Gut Bacterial Diversity of Insecticide-Susceptible and Insecticide-Resistant Megalurothrips usitatus (Thysanoptera: Thripidae) and Elucidation of Their Putative Functional Roles
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Populations
2.2. Insecticides and Toxicological Bioassay
2.3. Extraction of DNA from the Guts of the IS and IR M. usitatus
2.4. Amplicon Library Construction and Illumina Sequencing
2.5. Processing of the Sequence Reads and Data Analysis
3. Results
3.1. Toxicological Bioassay
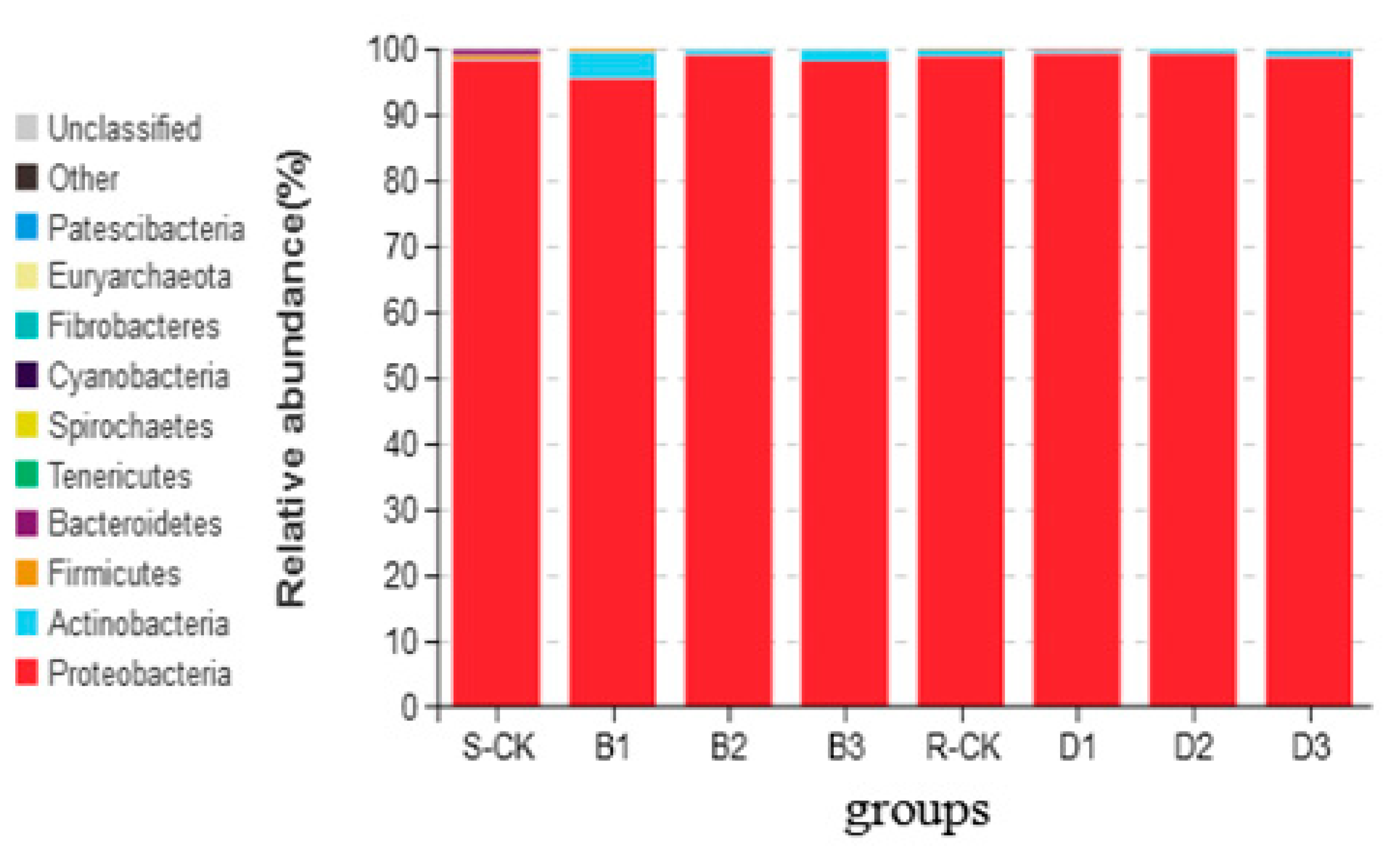
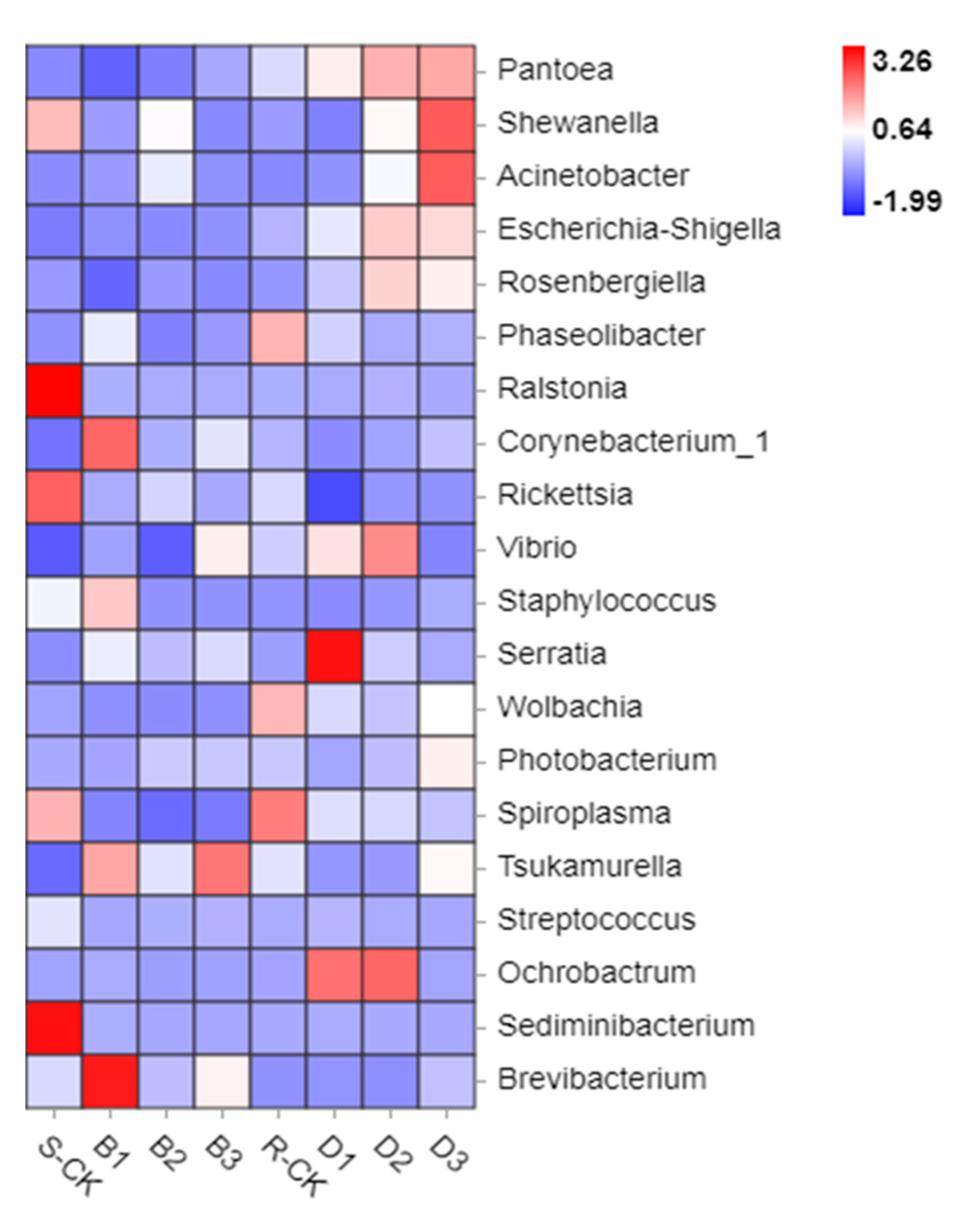
3.2. Illumina MiSeq Metagenomic Data and Taxonomic Assignments
3.3. Functional Analysis of Intestinal Bacteria of Two Populations of M. usitatus after Infection with B. brongniartii Isolate SB010
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, L.D.; Guo, L.H.; Ali, A.; Desneux, N.; Zang, L.S. Synergism of Adjuvants Mixed With Spinetoram for the Management of Bean Flower Thrips, Megalurothrips usitatus (Thysanoptera: Thripidae) in Cowpeas. J. Econ. Entomol. 2022, 115, 2013–2019. [Google Scholar] [CrossRef]
- Liu, P.P.; Qin, Z.F.; Feng, M.Y.; Zhang, L.; Huang, X.Z.; Shi, W.P. The male-produced aggregation pheromone of the bean flower thrips Megalurothrips usitatus in China: Identification and attraction of conspecifics in the laboratory and field. Pest Manag. Sci. 2020, 76, 2986–2993. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chan, F. The infestation of thrips on vegetables and their control. Chin. J. Entomol. 1987, 1, 45–53. [Google Scholar]
- Shukla, S.; Kalyani, G.; Kulkarni, N.; Waliyar, F.; Nigam, S. Mechanism of transmission of Tobacco streak virus by Scirtothrips dorsalis, Frankliniella schultzei and Megalurothrips usitatus in groundnut, Arachis hypogaea L. J. Oilseeds Res. 2005, 22, 215–217. [Google Scholar]
