Simple Summary
The ladybird beetle fauna of the Canary Islands is quite specific due to the presence of a number of species that do not occur anywhere else (the so-called endemic species). However, many ladybirds recorded in the archipelago are relatively recent arrivals from various parts of the world, as shown by our previous surveys carried out in several of the Canary Islands, including Fuerteventura, Lanzarote, Gran Canaria, and El Hierro. In this paper, we analyze the ladybird fauna of La Palma, one of the western islands of the archipelago, based on our field survey and already published data. The survey resulted in the recording of 26 species, seven of which had not previously been recorded on La Palma, and two of these seven had not been recorded on any of the Canary Islands. Combining our data and literature reports gives a figure of at least 35 ladybird species recorded to date on La Palma. This is fewer than on the central islands of the Canary archipelago (Gran Canaria and Tenerife), but more than on the other four islands (Fuerteventura, Lanzarote, La Gomera, and El Hierro). This study confirms previous observations that the Canary Islands are often colonized by exotic ladybird species.
Abstract
This paper provides new data on the ladybird beetles (Coccinellidae) of La Palma, one of the western islands of the Canarian archipelago. The field survey of 54 study sites resulted in recording 2494 ladybird individuals belonging to 26 species. Seven of the species recorded were new to La Palma, including two, Harmonia quadripunctata (Pontoppidan) and Nephus reunioni (Fürsch), which were not registered so far on any of the Canary Islands. Novius conicollis (Korschefsky) is synonymized with N. cruentatus (Mulsant). Taking our survey and literature reports into account, a total of at least 35 species of Coccinellidae have so far been recorded on La Palma. This richness in species is lower compared to that of the central islands of the Canarian archipelago, Gran Canaria (42 species) and Tenerife (41 species), but higher than that of the remaining four islands (between 22 and 27 species). The detection of two alien species new to La Palma, Nephaspis bicolor Gordon and Nephus reunioni (Fürsch), confirms earlier observations that colonization of the Canary Islands by ladybird species of exotic origins seems to be a frequent phenomenon.
1. Introduction
There are over 6000 species of Coccinellidae globally of which about 55 species were reported to occur on the Canary Islands [1,2,3,4]. A significant proportion of them are Canarian or Macaronesian endemics, but many species currently occurring on the Canaries are non-indigenous to this archipelago [3,5]. Some, such as Olla v-nigrum (Mulsant), Nephaspis bicolor Gordon, Pharoscymnus flexibilis (Mulsant), and Cheilomenes propinqua (Mulsant), have become established there only in recent years [2,3,4,6], indicating a high rate of colonization and a need to monitor the changes in the Canarian ladybird fauna.
Ladybirds found on the Canary Islands, both indigenous and non-indigenous, are almost exclusively predatory (only one mycophagous species, Vibidia duodecimguttata (Poda), was found by Uyttenboogaard [7] on Gran Canaria). No members of the herbivorous tribe Epilachnini have so far been recorded there, although in nearby Northwest Africa and Southwest Europe, several species of this tribe are found [8,9,10,11]. This discrepancy is consistent with Becker’s [12] finding that the ratio of predatory to herbivorous Coleoptera tends to be higher on oceanic islands than in the nearest mainland areas. According to Becker [12], the likely explanation for this is that predators may find it easier to establish and persist on islands than herbivores because the former are often food generalists in contrast to more food-specialized, host plant-dependent herbivores. It is also well known that generalist predators establishing themselves on islands may have especially destructive effects on those islands’ native biota [13].
This study is a continuation of our faunistic research on the Coccinellidae in the Canary Islands. Our recent surveys on the islands of Fuerteventura [6,14], Lanzarote [15], Gran Canaria [4], and El Hierro [16] revealed many ladybird species not previously recorded on those islands, including several alien species.
2. Materials and Methods
The Canary Islands lie in the northeast Atlantic Ocean near the African coast and are part of the Mediterranean Basin biodiversity hotspot [17]. La Palma is the northwesternmost island, and the most distant from the African mainland. This volcanic island has a relatively small area (706 km2) and reaches a maximum elevation of 2426 m.a.s.l. at Roque de los Muchachos. The mild subtropical Atlantic climate of the island is strongly influenced by humid trade winds that transport moisture from the northeast. As a result, the northern to eastern areas experience relatively stable humid conditions, while the western to southern regions are characterized by low and infrequent precipitation [18]. The vegetation of La Palma includes a wide range of habitats, such as laurisilva and Pinus canariensis forests, coastal habitats, and various kinds of scrub vegetation. In farmland areas, plants of agricultural interest are abundant, and in anthropogenic habitats, decorative plants sustained with irrigation are cultivated (Figure 1).
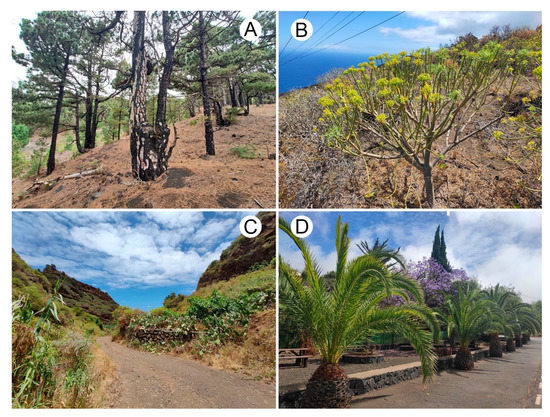
Figure 1.
Some of the habitats surveyed in this study: (A) A pine forest with Pinus canariensis; (B) Scrub vegetation with Euphorbia spp.; (C) Vegetation with Arundo donax and Opuntia ficus-indica near the roadside; (D) Decorative vegetation.
Ladybirds were recorded at 54 sites on La Palma (Table 1) using standard collecting methods, such as a beating tray, a sweeping net, or through direct observation. Although some of the caught ladybird individuals were released after their identification, each individual was noted. The majority of specimens were recorded by J. Romanowski and P. Ceryngier between 16 and 21 June 2021. Some material collected earlier, in 2013, 2014, 2018, and 2019, by A. Machado, T. Staněk, and J. Krátký was also used in this study. The voucher specimens are stored in the insect collection in the Institute of Biological Sciences, Cardinal Stefan Wyszyński University in Warsaw, and in the private collections of Jaroslav Větrovec. Species identification was based on morphological and anatomical features, including the form of reproductive organs (see [4,5,14,16]), and individuals collected in the larval and pupae stages were reared into adults in the laboratory. Habitus images were taken using a Leica MZ 16 stereo microscope with an IC 3D digital camera attached. The photographs of the genitalia were taken using an Olympus DP23 digital camera attached to an Olympus BX43F compound microscope. Final images were produced using Helicon Focus 5.0 × 64 and Adobe Photoshop CS6 software. The systematic arrangement of Coccinellidae used in this study follows Che et al. [19].

Table 1.
Collection sites of ladybird beetles on La Palma.
3. Results
Altogether, 2494 ladybird individuals (2390 adults, 88 larvae, and 16 pupae) belonging to 26 species (including one species identified to the genus level) were recorded in this study. Detailed data on all the recorded species are provided below. Morphological and anatomical details of several species of special interest are photographed.
- Microweiseinae Leng, 1920
- Serangiini Pope, 1962
Delphastus catalinae (Horn, 1895)
Material examined: From Cubo de la Galga, Fuente del Toro, Las Caletas, Mazo, Santa Cruz, and Tazacorte: 17.VI–21.VI.2021, a total of 94 specimens were collected mostly from Ficus sp., Nerium oleander L., Prunus dulcis (Mill.) D.A. Webb, and Punica granatum L.
Distribution: This species has been widely used as a biocontrol agent in various parts of the world. It is thought to occur natively in Colombia, Mexico, southern California (USA), and on the island of Trinidad [20]. In the Canary Islands, it was previously recorded on La Palma, La Gomera, Tenerife, Gran Canaria, Fuerteventura, and Lanzarote [4].
