First Discovery of the North American Leaf-Mining Moth Chrysaster ostensackenella (Lepidoptera: Gracillariidae) in Russia: The Genetic Diversity of a Novel Pest in Invaded vs. Native Range
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling, Rearing, Photographing
2.3. DNA Barcoding
3. Results
3.1. Novel Record and the Species Biology
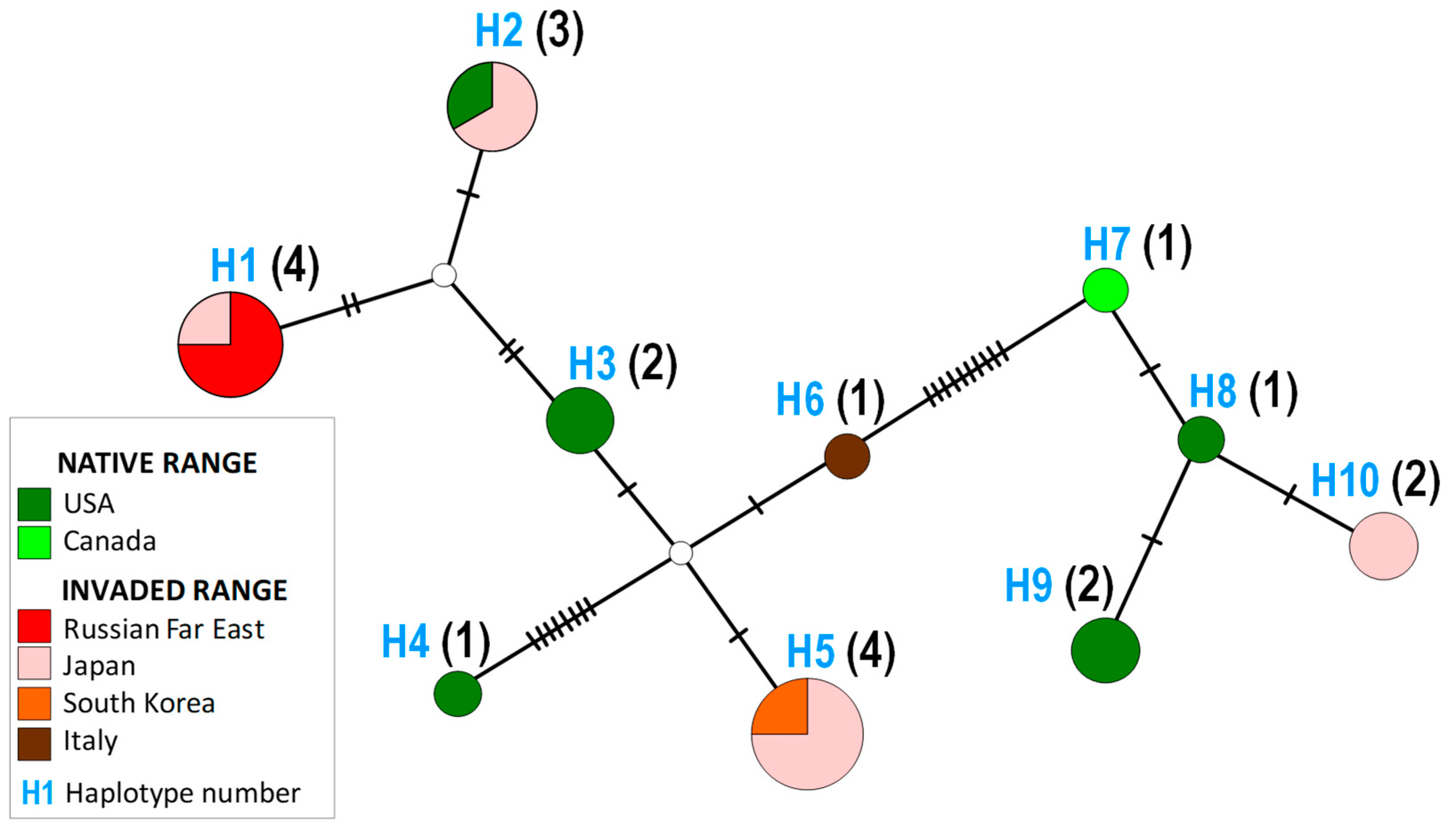
3.2. Genetic Data in Invaded vs. Native Rage
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maslyakov, V.Y.; Izhevskiy, S.S. Invasions of Herbivorous Insects in the European Part of Russia; IGRAN: Moscow, Russia, 2011; 272p. (In Russian) [Google Scholar]
- Kirichenko, N.; Augustin, S.; Kenis, M. Invasive leafminers on woody plants: A global review of pathways, impact, and management. J. Pest Sci. 2019, 92, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Steyn, L.A.I.; Geertsema, H.; Malan, A.P.; Addison, P.A. Review of leaf-mining insects and control options for their management, with special reference to Holocacista capensis (Lepidoptera: Heliozelidae) in vineyards in South Africa. S. Afr. J. Enol. Vitic. 2020, 40, 218–232. [Google Scholar] [CrossRef]
- Lopez-Vaamonde, C.; Kirichenko, N.; Cama, A.; Doorenweerd, C.; Godfray, H.C.J.; Guiguet, A.; Gomboc, S.; Huemer, P.; Landry, J.-F.; Laštuvka, A.; et al. Evaluating DNA barcoding for species identification and discovery in European gracillariid moths. Front. Ecol. Evol. 2021, 9, 626752. [Google Scholar] [CrossRef]
- Hering, E.M. Biology of the Leafminers; Junk’s: Gravenhage, The Hague, 1951; 420p. [Google Scholar]
- Connor, E.F.; Taverner, M. The evolution and adaptive significance of the leaf-mining habit. Oikos 1997, 79, 6–25. [Google Scholar] [CrossRef] [Green Version]
- Stiling, P.; Simberloff, D. Leaf abscission: Induced defense against pests or response to damage? Oikos 1989, 55, 43–49. [Google Scholar] [CrossRef]