- Sreekanth, M.; Sreeramulu, M.; Rao, R.; Babu, B.; Babu, T. Occurrence and distribution of thrips population and peanut bud necrosis virus (PBNV) incidence on greengram (Vigna radiata L. Wilczek) in Andhra Pradesh. In Proceedings of the Resources Management in Plant Protection during Twenty First Century, Hyderabad, India, 14–15 November 2002; Volume 2, pp. 116–120. [Google Scholar]
- Camara, I.; Cao, K.L.; Sangbaramou, R.; Wu, P.P.; Shi, W.P.; Tan, S.Q. Screening of Beauveria bassiana (Bals.) (Hypocreales: Cordycipitaceae) strains against Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and conditions for large-scale production. Egypt. J. Biol. Pest Control 2022, 32, 85. [Google Scholar] [CrossRef]
- Wu, J.H.; Yang, B.; Zhang, X.C.; Cuthbertson, A.G.S.; Ali, S. Synergistic Interaction between the Entomopathogenic Fungus Akanthomyces attenuatus (Zare & Gams) and the Botanical Insecticide Matrine against Megalurothrips usitatus (Bagrall). J. Fungi 2021, 7, 536. [Google Scholar]
- Chen, Y.; Yang, B.; Li, Z.; Yue, Y.; Tian, Q.; Chen, W.; Ali, S.; Wu, J. Immune-Related Genes of Megalurothrips usitatus (Bagrall) Against Beauveria brongniartii and Akanthomyces attenuatus Identified Using RNA Sequencing. Front Physiol. 2021, 12, 671599. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Zhang, X.C.; Tian, Q.H.; Ali, S.; Tang, L.D.; Wu, J.H. Toxicological and Biochemical Description of Synergism of Beauveria bassiana and Emamectin Benzoate against Megalurothrips usitatus (Bagrall). J. Fungi 2022, 8, 916. [Google Scholar] [CrossRef]
- Wu, J.H.; Yu, X.T.; Wang, X.S.; Tang, L.D.; Ali, S. Matrine Enhances the Pathogenicity of Beauveria brongniartii Against Spodoptera litura (Lepidoptera: Noctuidae). Front. Microbiol. 2019, 10, 1812. [Google Scholar] [CrossRef] [Green Version]
- Burgess, E.R.; Taylor, E.E.; Acevedo, A.; Tworek, M.; Nayduch, D.; Khurana, N.; Miller, J.S.; Geden, C.J. Diets of erythritol, xylitol, and sucrose affect the digestive activity and gut bacterial community in adult house flies. Entomol. Exp. Appl. 2021, 169, 878–887. [Google Scholar] [CrossRef]