- Coccinellinae Latreille, 1807
- Stethorini Dobzhansky, 1924
Stethorus tenerifensis Fürsch, 1987
Material examined: From Fuencaliente, Fuente del Toro, Las Caletas, Mazo, Malpaíses, Pino de la Virgen, Santa Cruz, and Tijarafe: 17.VI–20.VI.2021, a total of 15 exx. were collected from various plants including P. dulcis, N. oleander, Ficus sp., and Hedera sp.
Distribution: This is an endemic Macaronesian species, reported to be on all main islands of the Canary Islands [1,15] as well as Madeira [11].
Stethorus wollastoni Kapur, 1948
Material examined: From Llano Negro: 19.III.2018, a total of three exx. from Genista canariensis L. and Ilex canariensis Poir. were collected; from Fuente del Toro: 18.06.2021, one male was collected.
Distribution: This is a Macaronesian species reported on all the Canary Islands [1] and Madeira [11].
- Coccinellini Latreille, 1807
Adalia bipunctata (Linnaeus, 1758)
Material examined: From Fuente del Toro: 18.VI.2021, 10 exx. from P. dulcis, were collected; from Tijarafe: 18.VI.2021, five exx. from P. dulcis were collected; from El Paso: 20.VI.2021, one ex. was collected from Ficus sp.; from Mazo: 20.VI–21.VI.2021, two exx. (one adult, one pupa) from Ficus sp. were collected.
Distribution: This species is widespread in the Holarctic, and was also introduced to Australia, New Zealand, Africa, and South America [21,22,23]. In the Canary Islands, it has been found on La Palma, La Gomera, and Tenerife [1].
Remarks: Specimens of A. bipunctata collected in this study represent several color forms, including f. typica, sexpustulata, quadrimaculata, and fasciatopunctata. The lattermost is mainly known from the Middle East and central Asia [24,25]. Its presence on La Palma may, therefore, indicate the source of the Canarian population of A. bipunctata.
Coccinella miranda Wollaston, 1864
Material examined: From Los Braseros: 26.I.2013, 12 exx. were collected; from Barranco de las Angustias, Caldera de Taburiente, Las Manchas, Mirador de Mendo, Montaña de Tagoja, Montes de Luna, and Valencia: 13.III–19.III.2018, a total of 127 exx. were collected; from Barranco del Carmen Dorador, El Pilar, Fuencaliente, Mazo, Mirador Llano de las Ventas, Pared Vieja, Santa Cruz, Tijarafe, La Rosa, and Fuente Olén: 16.VI–21.VI.2021, a total of 169 exx. (167 adults, one pupa, one larva) were collected from Pinus canariensis, Fabaceae, and herbaceous plants.
Distribution: This is an endemic Canarian species reported to be on all islands of the archipelago except Lanzarote [1,15].
Coccinella septempunctata algerica Kovář, 1977
Material examined: From Pared Vieja: 16.VI.2021, two exx. were collected; from Malpaíses: 17.VI.2021, one ex. was collected; from the LP-4 roadside: 18.VI.2021, one ex. from Pinus canariensis was collected.
Distribution: This subspecies occurs mainly in North Africa. It is reported to be on all the Canary Islands [1].
Harmonia quadripunctata (Pontoppidan, 1763)
Material examined: From Barranco del Carmen Dorador: 18.VI.2021, one ex. was collected, and 19.VI.2021, three exx. were collected, all from Pinus canariensis.
Distribution: This is a Palaearctic species [23], not previously recorded on the Canary Islands.
Hippodamia variegata (Goeze, 1777)
Material examined: From El Pilar, Las Indias, Malpaíses, Mazo, Pared Vieja, Santa Cruz, and Tazacorte: 16.VI–21.VI.2021, a total of 38 specimens (26 adults, 12 larvae) were collected from N. oleander, Tamarix sp., Euphorbia sp., ferns, and herbaceous flowering plants.
Distribution: This species is widely distributed in the Palaearctic [23] and in many regions outside of its native range, such as South and North America, Africa, India, Australia, New Zealand, and even the remote Easter Island in the Pacific [26,27,28,29,30]. It is common on all islands of the Canary archipelago [1].
Myrrha octodecimguttata (Linnaeus, 1758)
Material examined: From El Pilar: 16.VI.2021, seven exx. were collected, and 21.VI.2021, eight exx. were collected, all from Pinus canariensis.
Distribution: This is a Palaearctic species [23], recorded in the Canary archipelago on La Gomera [1], and recently also on Gran Canaria [4] and El Hierro [16]. It is new to La Palma.
- Novini Mulsant, 1846
Novius cardinalis (Mulsant, 1850)
Material examined: From Breña Baja: 25.I.2013, one ex. was collected; from Barlovento, Barranco de los Hombres, and Las Caletas: 16.III-17.III.2018, total of three specimens from Euphorbia sp., Kleinia sp., and Vicia sp. were collected; from Buenavista de Arriba, Cudad Alta, Cubo de la Galga, Los Llanos, Mazo, and Santa Cruz: 18.VI–21.VI.2021, a total of 31 specimens (24 adults, three larvae, four pupae) were collected from N. oleander, Hibiscus sp., Phoenix canariensis, and palms.
Distribution: This species is native to Australia but introduced in many regions throughout the world [31]. It is present on all the Canary Islands [1,14,15].
Novius cruentatus (Mulsant, 1846)
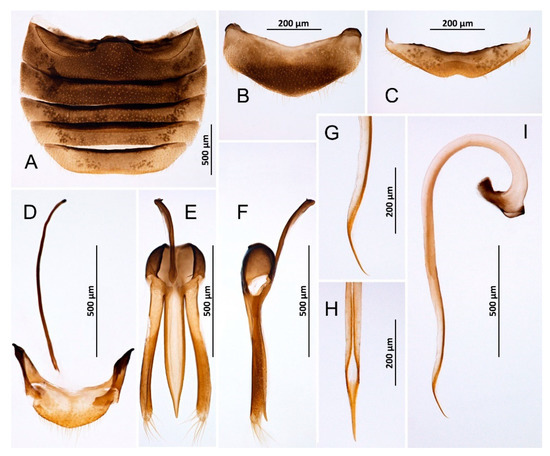
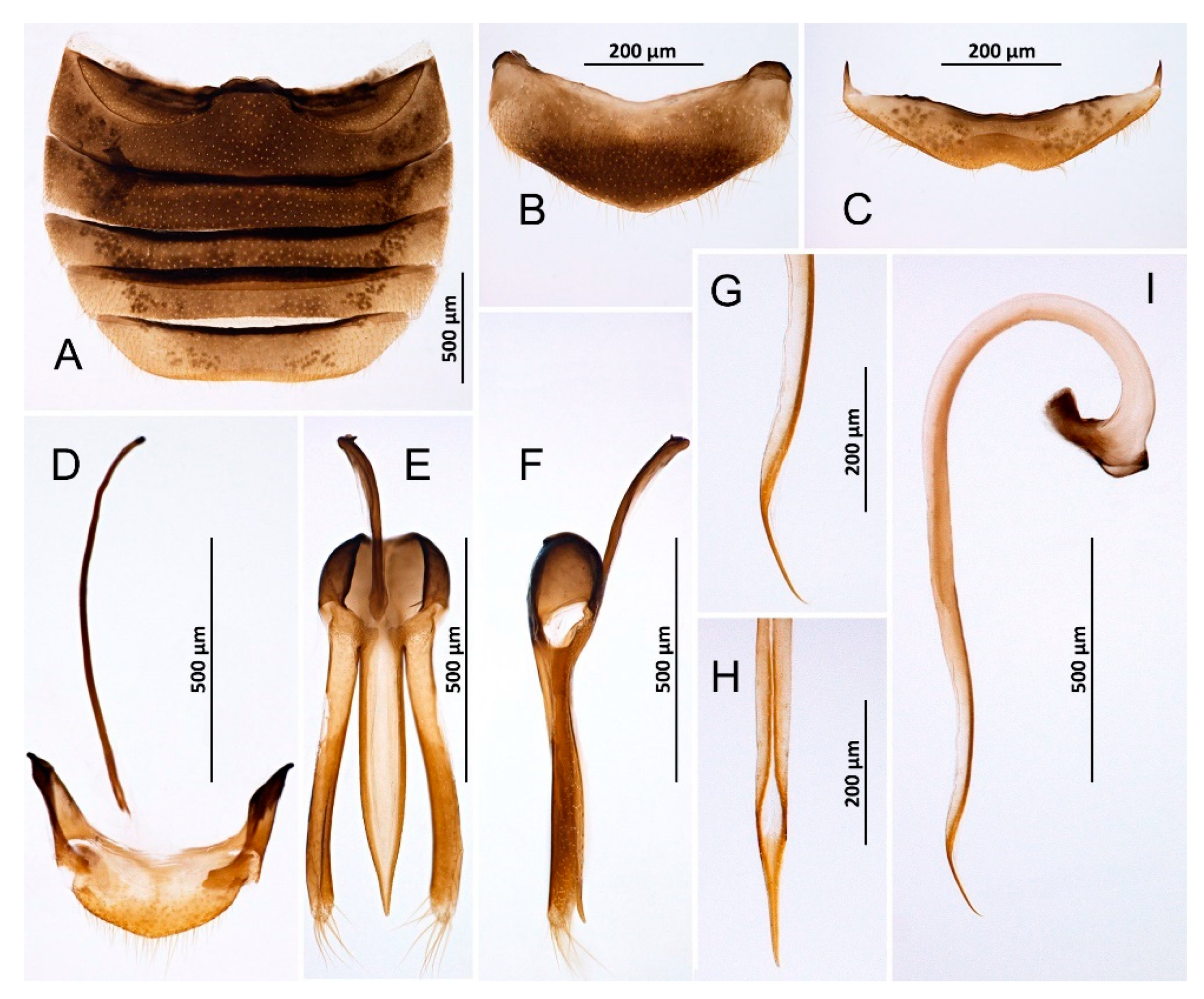
Figure 2.