- Wagner, D.; Doak, P.; Schneiderheinze, J. Impact of epidermal leaf mining by the aspen leaf miner (Phyllocnistis populiella) on the growth, physiology, and leaf longevity of quaking aspen. Oecologia 2008, 157, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.J.; Hughes, L. Leaf miners: The hidden herbivores. Austral Ecol. 2010, 35, 300–313. [Google Scholar] [CrossRef]
- Šefrová, H. Phyllonorycter issikii (Kumata, 1963)—Bionomics, ecological impact and spread in Europe (Lepidoptera, Gracillariidae). Acta Univ. Agric. Silvic. Mendel Brunen 2002, 50, 99–104. [Google Scholar]
- Gilbert, M.; Grégoire, J.-C.; Freise, J.F.; Heitland, W. Long-distance dispersal and human population density allow the prediction of invasive patterns in the horse chestnut leafminer Cameraria ohridella. J Anim. Ecol. 2004, 73, 459–468. [Google Scholar] [CrossRef]
- Musolin, D.L.; Kirichenko, N.I.; Karpun, N.N.; Aksenenko, E.V.; Golub, V.B.; Kerchev, I.A.; Mandelshtam, M.Y.; Vasaitis, R.; Volkovitsh, M.G.; Zhuravleva, E.N.; et al. Invasive insect pests of forests and urban trees in Russia: Origin, pathways, damage, and management. Forests 2022, 13, 521. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Hughes, C.; Zhang, H.; Kirk Hillier, N.; Morrison, A.; Johns, R. Impact of the invasive beech leaf-mining weevil, Orchestes fagi, on American beech in Nova Scotia, Canada. Front. For. Glob. Chang. 2020, 3, 46. [Google Scholar] [CrossRef]
- Kirichenko, N.I.; Karpun, N.N.; Zakharchenko, V.Y.; Mamaev, N.A.; Musolin, D.L. Alien invasive gracillariid moths as pests of woody plants in forest park zones of southern Russia. In Forests of Russia: Policy, Industry, Science, and Education. Proceedings of the VI Pan-Russian Scientific and Technical Conference; Dobrovolskiy, A.A., Ed.; SPbFTU: Saint Petersburg, Russia, 2021; Volume 1, pp. 208–211. (In Russian) [Google Scholar]
- De Prins, J.; De Prins, W. Global Taxonomic Database of Gracillariidae. 2023. Available online: http://www.gracillariidae.net/ (accessed on 16 May 2023).