- Chaitra, H.S.; Singh, A.; Pandiyan, K.; Kalia, V.K. Sex Biased Variance in the Structural and Functional Diversity of the Midgut Bacterial Community of Last Instar Larvae of Pectinophora gossypiella (Lepidoptera: Gelechiidae). Microb. Ecol. 2022, 83, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Dada, N.; Lol, J.C.; Benedict, A.C.; Lopez, F.; Sheth, M.; Dzuris, N.; Padilla, N.; Lenhart, A. Pyrethroid exposure alters internal and cuticle surface bacterial communities in Anopheles albimanus. ISME 2019, 13, 2447–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta-Mate, A.; Renelies-Hamilton, J.; Kryger, P.; Jensen, A.B.; Sinotte, V.M.; Poulsen, M. Resistance and Vulnerability of Honeybee (Apis mellifera) Gut Bacteria to Commonly Used Pesticides. Front Microbiol. 2021, 12, 717990. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.F.S.; Aneesh, E.M. Gut bacterium induced pesticide resistance in insects with special emphasis to mosquitoes. Int. J. Trop. Insect Sci. 2022, 42, 2051–2064. [Google Scholar] [CrossRef]
- Cheng, D.; Guo, Z.; Riegler, M.; Xi, Z.; Liang, G.; Xu, Y. Gut symbiont enhances insecticide resistance in a significant pest, the orient fruit fly Bactrocera dorsalis (Hendel). Microbiome 2017, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.Y.; Liu, Y.; He, Z.Q.; Wen, Q.; Chen, X.Y.; Khan, M.M.; Osman, M.; Mandour, N.S.; Qiu, B.L. Rickettsia Infection Benefits Its Whitefly Hosts by Manipulating Their Nutrition and Defense. Insects 2022, 13, 1161. [Google Scholar] [CrossRef]
- Ali, S.; Sajjad, A.; Shakeel, Q.; Farooqi, M.A.; Aqueel, M.A.; Tariq, K.; Ullah, M.I.; Iqbal, A.; Jamal, A.; Saeed, M.F.; et al. Influence of Bacterial Secondary Symbionts in Sitobion avenae on Its Survival Fitness against Entomopathogenic Fungi, Beauveria bassiana and Metarhizium brunneum. Insects 2022, 13, 1037. [Google Scholar] [CrossRef]