Novius cruentatus, male (A) Abdomen; (B) Abdominal tergite VIII; (C) Ventrite 6; (D) Abdominal segments IX and X; (E) Tegmen, inner; (F) Tegmen, lateral; (G) Tip of penis, lateral; (H) Tip of penis, inner; (I) Penis, lateral.

Figure 3.
Habitus. (A) Novius cruentatus; (B) Nephaspis bicolor; (C) Diomus sp.
Material examined: From Montaña de Tagoja: 15.III.2018, one ex. was collected; from Barranco del Carmen Dorador: 18.VI.2021, 28 exx. were collected (25 adults, three larvae), and 19.VI.2021, 24 exx. were collected, all from Pinus canariensis.
Distribution: This is a species mainly found in central and southern Europe [23]. It is reported on the Canary Islands either as N. cruentatus or N. conicollis, on El Hierro [1,32], La Palma [1,33,34], Tenerife [1,35], and Gran Canaria [1,4].
Remarks: N. cruentatus is a specialized predator of Palaeococcus fuscipennis (Burmeister), a giant scale (Hemiptera: Monophlebidae) associated with several pine species [36,37,38]. In the Canary Islands, N. cruentatus is usually found on Pinus canariensis ([4,34], this study). Korschefsky [33] considered the specimens collected on La Palma as representing a separate species, different from N. cruentatus and placed them in a newly described species, N. conicollis. However, examination of male genitalia in specimens collected in this study revealed no difference between them and the European specimens of N. cruentatus, and therefore we here synonymize Novius conicollis Korschefsky, 1935 with Novius cruentatus (Mulsant, 1846).
Novius canariensis Korschefsky, 1935
Material examined: From Las Caletas II: 17.III.2018, one ex. was collected (adult female); from Puerto de Puntagorda: 18.III.2018, one ex. was collected.
Distribution: This is an endemic Canarian species, reported to be on Gran Canaria [33], Tenerife [35], and El Hierro [16]. It is new to La Palma.
- Scymnini Mulsant, 1846
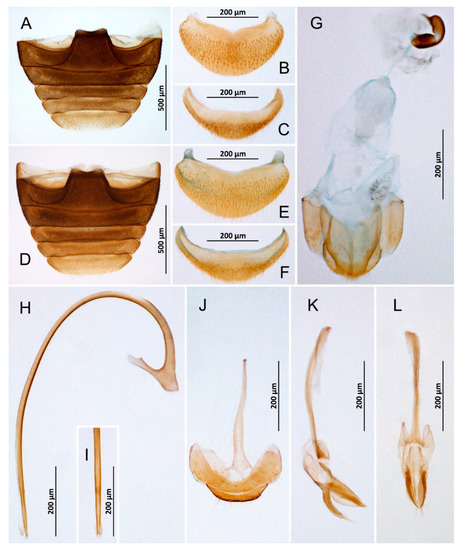
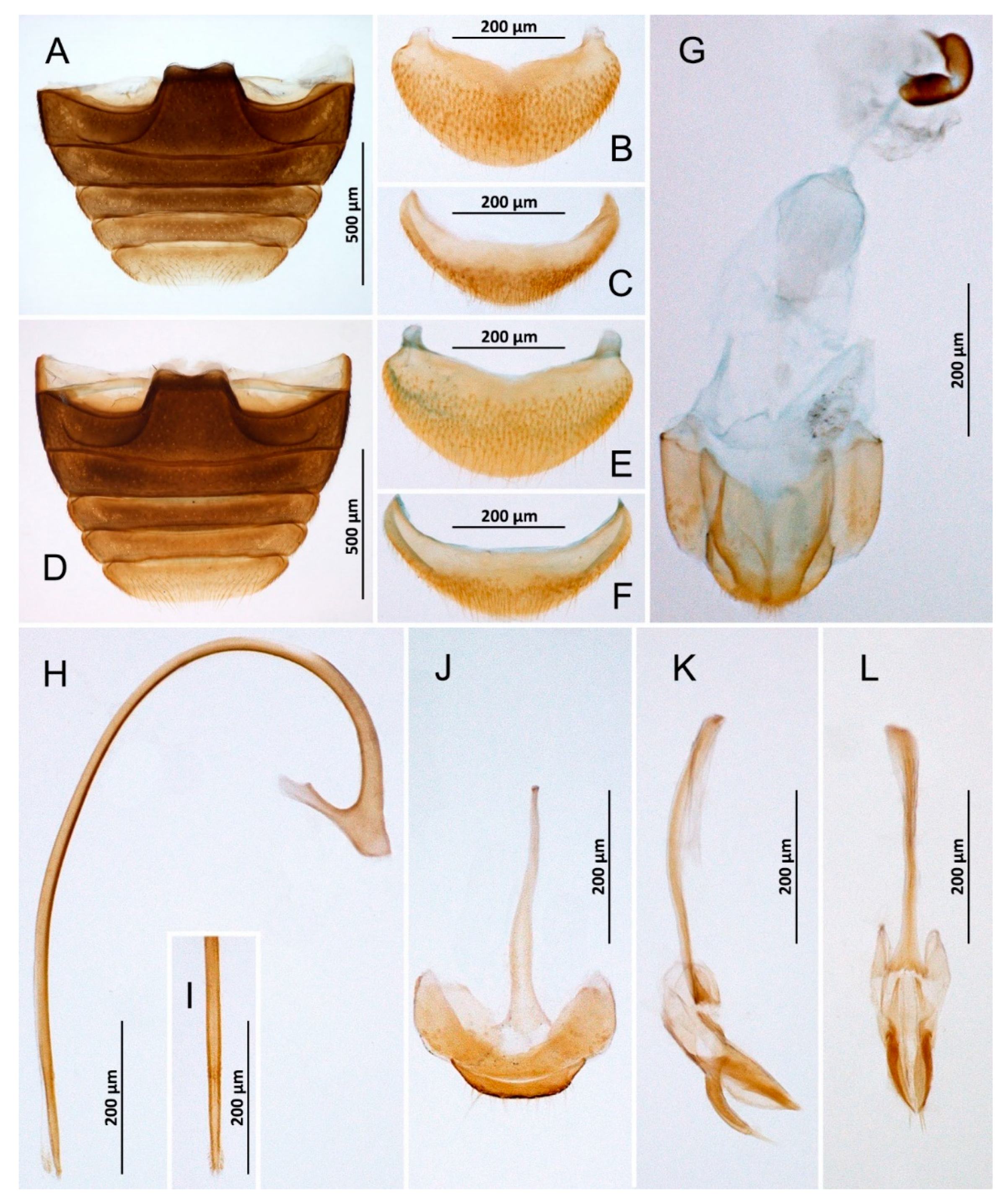
Figure 4.