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; Vonderlippe, M.; Weber, E. Biological flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Li, G.Q.; Xu, G.H.; Guo, K.; Du, S. Mapping the global potential geographical distribution of black locust (Robinia pseudacacia L.) using herbarium data and a maximum entropy model. Forests 2014, 5, 2773–2792. [Google Scholar] [CrossRef] [Green Version]
- Kolyada, N.A.; Kolyada, A.S. Robinia pseudoacacia L. (Fabaceae Lindl.) in the south of the Russian Far East. Rus. J. Biol. Invasions 2018, 9, 215–218. (In Russian) [Google Scholar] [CrossRef]
- Kolyada, N.A. Robinia pseudoacacia (Robinia pseudoacacia L., Fabaceae Lindl.) as part of a secondary plant community. Astrakhan Bull. Ecol. Educ. 2020, 3, 190–196. (In Russian) [Google Scholar]
- Tytar, V.; Nekrasova, O.; Marushchak, O.; Pupins, M.; Skute, A.; Čeirāns, A.; Kozynenko, I. The Spread of the Invasive Locust Digitate Leafminer Parectopa robiniella Clemens, 1863 (Lepidoptera: Gracillariidae) in Europe, with Special Reference to Ukraine. Diversity 2022, 14, 605. [Google Scholar] [CrossRef]
- Medzihorský, V.; Trombik, J.; Mally, R.; Turčáni, M.; Liebhold, A.M. Insect invasions track a tree invasion: Global distribution of black locust herbivores. J. Biogeogr. 2023, 50, 1285–1298. [Google Scholar] [CrossRef]
- Braun, A.F. Revision of the North American species of the genus Lithocolletis Hübner. Trans. Am. Entomol. Soc. 1908, 34, 311–312. [Google Scholar]
- Bai, H.Y.; Xu, J.S.; Dai, X.H. Three new species, two newly recorded species and one newly recorded genus of Lithocolletinae (Lepidoptera: Gracillariidae) from China. Zootaxa 2015, 4032, 229–235. [Google Scholar] [CrossRef]
- Liu, T.; Cai, Y.-P.; Wang, C.-Z.; Li, H.-H. Biology of Chrysaster ostensackenella (Fitch), a new invasive pest of black locust Robinia pseudoacacia L. plantations, and a new record of a related species, in China. Chin. J. Appl. Entomol. 2015, 52, 942–950. [Google Scholar]
- Koo, J.M.; Kim, S.K.; Cho, S.W. Chrysaster ostensackenella (Fitch, 1859) (Lepidoptera: Gracillariidae) New to Korea. Korean J. Appl. Entomol. 2019, 58, 225–228. [Google Scholar]
- Sawada, M.; Sakurai, M. The first record of an alien species, Chrysaster ostensackenella (Fitch, 1859) (Lepidoptera: Gracillariidae), in Japan. Jpn. J. Ent. 2022, 25, 106–110. [Google Scholar]
- Huemer, P.; Mayr, T. Chrysaster ostensackenella (Fitch, 1859), a potentially invasive species newly recorded from Europe (Lepidoptera, Gracillariidae). Check List 2022, 18, 1237–1242. [Google Scholar] [CrossRef]
- Kolyada, N.A. Clarification of secondary area boundaries of North American potentially invasive plant species in the south of the Russian Far East. Sib. J. For. Sci. (Sibirskij Lesnoj Zurnal) 2021, 1, 68–76. (In Russian) [Google Scholar]
- ESRI ArcGIS Pro Software. Available online: https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview (accessed on 30 May 2023).
- Robinson, G.S. The preparation of slides of Lepidoptera genitalia with special reference to the Microlepidoptera. Entomologist’s Gaz. 1976, 27, 127–132. [Google Scholar]
- de Waard, J.R.; Ivanova, N.V.; Hajibabaei, M.; Hebert, P.D.N. Assembling DNA barcodes: Analytical methods. In Methods in Molecular Biology: Environmental Genetics; Cristopher, M., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molec. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. PopART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Gomboc, S. (independent researcher, Slovenia) On findings of the upper side blotch mines of Macrosaccus robiniella on Robinia pseudoacacia in Slovenia. Personal communication, 2023.