- Łukasik, P.; Guo, H.; van Asch, M.; Ferrari, J.; Godfray, C.H. Protection against a fungal pathogen conferred by the aphid facultative endosymbionts Rickettsia and Spiroplasma is expressed in multiple host genotypes and species and is not influenced by co-infection with another symbiont. J. Evol. Biol. 2013, 26, 2654–2661. [Google Scholar] [CrossRef]
- Parker, B.J.; Sprag, C.; Altincicek, B.; Gerardo, N.M. Symbiont-mediated protection against fungal pathogens in pea aphids: A role for pathogen specificity? Appl. Environ. Microbiol. 2013, 79, 2455–2458. [Google Scholar] [CrossRef] [Green Version]
- Duguma, D.; Hall, M.W.; Smartt, C.T.; Debboun, M.; Neufeld, J.D. Microbiota variations in Culex nigripalpus disease vector mosquito of West Nile virus and Saint Louis Encephalitis from different geographic origins. PeerJ 2019, 6, e6168. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [Green Version]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Chen, H.; Boutros, P.C. VennDiagram: A package for the generation of highly-customizable Venn and Euler diagrams in R. BMC Bioinform. 2011, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Pruesse, E.; Quast, C.; Knittel, K.; Fuchs, B.M.; Ludwig, W.; Peplies, J.; Glöckner, F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007, 35, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Green genes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [Green Version]
- Ankenbrand, M.J.; Keller, A.; Wolf, M.; Schultz, J.; Förster, F. ITS2 database V: Twice as much. Mol. Biol. Evol. 2015, 32, 3030–3032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickham, H.; Chang, W. ggplot2: An Implementation of the Grammar of Graphics. R Package Version 0.7. 2008, Volume 3. Available online: Http://CRAN.R-Project.Org/Package=Ggplot2 (accessed on 24 May 2021).
- Kolde, R. Package ‘Pheatmap’. R Package. 2015, Volume 1, p. 790. Available online: https://cran.ms.unimelb.edu.au/web/packages/pheatmap/pheatmap.pdf (accessed on 15 June 2023).