Nephaspis bicolor. (A) Abdomen, male; (B) Abdominal tergite VIII, male; (C) Ventrite 6, male; (D) Abdomen, female; (E) Tergite VIII, female; (F) Ventrite 6, female; (G) Female terminalia and genitalia; (H) Penis, lateral; (I) Tip of penis, inner; (J) Abdominal segments IX and X, male; (K) Tegmen, lateral; (L) Tegmen, inner.
Material examined: From Santa Cruz: 21.VI.2021, 10 exx. were collected from a palm tree heavily infested with coccids and aleyrodids.
Distribution: This species is native to Trinidad and was introduced into Hawaii [39] and the Canary Islands [2]. Between 2005 and 2010, N. bicolor was introduced on three islands of the archipelago, El Hierro, La Palma, and Tenerife, and in 2015 it was found to be well established on Tenerife [2]. Our present record indicates that this species has also been established on La Palma.
Nephus (Nephus) flavopictus (Wollaston, 1854)
Material examined: From Las Caletas II: 28.I.2013, one ex. was collected; from Cubo de la Galga: 8.II.2014, one ex. was collected; from Puerto Naos: 9.II.2014, two exx. were collected; from Montes de Luna: 13.III.2018, one ex. was collected; from Cueva de los Palmeros: 14.III.2018, one ex. was collected; from Barranco de los Hombres: 16.III.2018, three exx. were collected; from Puntagorda: 18.III.2018, one ex. was collected; from Playa de Nogales: 19.VI.2019, four exx. were collected; from Barranco del Carmen Dorador, Buenavista de Arriba, Fuencaliente, Fuente del Toro, Las Caletas, Malpaíses, Mazo, Montaña de Mago, Puerto Espíndola, San Andrés, Santa Cruz, Tazacorte, and Tijarafe: 17.VI–21.VI.2021, a total of 50 exx. were collected mostly from succulents, Euphorbia sp., Ficus sp., N. oleander, and Hedera sp.
Distribution: This is a Macaronesian species, reported on all the Canary Islands [1].
Nephus (Nephus) incisus (Har. Lindberg, 1950)
Material examined: From Las Caletas: 17.VI.2021, one ex. was collected; from Mazo: 20.VI.2021, one ex. was collected.
Distribution: Until recently, this species was considered to be endemic to the Canary Islands, but new data showed its presence in continental Spain, Algeria, and Cape Verde [40]. It is found on all islands of the Canary archipelago [1,14,15,16].
Nephus (Geminosipho) reunioni (Fürsch, 1974)
Material examined: From Fuente del Toro: 18.VI.2021, one ex. was collected (larva bred to adulthood) from N. oleander; from Los Llanos: 18.VI.2021, 36 exx. were collected; from Santa Cruz: 19.VI.2021, three exx. were collected from P. granatum, and 21.VI.2021, 40 exx. were collected mostly from N. oleander; from Mazo: 21.VI.2021, one ex. was collected from Artemisia sp.
Distribution. This species is endemic to the Mascarene Island of Réunion [41]. In Macaronesia, it was previously reported in the Azores and Madeira [11], but not on the Canary Islands.
Scymnus (Mimopullus) cercyonides Wollaston, 1864
Material examined: From El Tablado: 27.I.2013, one ex. was collected, 13.II.2014, two exx. were collected, and 16.III.2018, two exx. were collected; from Puerto de Puntagorda: 18.III.2018, two exx. were collected; from Mazo: 16.VI.2021, one ex. was collected from Euphorbia sp.; from Las Caletas: 17.VI.2021, one ex. was collected from N. oleander.
Distribution: This is an endemic Canarian species, reported to be on all islands of the archipelago except the easternmost Fuerteventura and Lanzarote [1].
Scymnus (Pullus) canariensis Wollaston, 1864
Material examined: From Los Braseros: 26.I.2013, two exx. were collected; from Montes de Luna: 13.III.2018, 20 exx. were collected; from La Fajana: 16.III.2018, 13 exx. were collected; from Las Caletas II: 17.III.2018, 12 exx. were collected; from Puntagorda: 18.III.2018, 29 exx. were collected; from Barranco del Carmen Dorador, Breña Alta, Buenavista de Arriba, Cudad Alta, Cubo de la Galga, El Granel, El Pilar, El Paso, Fagundo, Fuencaliente, Fuente del Toro, Garafía, Las Caletas, Las Indias, Los Llanos, Malpaíses, Mazo, Mirador de los Dragos, Mirador Llano de las Ventas, Mirador del Time, Montaña de Mago, Pared Vieja, Pico de la Nieve, Pino de la Virgen, Puerto Espíndola, San Andrés, San Juan, Santa Cruz, Tazacorte, and Tijarafe: 16.VI–21.VI.2021, a total of 1147 exx. were collected from various plants including N. oleander, Prunus dulcis, Hibiscus sp., Phoenix canariensis, Pinus canariensis, Euphorbia sp., Hedera sp., Olea europaea L., Arundo donax L., and herbaceous vegetation.
Distribution: This is an endemic Canarian species, recorded throughout the Canary Islands [1].
Scymnus (Scymnus) nubilus Mulsant, 1850
Material examined: From Fuente del Toro, Los Llanos, Mazo, Mirador del Time, Puerto Espíndola, San Andrés, Santa Cruz, and Tazacorte: 16.VI–21.VI.2021, a total of 48 exx. were collected mostly from N. oleander, Ficus sp., A. donax, Tamarix sp., Ricinus communis L., and Phoenix canariensis.
Distribution: This species is widely distributed in the Mediterranean Basin and the Middle East [23]. It is previously recorded on all islands of the Canary archipelago except La Palma [1,14,15].