- Khapugin, A.A. A global systematic review of publications concerning the invasion biology of four tree species of temperate Eurasia. Hacquetia 2019, 18, 233–270. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.D. Addressing geographical bias: A review of Robinia pseudoacacia (black locust) in the Southern Hemisphere. S. Afr. J. Bot. 2019, 125, 481–492. [Google Scholar] [CrossRef]
- Nicolescu, V.N.; Rédei, K.; Mason, W.L.; Vor, T.; Pöetzelsberger, E.; Bastein, J.-C.; Brus, R.; Benčať, T.; Đodan, M.; Cvjetkovic, B.; et al. Ecology, growth and management of black locust (Robinia pseudoacacia L.), a non-native species integrated into European forests. J. For. Res. 2020, 31, 1081–1101. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Rogers, P.C.; Huang, J. Black locust (Robinia pseudoacacia L.) range shifts in China: Application of a global model in climate change futures. Clim. Chang. Ecol. 2021, 2, 100036. [Google Scholar] [CrossRef]
- Lombaert, E.; Guillemaud, T.; Cornuet, J.M.; Malausa, T.; Facon, B.; Estouop, A. Bridgehead effect in the worldwide invasion of the biocontrol harlequin ladybird. PLoS ONE 2010, 5, e9743. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, S.E.; Semenyutina, A.V. Prospects of species and forms of the genus Robinia L. for forest protection and landscaping. Adv. Curr. Nat. Sci. 2020, 8, 11–17. [Google Scholar]
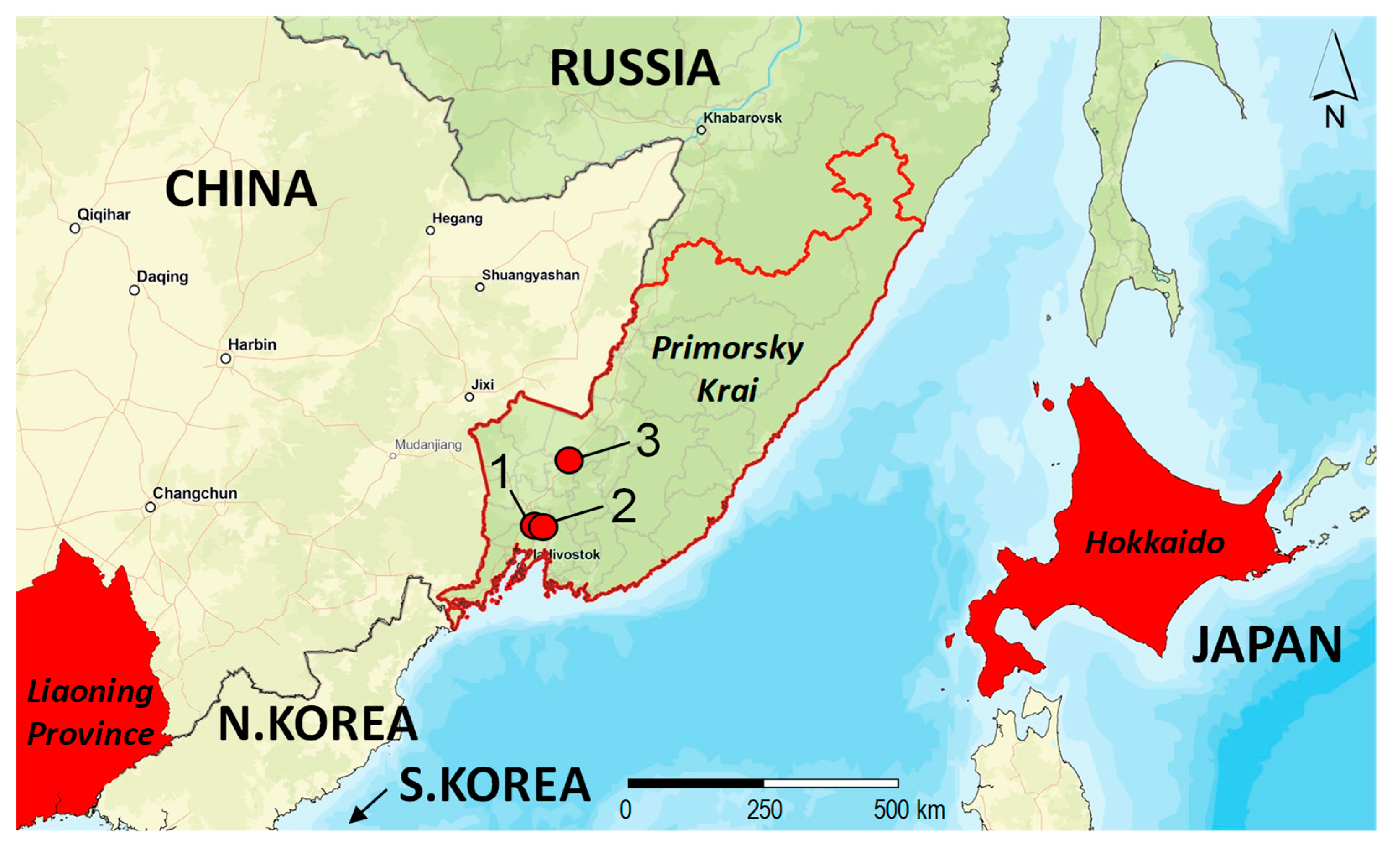



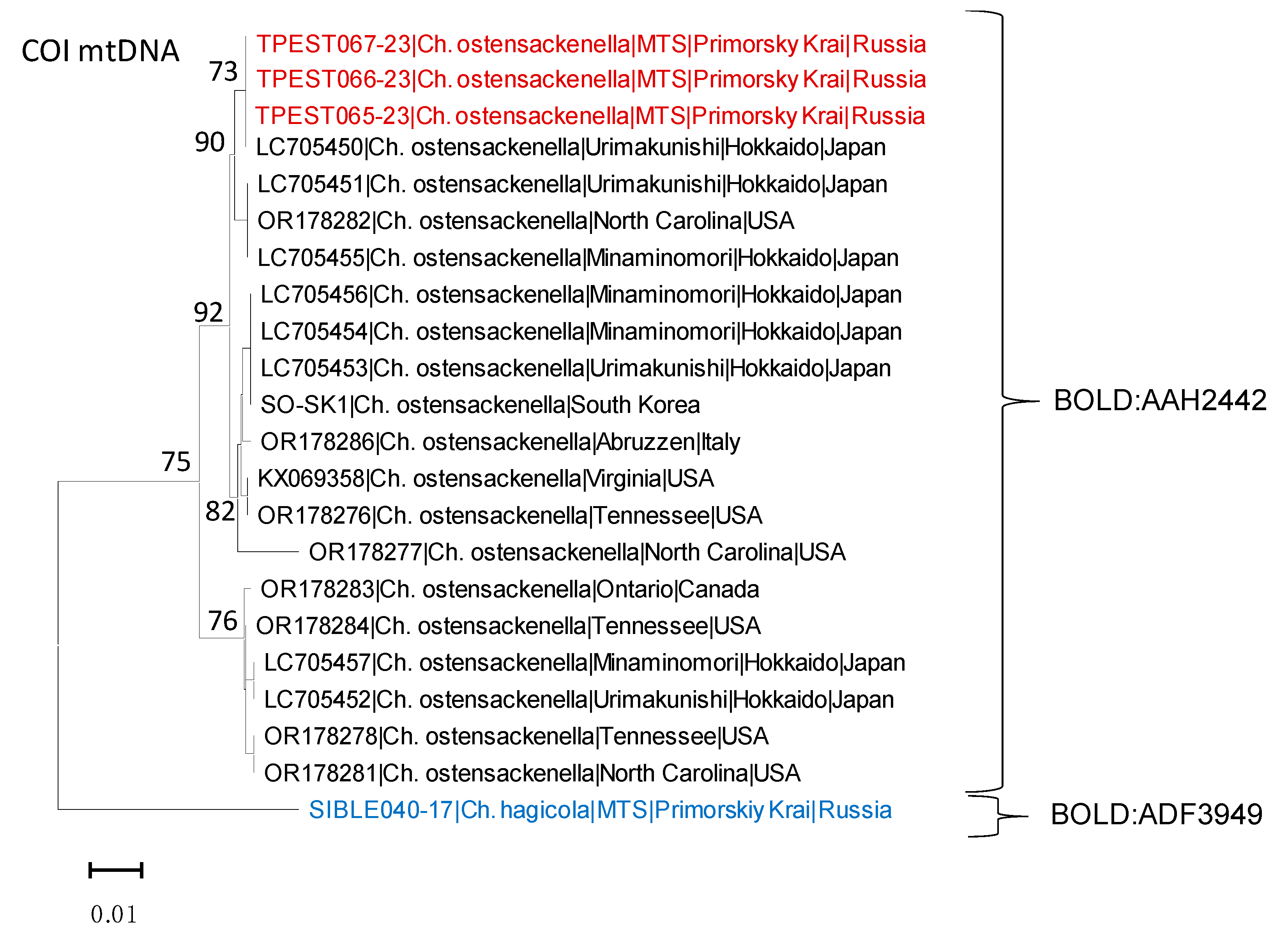

| No. | Sample ID | Country | Region, Place | Collection Date | Collectors 1 | GenBank Accession Number |
