | 0.03 | 1.18 |
| 13 | trans-Sabinene hydrate | 1098/1099 | 5.74 | |||||
| 14 | Camphor | 1145/1146 | 0.15 | 8.31 | ||||
| 15 | cis-Isocitral | 1165/1164 | 1.04 | |||||
| 16 | Borneol | 1169/1169 | 0.13 | 2.39 | 0.4 | |||
| 17 | Terpinen-4-ol | 1177/1177 | 13.08 | 0.63 | 2.59 | 0.25 | ||
| 18 | trans-Isocitral | 1179/1180 | 1.87 | |||||
| 19 | α-Terpineol | 1188/1188 | 2.36 | 1.09 | 1.07 | 0.19 | ||
| 20 | cis-Dihydro carvone | 1193/1192 | 0.14 | 1.05 | ||||
| 21 | Neral | 1239/1238 | 0.03 | 30.48 | ||||
| 22 | Carvone | 1242/1243 | 0.07 | 78.01 | 0.03 | |||
| 23 | Linalyl acetate | 1252/1257 | 0.26 | 39.78 | 0.01 | |||
| 24 | Geraniol | 1257/1257 | 0.18 | 6.31 | ||||
| 25 | Geranial | 1268/1268 | 0.04 | 42.07 | ||||
| 26 | Lavandulyl acetate | 1289/1290 | 3.28 | |||||
| 27 | Thymol | 1290/1290 | 7.42 | |||||
| 28 | Eugenol | 1359/1359 | 75.86 | 0.04 | ||||
| 29 | Geranyl acetate | 1380/1379 | 0.58 | 4.08 | ||||
| 30 | β-Bourbonene | 1388/1388 | 0.03 | 1.56 | 0.03 | 0.01 | ||
| 31 | trans-Caryophyllene | 1420/1419 | 2.26 | 0.80 | 1.77 | 9.84 | tr. | 1.51 |
| 33 | α-Humulene | 1454/1454 | 0.07 | 1.09 | 0.16 | |||
| 34 | γ-Cadinene | 1512/1513 | 0.01 | 0.23 | 1.52 | |||
| 35 | Eugenol acetate | 1520/1521 | 11.64 |
| Distance Moved (d) | Time Spent (t) | (d + t)/2 | |||||
|---|---|---|---|---|---|---|---|
| Chemical Compound | Dose (mM) | Cd/Sd | Effect | Ct/St | Effect | ((Cd/Sd) + (Ct/St))/2 | Effect |
| Carvone | 0.01 | 1.47 | No | 1.54 | No | 1.51 | Low |
| 0.1 | 1.76 * | Low | 2.18 | No | 1.97 | Low | |
| 1 | 5.02 * | High | 3.62 * | Average | 4.32 | High | |
| Limonene | 0.01 | 0.85 | No | 0.77 | No | 0.81 | No |
| 0.1 | 1.17 | No | 1.29 | No | 1.23 | No | |
| 1 | 1.24 | No | 1.55 | No | 1.40 | No | |
| Myrcene | 0.01 | 0.98 | No | 1.21 | No | 1.10 | No |
| 0.1 | 1.09 | No | 1.31 | No | 1.20 | No | |
| 1 | 0.97 | No | 0.92 | No | 0.95 | No | |
| γ-Terpinene | 0.01 | 1.13 | No | 1.18 | No | 1.155 | No |
| 0.1 | 1.25 | No | 1.37 | No | 1.31 | No | |
| 1 | 0.93 | No | 1.04 | No | 0.985 | No | |
| cis-Sabinene hydrate | 0.01 | 1.37 | No | 1.83 | No | 1.6 | No |
| 0.1 | 1.30 | No | 1.34 | No | 1.32 | No | |
| 1 | 1.92 * | Low | 1.46 | No | 1.69 | Low | |
| 4-Terpineol | 0.01 | 1.81 * | Low | 2.82 | No | 2.32 | Low |
| 0.1 | 3.60 * | Average | 4.21 * | High | 3.91 | Average | |
| 1 | 2.06 * | Average | 3.61 * | Average | 2.84 | Average | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bumbulytė, G.; Būdienė, J.; Būda, V. Essential Oils and Their Components Control Behaviour of Yellow Mealworm (Tenebrio molitor) Larvae. Insects 2023, 14, 636. https://doi.org/10.3390/insects14070636
Bumbulytė G, Būdienė J, Būda V. Essential Oils and Their Components Control Behaviour of Yellow Mealworm (Tenebrio molitor) Larvae. Insects. 2023; 14(7):636. https://doi.org/10.3390/insects14070636
Chicago/Turabian StyleBumbulytė, Gabrielė, Jurga Būdienė, and Vincas Būda. 2023. "Essential Oils and Their Components Control Behaviour of Yellow Mealworm (Tenebrio molitor) Larvae" Insects 14, no. 7: 636. https://doi.org/10.3390/insects14070636
APA StyleBumbulytė, G., Būdienė, J., & Būda, V. (2023). Essential Oils and Their Components Control Behaviour of Yellow Mealworm (Tenebrio molitor) Larvae. Insects, 14(7), 636. https://doi.org/10.3390/insects14070636