- Diomini Gordon, 1999
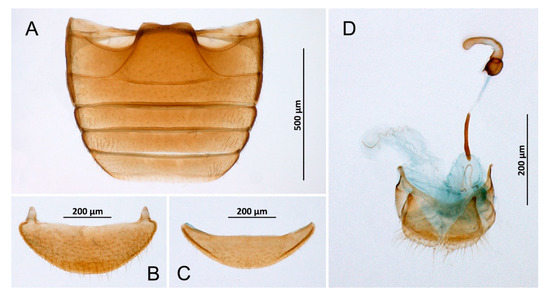
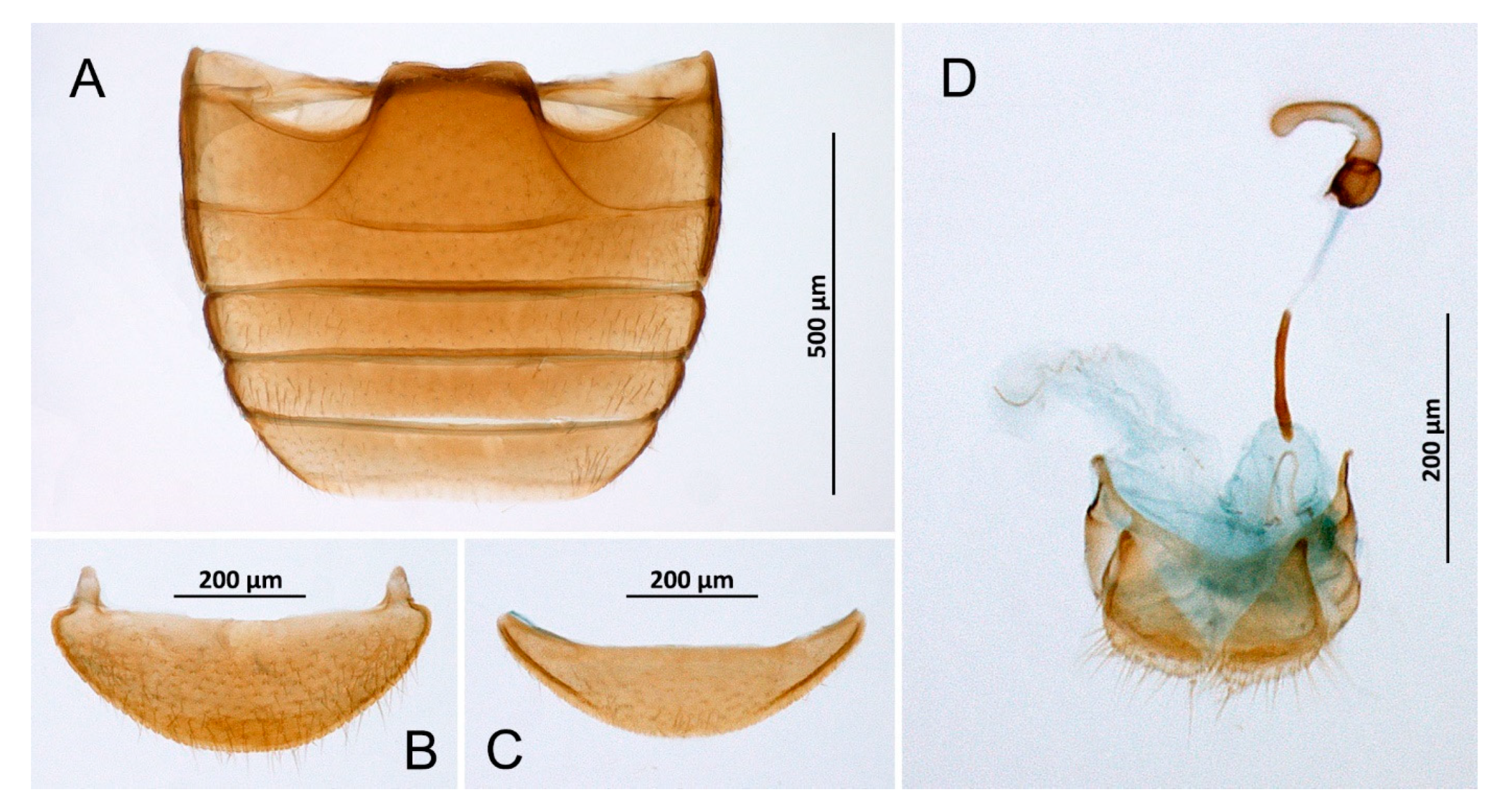
Figure 5.
Diomus sp., female. (A) Abdomen; (B) Abdominal tergite VIII; (C) Ventrite 6; (D) Terminalia and genitalia.
Material examined: From Santa Cruz: 21.VI.2021, one ex. was collected (female) from a palm tree.
Remarks: We are unable to determine the species affiliation of this single female. The genus is represented in the Canary archipelago by one species: Diomus gillerforsi Fürsch, recorded on La Palma and Tenerife [1,42].
- Azyini Mulsant, 1850
Cryptolaemus montrouzieri Mulsant, 1853
Material examined: From Cudad Alta, Fuencaliente, Fuente del Toro, Malpaíses, Mazo, Mirador de los Dragos, Santa Cruz, and Tijarafe: 17.VI–21.VI.2021, a total of 150 exx. were collected (112 adults, 10 larvae, 28 pupae) from N. oleander, Dracaena sp., Opuntia ficus-indica (L.) Mill., P. dulcis, Phoenix canariensis, and Euphorbia sp.
Distribution: This species is a well-known biocontrol agent of Australian origin, established in many warmer regions worldwide [43]. It has been recorded on all seven islands of the Canary archipelago [1,14,15].
- Chilocorini Mulsant, 1846
Chilocorus canariensis Crotch, 1874
Material examined: From Pico de la Cruz: 11.II.2014, one ex. was collected; from La Fajana: 16.III.2018, one ex. was collected; from Las Caletas II: 17.III.2018, two exx. were collected; from Las Caletas: 17.VI.2021, three exx. were collected from Euphorbia sp.; from San Andrés: 19.VI.2021, one ex. was collected; from Santa Cruz: 19.VI.2021, eight larvae were collected from palms.
Distribution: This species is endemic to the Canary Islands, reported to occur on all islands of the archipelago [1].
Parexochomus nigripennis (Erichson, 1843)
Material examined: from Barranco del Carmen Dorador: 19.VI.2021, eight larvae were collected.
Distribution: This species occurs in the Mediterranean and Middle Eastern regions as well as in northwestern India, Pakistan, and the Afrotropical region [23,44]. It has been previously recorded on all islands of the Canary archipelago except La Palma [1].
- Sticholotidini Pope, 1962
Pharoscymnus decemplagiatus (Wollaston, 1857)
Material examined: From Las Caletas II: 12.II.2014, one ex. was collected; from Montes de Luna: 13.III.2018, five exx. were collected; from La Fajana: 16.III.2018, one ex. was collected; from Puntagorda: 18.III.2018, three exx. were collected from Cistus sp.; from Barranco del Carmen Dorador, Fagundo, Fuente del Toro, Las Caletas, Mazo, Mirador de los Dragos, Montaña de Mago, San Andrés, Santa Cruz, Tazacorte, and Tijarafe: 17.VI–20.VI.2021, a total of 151 specimens (124 adults and 27 larvae) were collected from N. oleander, Tamarix sp., Pinus canariensis, and Dracaena sp.
Distribution: This is a Macaronesian species, long known from Madeira [45] and the Canaries [46], and recently also found in the Azores [47]. In the Canary archipelago, it has been recorded on all islands [1,4,14,15].
- Coccidulini Mulsant, 1846
Rhyzobius litura (Fabricius, 1787)
Material examined: From Los Braseros: 26.I.2013, one ex. was collected; from El Tablado: 29.I.2013, three exx. were collected; from Puntagorda: 29.I.2013, two exx. were collected; from Montes de Luna: 30.I.2013, one ex. was collected; from San Juan de Puntallana: 8.II.2014, two exx. were collected; from Barranco de los Hombres: 13.II.2014, three exx. were collected; from Montaña de Tagoja: 15.III.2018, one ex. was collected; from Barlovento: 16.3.2018, five exx. were collected; from La Fajana: 16.III.2018, one ex. was collected; from Tijarafe: 18.III.2018, two exx. were collected; from El Granel: 19.VI.2021, one ex. was collected from Rubus sp.
Distribution: This species is widely distributed in Europe and North Africa and is also reported to occur in the Asiatic part of Turkey [23]. It is found on all the Canary Islands [1].
Rhyzobius lophanthae (Blaisdell, 1892)
Material examined: From Barranco de los Hombres: 13.II.2014, one ex. was collected; from Puerto de Puntagorda: 18.III.2018, one ex. was collected; from Buenavista de Arriba, Cudad Alta, El Paso, Fuencaliente, Fuente del Toro, Las Caletas, Las Indias, Los Llanos, Malpaíses, Mazo, Mirador de los Dragos, San Andrés, Santa Cruz, and Tijarafe: 16.VI–21.VI.2021, a total of 82 exx. were collected mostly from Phoenix canariensis, Dracaena sp., Cycas sp., Hibiscus sp., Ficus sp., Euphorbia sp., and Tamarix sp.