|---|---|---|---|---|---|---|
| Original DNA barcodes | ||||||
| 1 | NK1931 | Russia | Primorsky Krai, MTS | 02 August 2022 | NAK | OR178285 |
| 2 | NK1930 | Russia | Primorsky Krai, Khorol | 02 August 2022 | NAK | OR178279 |
| 3 | NK1929 | Russia | Primorsky Krai, MTS | 02 August 2022 | NAK | OR178280 |
| Borrowed DNA barcodes | ||||||
| 4 | DNA-ATBI-3029 | USA | Tennessee | 19 May 2005 | PH, J-FL | OR178278 |
| 5 | DRD-05-0247 | USA | Virginia | 26 June 2005 | JWB | KX069358 |
| 6 | RMNH.INS.544312 | USA | North Carolina | 27 September 2010 | EJN, CD | OR178282 |
| 7 | RMNH.INS.18247 | USA | North Carolina | 27 September 2010 | EJN, CD | OR178277 |
| 8 | RMNH.INS.18245 | USA | North Carolina | 27 September 2010 | EJN, CD | OR178281 |
| 9 | CNCLEP00027381 | USA | Tennessee | 13 August 2006 | JWB | OR178284 |
| 10 | DNA-ATBI-3030 | USA | Tennessee | 19 May 2005 | PH, J-FL | OR178276 |
| 11 | BIOUG35791-H02 | Canada | Ontario | 09 June 2014 | CBG | OR178283 |
| 12 | TLMF Lep 31353 | Italy | Abruzzen | 31 June 2021 | AM | OR178286 |
| 13 | CO-SK1 | South Korea | not indicated | 06-07.2017 | JMK, SKK, HEL | see [25] |
| 14 | MS044 | Japan | Hokkaido, Mi | 19 September 2021 | MS, MSak | LC705457 |
| 15 | MS043 | Japan | Hokkaido, Mi | 19 September 2021 | MS, MSak | LC705456 |
| 16 | MS042 | Japan | Hokkaido, Mi | 19 September 2021 | MS, MSak | LC705455 |
| 17 | MS041 | Japan | Hokkaido, Mi | 19 September 2021 | MS, MSak | LC705454 |
| 18 | MS036 | Japan | Hokkaido, Uri | 6 August 2021 | MS, MSak | LC705453 |