- Aßhauer, K.P.; Wemheuer, B.; Daniel, R.; Meinicke, P. Tax4Fun: Predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 2015, 31, 2882–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.R.; Wu, Y.Y.; Dai, P.L.; Wang, Q.; Zhou, T. Effects of the sublethal doses of imidacloprid on the bacterial diversity in the midgut of Apis mellifera ligustica (Hymeno-ptera: Apidae). Acta Entomol. Sin. 2015, 58, 139–146. [Google Scholar]
- Li, W.H. Mechanism of Resistance to Nitenpyram Mediated by Symbionts in Nilaparvata lugens. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Lin, P. Microbes of Grapholitha molesta and Cydia pomonella: Effects of Different Conditions Stresses on Diversity of Intestinal. Master’s Thesis, Shihezi University, Shihezi, China, 2021. [Google Scholar]
- Zhao, T.Y. Study on the Relationship between Gut Bacteria of Ectropis obliqua Larva and Bifenthrin Resistance. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2020. [Google Scholar]
- Hunter, M.S.; Perlman, S.J.; Kelly, S.E. A bacterial symbiont in the Bacteroidetes induces cytoplasmic incompatibility in the parasitoid wasp Encarsia pergandiella. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 2185–2190. [Google Scholar] [CrossRef]
- Oliver, K.M.; Russell, J.A.; Moran, N.A.; Hunter, M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. USA 2003, 100, 1803–1807. [Google Scholar] [CrossRef]
- Oliver, K.M.; Moran, N.A.; Hunter, M.S. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. USA 2005, 102, 12795–12800. [Google Scholar] [CrossRef]
- Hagimori, T.; Abe, Y.; Miura, K. The first finding of a Rickettsia bacterium associated with parthenogenesis induction among insects. Curr. Microbiol. 2006, 52, 97–101. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Karaca, M.M.; Döker, İ.; Karut, K. Monitoring insecticide resistance and endosymbiont composition in greenhouse populations of Bemisia tabaci (Gennadius)(Hemiptera: Aleyrodidae) from Mersin, Turkey. Phytoparasitica 2020, 48, 659–672. [Google Scholar] [CrossRef]
- Dillon, R.; Charnley, A. Chemical barriers to gut infection in the desert locust: In vivo production of antimicrobial phenols associated with the bacterium Pantoea agglomerans. J. Invertebr. Pathol. 1995, 66, 72–75. [Google Scholar] [CrossRef]
- Ramya, S.L.; Venkatesan, T.; Murthy, K.S.; Jalali, S.K.; Varghese, A. Degradation of acephate by Enterobacter asburiae, Bacillus cereus and Pantoea agglomerans isolated from diamondback moth Plutella xylostella (L.), a pest of cruciferous crops. J. Environ. Biol. 2016, 37, 611. [Google Scholar]
- Wang, X.; Yang, X.; Zhou, F.; Qiang Tian, Z.; Cheng, J.; Michaud, J.; Liu, X. Symbiotic bacteria on the cuticle protect the oriental fruit moth Grapholita molesta from fungal infection. Biol. Control 2022, 169, 104895. [Google Scholar] [CrossRef]
- Shukla, S.P.; Beran, F. Gut microbiota degrades toxic isothiocyanates in a flea beetle pest. Mol. Ecol. 2020, 29, 4692–4705. [Google Scholar] [CrossRef]
- Duron, O.; Labbé, P.; Berticat, C.; Rousset, F.; Guillot, S.; Raymond, M.; Weill, M. High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 2006, 60, 303–314. [Google Scholar] [PubMed]
- Panteleev, D.Y.; Goryacheva, I.; Andrianov, B.; Reznik, N.; Lazebny, O.; Kulikov, A. The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Russ. J. Genet. 2007, 43, 1066–1069. [Google Scholar] [CrossRef]
- Teixeira, L.; Ferreira, Á.; Ashburner, M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 2008, 6, e1000002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osborne, S.E.; Leong, Y.S.; O’neill, S.L.; Johnson, K.N. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathog. 2009, 5, e1000656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halpern, M.; Fridman, S.; Atamna-Ismaeel, N.; Izhaki, I. Rosenbergiella nectarea gen. nov., sp. nov., in the family Enterobacteriaceae, isolated from floral nectar. Int. J. Syst. Evol. Microbiol. 2013, 63, 4259–4265. [Google Scholar] [CrossRef]