Distribution: This is an Australian species, introduced in many regions of the world as a biocontrol agent [48]. It is found on all islands of the Canary archipelago [1,49].
4. Discussion
Prior to this study, 28 ladybird species were reported to occur on La Palma (Table 2). We failed to find 10 of them but found seven species not previously reported as occurring on this island, including two (Harmonia quadripunctata and Nephus reunioni) that are new to the whole Canary archipelago. Another species recorded by us, Nephaspis bicolor, was released several years ago on La Palma [2] but had not been found as established there prior to this study. We also collected a single female of the Diomus sp. whose species affiliation could not be determined, but whose spermatheca is different from that of the Mediterranean and Middle Eastern D. rubidus (Motschulsky). Perhaps this female belongs to D. gillerforsi Fürsch, a species described on the basis of one male collected on Tenerife [42]. To solve this problem, more material representing both sexes is needed.

Table 2.
Coccinellidae recorded on La Palma. Species new to La Palma are in bold print.
Altogether, the literature reports and our data indicate the occurrence of 35–36 species of Coccinellidae on La Palma. This is fewer than those reported so far on Gran Canaria (42 species) and Tenerife (41 species) [4], but clearly more than on Fuerteventura (27 species), La Gomera (24 species) [1,49], Lanzarote (23 species) [15], and El Hierro (22 species) [16].
Of the species recorded in this study as new to La Palma, one, N. canariensis, is considered to be endemic to the Canary Islands [4]; four, H. quadripunctata, M. octodecimguttata, S. nubilus, and P. nigripennis, occur both on the Canary Islands and the neighboring regions of North Africa and Europe [23]; and two, N. bicolor and N. reunioni, are alien species, native to the remote islands of Trinidad (Lesser Antilles in the Atlantic Ocean) and Réunion (Mascarenes in the Indian Ocean), respectively [39,41].
The La Palma population of N. bicolor is almost certainly derived from individuals introduced to the Canary Islands for biocontrol of whiteflies (Hemiptera: Aleyrodidae). As reported by Rizza Hernández and Hernández Suárez [2], between 2005 and 2010, N. bicolor imported from Trinidad was released at several sites on the islands of La Palma, El Hierro, and Tenerife, and in 2015, abundant, established populations were recorded on Tenerife. On La Palma, a total of only 36 individuals were released in Puerto Naos on the western coast of the island in 2008 and 2009. After about 12 years, we found this ladybird in Santa Cruz, on the opposite (eastern) coast. The distance between these two localities is not great in a straight line (less than 20 km) but, as the island is baffled meridionally by a steep mountain chain, the direct spread of the beetle from west to east may have been impeded. We, therefore, suppose that N. bicolor has dispersed along the coastal areas of La Palma or arrived there from Tenerife.
Nephus reunioni was introduced in the 1970s and 1980s to some Mediterranean countries and the former USSR as a biocontrol agent against mealybugs (Hemiptera: Pseudococcidae), and subsequently established in Albania, France, Greece, Italy, Portugal, and Spain [61]. Later, its occurrence in the Azores and Madeira was documented [11,62].
In addition to the two species mentioned above, five other alien species have been recorded on La Palma. Four of them, American Delphastus catalinae and Australian Novius cardinalis, Cryptolaemus montrouzieri, and Rhyzobius lophanthae were found in this study, but we did not manage to collect the fifth species, American Olla v-nigrum. Most of these alien ladybirds are specialized predators of whiteflies (N. bicolor, D. catalinae) or scale insects (N. reunioni, N. cardinalis, C. montrouzieri, and R. lophanthae), but O. v-nigrum is a generalist predator, most often preying on aphids and psyllids, with a high potential invasiveness [5]. To date, it has become established on many oceanic islands and archipelagos in the Pacific and Indian Oceans (Easter Island, Hawaii, Midway, Guam, Tahiti, New Caledonia, Japan, and Réunion) [63,64,65], and has recently started to spread in Macaronesia. On the Canary Islands, O. v-nigrum has been observed since 2014, first on Tenerife and La Palma and then on Lanzarote and Gran Canaria [4,15,66]. In 2020, it was also recorded in Madeira [5].
Another generalist predator, the Asiatic Harmonia axyridis (Pallas), can also be expected to make an imminent appearance on La Palma and other islands in the Canary archipelago. Specimens of this well-known, now nearly cosmopolitan invasive species [67] were already recorded on Tenerife in 2003 and 2004 [49,68] and recently in 2022 [69]. H. axyridis could not establish, despite its deliberate introductions in the Azores between 1988 and 1995 [70], and the first findings of a reproducing population of H. axyridis in Macaronesia have been reported from Funchal (Madeira) in 2019 and 2020 [5,11].
Author Contributions
Conceptualization, J.R. and P.C.; methodology, J.R., P.C. and K.S.; validation, J.R., P.C. and K.S.; formal analysis, J.R., P.C. and K.S.; investigation, J.R., P.C., J.V. and K.S.; resources, J.R., P.C. and J.V., data curation, J.R.; writing—original draft preparation, J.R. and P.C.; writing—review and editing, all authors; visualization, K.S. and J.R.; supervision, J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the En Arche foundation, grant number 2020/01/15, to J.R.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank Kamila and Mathias Franz for remarks on the manuscript, to Tomasz Czerwiński for taking photographs of the genitalia and habitus of species studied, and the anonymous reviewers for their valuable comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Oromí, P.; de la Cruz, S.; Báez, M. Lista de Especies Silvestres de Canarias. Hongos, Plantas y Animales Terrestres; Arechavaleta, M., Rodríguez, S., Zurita, N., García, A., Eds.; Gobierno de Canarias: Tenerife, Spain, 2010; pp. 254–301. (In Spanish) [Google Scholar]
- Rizza Hernández, R.; Hernández Suárez, E. Éxito en la instalación de Nephaspis bicolor en el Control Biológico Clásico de las moscas blancas espirales en Canarias. Rev. Agropecu. 2019, 23, 85–93. [Google Scholar]
- Eizaguirre, S. Fauna canaria de coccinélidos (Coleoptera: Coccinellidae), addenda et corrigenda. Bol. Soc. Entomol. Aragon. 2020, 66, 103–106. [Google Scholar]
- Romanowski, J.; Ceryngier, P.; Vĕtrovec, J.; Piotrowska, M.; Szawaryn, K. Endemics versus newcomers: The ladybird beetle (Coleoptera: Coccinellidae) fauna of Gran Canaria. Insects 2020, 11, 641. [Google Scholar] [CrossRef]