| 19 | MS035 | Japan | Hokkaido, Uri | 6 August 2021 | MS, MSak | LC705452 |
| 20 | MS034 | Japan | Hokkaido, Uri | 6 August 2021 | MS, MSak | LC705451 |
| 21 | MS033 | Japan | Hokkaido, Uri | 6 August 2021 | MS, MSak | LC705450 |
| Outgroup | ||||||
| 22 | NK551 | Russia | Primorsky Krai | 23 June 2016 | NIK | MK403724 |
| Country, Region 1 | Country, Region | |||||||
|---|---|---|---|---|---|---|---|---|
| Russia, Primorsky Krai | Japan, Hokkaido | USA, North Carolina | South Korea | USA, Tennessee | USA, Virginia | Italy, Abruzzen | Canada, Ontario | |
| Russia, Primorsky Krai | (0) | |||||||
| Japan, Hokkaido | 0–2.08 | (0–2.08) | ||||||
| USA, North Carolina | 0.47–2.08 | 0–3.41 | (1.86–3.29) | |||||
| South Korea | 0.63 | 0–1.92 | 0.79–1.92 | (—) | ||||
| USA, Tennessee | 0.63–2.08 | 0.16–2.08 | 0–3.29 | 0.32–1.92 | (0.16–1.92) | |||
| USA, Virginia | 0.63 | 0.32–1.92 | 0.46–1.92 | 0.32 | 0–1.92 | (0) | ||
| Italy, Abruzzen | 0.95 | 0.32–1.92 | 0.79–1.92 | 0.32 | 0.32–1.92 | 0.32 | (—) | |
| Canada, Ontario | 2.08 | 0.32–2.08 | 0.33–3.08 | 1.92 | 0.32–1.92 | 1.92 | 1.60 | (—) |
| Haplotype | Specimens Process ID and Origin 1 | Ratio | |
|---|---|---|---|
| Native Range | Invaded Range 2 | ||
| H1 | — | LC705450|Uri|Japan OR178285|MTS|Russia OR178279|Khorol|Russia OR178280|MTS|Russia | 0:4 |
| H2 | OR178282|North Carolina|USA | LC705455|Mi|Japan LC705451|Uri|Japan | 1:2 |
| H3 | KX069358|Virginia|USA OR178276|Tennessee|USA | — | 2:0 |
| H4 | OR178277|North Carolina|US | — | 1:0 |
| H5 | — | LC705456|Mi|Japan LC705454|Mi|Japan LC705453|Uri|Japan S. Korea | 0:4 |