- Lenaerts, M.; Álvarez-Pérez, S.; De Vega, C.; Van Assche, A.; Johnson, S.D.; Willems, K.A.; Herrera, C.M.; Jacquemyn, H.; Lievens, B. Rosenbergiella australoborealis sp. nov., Rosenbergiella collisarenosi sp. nov. and Rosenbergiella epipactidis sp. nov., three novel bacterial species isolated from floral nectar. Syst. Appl. Microbiol. 2014, 37, 402–411. [Google Scholar] [CrossRef]
- Hongoh, Y.; Ishikawa, H. Evolutionary studies on uricases of fungal endosymbionts of aphids and planthoppers. J. Mol. Evol. 2000, 51, 265–277. [Google Scholar] [CrossRef]
- Akbar, S.; Sultan, S.; Kertesz, M. Determination of cypermethrin degradation potential of soil bacteria along with plant growth-promoting characteristics. Curr. Microbiol. 2015, 70, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Welte, C.U.; De Graaf, R.M.; Van Den Bosch, T.J.; Op Den Camp, H.J.; Van Dam, N.M.; Jetten, M.S. Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyses the conversion of the plant toxin 2-phenylethyl isothiocyanate. Environ. Microbiol. 2016, 18, 1379–1390. [Google Scholar] [CrossRef] [PubMed]
- Rivero, A.; Magaud, A.; Nicot, A.; Vézilier, J. Energetic cost of insecticide resistance in Culex pipiens mosquitoes. J. Med. Entomol. 2011, 48, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.R.; Potter, M.F.; Haynes, K.F. Insecticide resistance in the bed bug comes with a cost. Sci. Rep. 2015, 5, 10807. [Google Scholar] [CrossRef] [Green Version]



| Insecticide | Population | Slope ± SE | LC50 (95% CL) a | LCR50 (95%CL) b |
|---|---|---|---|---|
| Acetamiprid- | IS | 1.00 ± 0.18 | 8.63 (4.18–10.57) | 138.04 (172.24–186.48) * |
| IR | 1.57 ± 0.17 | 1191.28 (719.97–1971.14) | ||
| B. brongniartii | IS | 0.38 ± 2.70 | 1.2 × 106 (2.5 × 105–6.0 × 106) | 55.62 (10.26–30,150.03) * |
| IR | 0.31 ± 2.56 | 6.8 × 107 (2.6 × 106–1.8 × 109) |
| Sample | Raw Tags | Clean Tags | Effective Tags | Effective Ratio (%) | OTUs (Individual) |
|---|---|---|---|---|---|
| S-CK | 102,074 | 96,541 | 92,964 | 74 | 191 |
| B1 | 109,466 | 105,369 | 99,172 | 74 | 81 |
| B2 | 109,422 | 104,815 | 98,539 | 73 | 92 |
| B3 | 104,250 | 100,345 | 94,579 | 74 | 93 |
| R-CK | 106,162 | 101,810 | 95,872 | 73 | 95 |
| D1 | 105,975 | 102,716 | 98,807 | 76 | 142 |
| D2 | 102,590 | 98,920 | 93,522 | 74 | 76 |
| D3 | 103,351 | 99,454 | 94,125 | 74 | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Chen, Y.; Zhou, C.; Li, H.; Ali, S.; Wu, J. Gut Bacterial Diversity of Insecticide-Susceptible and Insecticide-Resistant Megalurothrips usitatus (Thysanoptera: Thripidae) and Elucidation of Their Putative Functional Roles. Insects 2023, 14, 669. https://doi.org/10.3390/insects14080669
Zhu B, Chen Y, Zhou C, Li H, Ali S, Wu J. Gut Bacterial Diversity of Insecticide-Susceptible and Insecticide-Resistant Megalurothrips usitatus (Thysanoptera: Thripidae) and Elucidation of Their Putative Functional Roles. Insects. 2023; 14(8):669. https://doi.org/10.3390/insects14080669
Chicago/Turabian StyleZhu, Bifeng, Yueyin Chen, Chenyan Zhou, Haolong Li, Shaukat Ali, and Jianhui Wu. 2023. "Gut Bacterial Diversity of Insecticide-Susceptible and Insecticide-Resistant Megalurothrips usitatus (Thysanoptera: Thripidae) and Elucidation of Their Putative Functional Roles" Insects 14, no. 8: 669. https://doi.org/10.3390/insects14080669
APA StyleZhu, B., Chen, Y., Zhou, C., Li, H., Ali, S., & Wu, J. (2023). Gut Bacterial Diversity of Insecticide-Susceptible and Insecticide-Resistant Megalurothrips usitatus (Thysanoptera: Thripidae) and Elucidation of Their Putative Functional Roles. Insects, 14(8), 669. https://doi.org/10.3390/insects14080669