- Romanowski, J.; Ceryngier, P. Non-indigenous ladybird beetles (Coleoptera: Coccinellidae) in Madeira and other Macaronesian archipelagos. BioInvasions Rec. 2022, 11, 876–886. [Google Scholar] [CrossRef]
- Romanowski, J.; Ceryngier, P.; Szawaryn, K. First records of Pharoscymnus flexibilis (Mulsant, 1853) (Coleoptera: Coccinellidae) on Fuerteventura, Canary Islands. Coleopt. Bull. 2018, 72, 858–860. [Google Scholar] [CrossRef]
- Uyttenboogaart, D.L. Contributions to the knowledge of the fauna of the Canary-Islands. Synopsis of the results of the collecting-excursions 1925 and 1927. Tijdschr. Entomol. 1930, 73, 211–235. [Google Scholar]
- Eizaguirre, S. Coleoptera, Coccinellidae. Fauna Ibérica, Volume 40; Museo Nacional de Ciencias Naturales, CSIC: Madrid, Spain, 2015; 498p. [Google Scholar]
- Chavanon, G. Catalogue des Coléoptères de la région orientale du Maroc (Province de Guercif exceptée). Trav. Inst. Sci. Zool. 2018, 57, 1–192. [Google Scholar]
- Lakhal, M.A.; Ghezali, D.; Nedvĕd, O.; Doumandji, S. Checklist of ladybirds of Algeria with two new recorded species (Coleoptera, Coccinellidae). ZooKeys 2018, 774, 41–52. [Google Scholar] [CrossRef]
- Soares, A.O.; Calado, H.R.; Franco, J.C.; Aguiar, A.F.; Andrade, M.M.; Zina, V.; Ameixa, O.M.C.C.; Borges, I.; Magro, A. An annotated checklist of ladybeetle species (Coleoptera, Coccinellidae) of Portugal, including the Azores and Madeira Archipelagos. ZooKeys 2021, 1053, 107–144. [Google Scholar] [CrossRef]
- Becker, P. Island colonization by carnivorous and herbivorous Coleoptera. J. Anim. Ecol. 1975, 44, 893–906. [Google Scholar] [CrossRef]
- Gillespie, R.G.; Roderick, G.K. Arthropods on islands: Colonization, speciation, and conservation. Annu. Rev. Entomol. 2002, 47, 595–632. [Google Scholar] [CrossRef]
- Romanowski, J.; Ceryngier, P.; Vĕtrovec, J.; Szawaryn, K. The Coccinellidae (Coleoptera) from Fuerteventura, Canary Islands. Zootaxa 2019, 4646, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, J.; Ceryngier, P.; Szawaryn, K. New data on the Coccinellidae (Coleoptera) from Lanzarote, Canary Islands. Coleopt. Bull. 2020, 74, 188–194. [Google Scholar] [CrossRef]
- Romanowski, J.; Ceryngier, P.; Zmuda, C.; Vĕtrovec, J.; Szawaryn, K. The Coccinellidae (Coleoptera) from El Hierro, Canary Islands. Bonn Zool. Bull. 2020, 69, 249–261. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Whittaker, R.J. The Canaries: An important biogeographical meeting place. J. Biogeogr. 2008, 35, 379–387. [Google Scholar] [CrossRef]
- Che, L.; Zhang, P.; Deng, S.; Escalona, H.E.; Wang, X.; Li, Y.; Pang, H.; Vandenberg, N.; Ślipiński, A.; Tomaszewska, W.; et al. New insights into the phylogeny and evolution of lady beetles (Coleoptera: Coccinellidae) by extensive sampling of genes and species. Mol. Phylogenet. Evol. 2021, 156, e107045. [Google Scholar] [CrossRef]
- Hoelmer, K.A.; Pickett, C.H. Geographic origin and taxonomic history of Delphastus spp. (Coleoptera: Coccinellidae) in commercial culture. Biocontrol Sci. Technol. 2003, 13, 529–535. [Google Scholar] [CrossRef]
- Belicek, J. Coccinellidae of western Canada and Alaska with analyses of the transmontane zoogeographic relationships between the fauna of British Columbia and Alberta (Insecta: Coleoptera: Coccinellidae). Quest. Entomol. 1976, 12, 283–409. [Google Scholar]
- Gordon, R.D. The Coccinellidae (Coleoptera) of America north of Mexico. J. N. Y. Entomol. Soc. 1985, 93, 1–912. [Google Scholar]
- Kovář, I.; Löbl, I.; Smetana, A. (Eds.) Catalogue of Palaearctic Coleoptera; Apollo Books: Stentrup, Denmark, 2007; Volume 4, pp. 71–74, 568–630. [Google Scholar]
- Bielawski, R. Coccinellidae (Coleoptera) of Mongolia. Annal. Zool. 1984, 38, 281–460. [Google Scholar]
- Nedvĕd, O.; Biranvand, A.; Shakarami, J.; Şenal, D. Potential Müllerian mimicry between Adalia bipunctata (Linnaeus) and Oenopia conglobata (Linnaeus) (Coleoptera: Coccinellidae) in Iran. Coleopt. Bull. 2020, 74, 161–167. [Google Scholar] [CrossRef]
- Ellis, D.R.; Prokrym, D.R.; Adams, R.G. Exotic lady beetle survey in northeastern United States: Hippodamia variegata and Propylea quatuordecimpunctata (Coleoptera: Coccinellidae). Entomol. News 1999, 110, 73–84. [Google Scholar]
- Franzmann, B.A. Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae), a predacious ladybird new in Australia. Aust. J. Entomol. 2002, 41, 375–377. [Google Scholar] [CrossRef]
- Poorani, J. First record of Hippodamia variegata (Goeze) (Coleoptera: Coccinellidae) from South India. J. Biol. Control 2007, 21, 295–296. [Google Scholar] [CrossRef]
- Grez, A.A.; Rand, T.A.; Zaviezo, T.; Castillo-Serey, F. Land use intensification differentially benefits alien over native predators in agricultural landscape mosaics. Divers. Distrib. 2013, 19, 749–759. [Google Scholar] [CrossRef]
- Bustamante-Navarrete, A.; Marquina-Montesinos, E.L.; Elme-Tumpay, A. Primer registro de Hippodamia variegata (Goeze 1777) (Coleoptera: Coccinellidae) en el Perú. Arq. Entomol. 2017, 17, 347–350. (In Spanish) [Google Scholar] [CrossRef]
- Cock, M.J.W.; van Lenteren, J.C.; Brodeur, J.; Barratt, B.I.P.; Bigler, F.; Bolckmans, K.; Cônsoli, F.L.; Haas, F.; Mason, P.G.; Parra, J.R.P. Do new Access and Benefit Sharing procedures under the Convention on Biological Diversity threaten the future of biological control? BioControl 2010, 55, 199–218. [Google Scholar] [CrossRef]
- Franz, H. Die Ergebnisse meiner langjährigen Aufsammlungen der Coleopterenfauna auf der Insel Hierro (Kanarische Inseln). Sitzungsber. Abt. I 1995, 202, 71–138. (In German) [Google Scholar]
- Korschefsky, R. Zwei neue Novius-Arten von den Canaren. Comment. Biol. 1935, 6, 1–3. (In German) [Google Scholar]
- Nicolas, V.; Rae, S. Nouvelles observations de Coccinelles (Coleoptera, Coccinellidae) dans l’archipel des Canaries. Harmonia 2012, 9, 5–9. (In French) [Google Scholar]
- Israelson, G.; Machado, A.; Oromí, P.; Palm, T. Novedades para la fauna coleopterológica de las Islas Canarias. Vieraea 1982, 11, 109–134. (In Spanish) [Google Scholar]
- Mendel, Z.; Assael, F.; Zeidan, S.; Zehavi, A. Classical biological control of Palaeococcus fuscipennis (Burmeister) (Homoptera: Margarodidae) in Israel. Biol. Control 1998, 12, 151–157. [Google Scholar] [CrossRef]
- Ülgentürk, S.; Evren, N.; Ayhan, B.; Dostbil, Ö.; Dursun, O.; Civelek, H.S. Scale insect (Hemiptera: Coccoidea) species on pine trees of Turkey. Turk. J. Zool. 2012, 36, 623–636. [Google Scholar] [CrossRef]
- Klausnitzer, B. Palaeococcus fuscipennis (Burmeister, 1835) (Sternorrhyncha, Coccina, Margarodidae) in der Oberlausitz. Entomol. Nachr. Ber. 2019, 63, 300–301. [Google Scholar]