| H6 | — | OR178286|Italy | 0:1 |
| H7 | OR178283|Canada | — | 1:0 |
| H8 | OR178284|Tennessee|USA | — | 1:0 |
| H9 | OR178278|Tennessee|USA OR178281|North Carolina|USA | — | 2:0 |
| H10 | — | LC705457|Mi|Japan LC705452|Uri|Japan | 0:2 |
| Sum of haplotypes 3 | 6 | 5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko, N.I.; Kolyada, N.A.; Gomboc, S. First Discovery of the North American Leaf-Mining Moth Chrysaster ostensackenella (Lepidoptera: Gracillariidae) in Russia: The Genetic Diversity of a Novel Pest in Invaded vs. Native Range. Insects 2023, 14, 642. https://doi.org/10.3390/insects14070642
Kirichenko NI, Kolyada NA, Gomboc S. First Discovery of the North American Leaf-Mining Moth Chrysaster ostensackenella (Lepidoptera: Gracillariidae) in Russia: The Genetic Diversity of a Novel Pest in Invaded vs. Native Range. Insects. 2023; 14(7):642. https://doi.org/10.3390/insects14070642
Chicago/Turabian StyleKirichenko, Natalia I., Nina A. Kolyada, and Stanislav Gomboc. 2023. "First Discovery of the North American Leaf-Mining Moth Chrysaster ostensackenella (Lepidoptera: Gracillariidae) in Russia: The Genetic Diversity of a Novel Pest in Invaded vs. Native Range" Insects 14, no. 7: 642. https://doi.org/10.3390/insects14070642
APA StyleKirichenko, N. I., Kolyada, N. A., & Gomboc, S. (2023). First Discovery of the North American Leaf-Mining Moth Chrysaster ostensackenella (Lepidoptera: Gracillariidae) in Russia: The Genetic Diversity of a Novel Pest in Invaded vs. Native Range. Insects, 14(7), 642. https://doi.org/10.3390/insects14070642