- Gordon, R.D. South American Coccinellidae (Coleoptera)—Part V: A taxonomic revision of the genus Nephaspis Casey. Frust. Entomol. 1996, 19, 1–50. [Google Scholar]
- Biranvand, A.; Větrovec, J.; Merzoug, A.; Boualem, M.; Fekrat, L.; Ceryngier, P. Complementary data on the distribution of Nephus (Nephus) incisus (Har. Lindberg, 1950) (Coleoptera: Coccinellidae) in the Palaearctic and Afrotropical regions. Zootaxa 2023, 5244, 287–292. [Google Scholar] [CrossRef]
- Poussereau, J.; Coutanceau, J.-P.; Nicolas, V.; Gomy, Y. Les coccinelles de l’île de la Réunion; Orphie: Chevagny-Sur-Guye, France, 2018; 222p. [Google Scholar]
- Fürsch, H. Die Scymninae der Kanaren, Azoren und Madeiras (Coleoptera Coccinellidae). Acta Coleopt. 1987, 3, 1–14. (In German) [Google Scholar]
- Kairo, M.T.K.; Paraiso, O.; Gautam, R.D.; Peterkin, D.D. Cryptolaemus montrouzieri (Mulsant) (Coccinellidae: Scymninae): A review of biology, ecology, and use in biological control with particular reference to potential impact on non-target organisms. CABI Rev. 2013, 8, 1–20. [Google Scholar] [CrossRef]
- Poorani, J. An annotated checklist of the Coccinellidae (Coleoptera) (excluding Epilachninae) of the Indian subregion. Orient. Insects 2002, 36, 307–383. [Google Scholar] [CrossRef]
- Wollaston, T.V. Catalogue of the Coleopterous Insects of Madeira in the Collection of the British Museum; Order of the Trustees: London, UK, 1857; 234p. [Google Scholar]
- Wollaston, T.V. Catalogue of the Coleopterous Insects of the Canaries in the Collection of the British Museum; Order of the Trustees: London, UK, 1864; 648p. [Google Scholar]
- Calado, H.R.M.G. Ciência Cidadã nos Açores: O uso de Joaninhas (Coleoptera: Coccinellidae) como Espécies-Modelo. Dissertação de Mestrado em Biodiversidade e Biotecnologia. Master’s Thesis, Universidade dos Açores, Ponta Delgada, Portugal, 2018; 82p. [Google Scholar]
- Tomaszewska, W. Rhyzobius Stephens, 1829 (Coleoptera: Coccinellidae), a Revision of the World Species. In Fauna Mundi; Natura optima dux Foundation: Warszawa, Poland, 2010; Volume 2, 475p. [Google Scholar]
- Eizaguirre, S. Revisión de los coleópteros coccinélidos de las islas Canarias (Coleoptera: Coccinelidae). Bol. Soc. Entomol. Aragon. 2007, 41, 101–118. (In Spanish) [Google Scholar]
- García, J.; García, R.; Báez, M.; Oromí, P. Análisis de la diversidad de coleópteros en la Zona de Especial Conservación “Montaña de la Breña”. Rev. Acad. Canar. Cienc. 2018, 30, 19–32. (In Spanish) [Google Scholar]
- Uyttenboogaart, D.L. Report on Canarian Coleoptera collected by R. Frey and R. Storå in 1931 for the Museum Zoologicum Universitatis Helsingfors. Comment. Biol. 1935, 6, 5–17. [Google Scholar]
- Oromí, P.; García, R. Contribución al conocimiento de la fauna de coleópteros de Canarias y su distribución. Vieraea 1995, 24, 175–186. (In Spanish) [Google Scholar]
- Torres del Castillo, R.; Méndez, P.; Carnero, A.; Fernández, M. Plagas de los cultivos de tagasaste (Chamaecytisus proliferus (L. fil.) Link ssp. palmensis (Christ.) Kunkel) en Canarias. Bol. San. Veg. Plagas 1992, 18, 483–489. (In Spanish) [Google Scholar]
- Nicolas, V. Contribution à la connaissance des coccinelles (Coleoptera Coccinellidae) de l’archipel des Canaries. Harmonia 2010, 4, 17–20. (In French) [Google Scholar]
- Fernández, J.M. Coleópteros Canarios. Fáunula de la isla de la Palma. Graellsia 1951, 8, 3–15. (In Spanish) [Google Scholar]
- Kovář, I. A new species of the genus Coccinella (Coleoptera) from North Africa. Acta Entomol. Mus. Nat. Pragae 1977, 39, 231–235. [Google Scholar]
- Hristova Gueorguieva, H. Estudio de Enemigos Naturales de Trioza erytreae (Del Guercio) en Canarias. Master’s Thesis, Universidad Miguel Hernández de Elche, Elche, Spain, 2014. (In Spanish). [Google Scholar]
- Machado, A.; Oromí, P. Elenco de los Coleópteros de las Islas Canarias/Catalogue of the Coleoptera of the Canary Islands; Instituto de Estudios Canarios: La Laguna, Spain, 2000; 306p. (In Spanish) [Google Scholar]
- Lindberg, H. Beitrag zur Kenntnis der Käferfauna der Kanarischen Inseln. Comment. Biol. 1950, 10, 1–20. (In German) [Google Scholar]
- Israelson, G. Some additions to the Coleopterous fauna of the Canary Islands (Coleoptera). Eos 1969, 44, 149–157. [Google Scholar]
- Gerber, E.; Schaffner, U. Review of Invertebrate Biological Control Agents Introduced into Europe; CABI: Wallingford, UK, 2016; xiv+194p. [Google Scholar]
- Soares, A.O. Coleoptera—Coccinellidae. In Listagem dos Organismos Terrestres e Marinhos dos Açores/A List of the Terrestrial and Marine Biota from the Azores; Borges, P.A.V., Costa, A., Cunha, R., Gabriel, R., Gonçalves, V., Martins, A.F., Melo, I., Parente, M., Raposeiro, P., Rodrigues, P., et al., Eds.; Princípia: Cascais, Portugal, 2010; p. 225. [Google Scholar]
- Vandenberg, N.J. Revision of the New World lady beetles of the genus Olla and description of a new allied genus. Ann. Entomol. Soc. Am. 1992, 85, 370–392. [Google Scholar] [CrossRef]
- Vandenberg, N.J. Contributions to the knowledge of Olla Casey (Coleoptera: Coccinellidae: Coccinellini): New species from the Galapagos Islands, updates on the distribution of O. v-nigrum (Mulsant). Proc. Entomol. Soc. Wash. 2004, 106, 619–626. [Google Scholar]
- González, G.; Cotoras, D.D.; Grez, A.A. Annotated list of island lady beetles from Chile (Coleoptera: Coccinellidae). Zootaxa 2020, 4852, 501–526. [Google Scholar] [CrossRef] [PubMed]
- Suárez, D.; Hernández-Teixidor, D.; Pérez-Delgado, A.J.; Roca-Cusachs, M.; Oromí, P. Nuevos registros de distribución de insectos (Insecta, Coleoptera and Diptera) en las Islas Canarias. Bol. Asoc. Esp. Entomol. 2018, 42, 1–11. (In Spanish) [Google Scholar]
- Camacho-Cervantes, M.; Ortega-Iturriaga, A.; del-Val, E. From effective biocontrol agent to successful invader: The harlequin ladybird (Harmonia axyridis) as an example of good ideas that could go wrong. Peer J. 2017, 5, e3296. [Google Scholar] [CrossRef] [PubMed]
- Machado, A. El sarantontón asiático Harmonia axyridis (Pallas, 1773) presente en Canarias (Coleoptera: Coccinellidae). Vieraea 2006, 34, 71–72. (In Spanish) [Google Scholar] [CrossRef]
- Ceryngier, P.; Franz, K.W.; Romanowski, J. Distribution, host range and host preferences of Dinocampus coccinellae (Hymenoptera: Braconidae): A worldwide database. Eur. J. Entomol. 2023, 120, 26–34. [Google Scholar] [CrossRef]
- Soares, A.O.; Honĕk, A.; Martinkova, Z.; Skuhrovec, J.; Cardoso, P.; Borges, I. Harmonia axyridis failed to establish in the Azores: The role of species richness, intraguild interactions and resource availability. BioControl 2017, 62, 423–434. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).