Validamycin Inhibits the Synthesis and Metabolism of Trehalose and Chitin in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Injection of Validamycin
2.3. Measurement of Trehalase Activity
2.4. Measurement of Carbohydrate Content
2.5. Measurement of Chitin Content
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Modelling of Trehalose-6-Phosphate Phosphatase C1 (BdTPPC1) and BdTPPC1−Validamycin Docking
2.8. Heterologous Expression of Recombinant BdTPPC1
2.9. Inhibition of Recombinant BdTPPC1 Activity by Validamycin
2.10. Statistical Analyses
3. Results
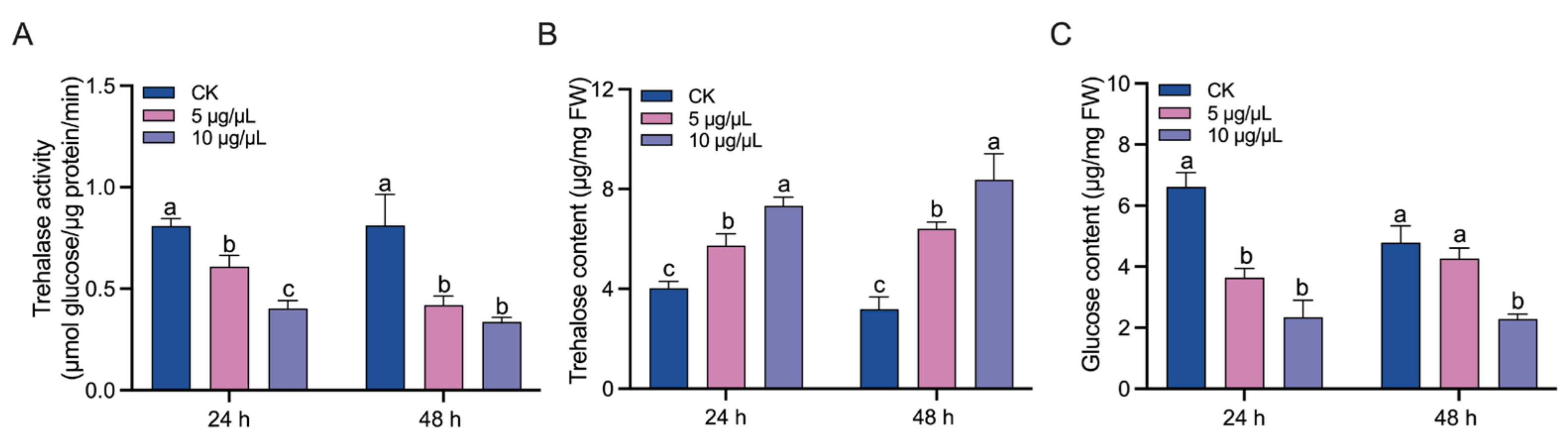
3.1. Inhibition of Trehalase Activity by Validamycin
3.2. Inhibition of Chitin Biosynthesis by Validamycin
3.3. Effect of Validamycin Injection on the Expressions of Chitin-Degradation-Related Genes
3.4. Effect of Validamycin Injection on the Expressions of Trehalose Biosynthesis Related Genes
3.5. Modelling of BdTPPC1 and BdTPPC1−Validamycin Docking
3.6. Phenotypes of B. dorsalis after Validamycin Injection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avonce, N.; Mendoza-Vargas, A.; Morett, E.; Iturriaga, G. Insights on the evolution of trehalose biosynthesis. BMC Evol. Biol. 2006, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Shukla, E.; Thorat, L.J.; Nath, B.B.; Gaikwad, S.M. Insect trehalase: Physiological significance and potential applications. Glycobiology 2015, 25, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wei, P.; Chen, J.; Wang, S.-G.; Zhang, W.-Q. Progress in gene features and functions of insect trehalases. Acta Entomol. Sinica 2012, 55, 1315–1321. [Google Scholar]
- Asano, N. Glycosidase inhibitors: Update and perspectives on practical use. Glycobiology. 2003, 13, 93R–104R. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.N. Trehalose—The insect ‘blood’ sugar. Adv. Insect Physiol. 2003, 31, 205–285. [Google Scholar]
- Gong, T.; Li, L.L.; Zhao, Z.Z.; Liu, D.H. Advances in trehalose biosynthesis pathways and application of molecular biology technique. Chin. Agric. Sci. Bull. 2016, 32, 62–67. [Google Scholar]
- Tournu, H.; Fiori, A.; Van Dijck, P. Relevance of trehalose in pathogenicity: Some general Rules, yet many exceptions. PLoS Pathog. 2013, 9, e1003447. [Google Scholar] [CrossRef]
- Tang, B.; Wei, P.; Zhao, L.; Shi, Z.K.; Shen, Q.D.; Yang, M.M.; Xie, G.Q.; Wang, S.G. Knockdown of five trehalase genes using RNA interference regulates the gene expression of the chitin biosynthesis pathway in Tribolium castaneum. BMC Biotechnol. 2016, 16, 16–67. [Google Scholar] [CrossRef]
- Ai, D.; Cheng, S.H.; Chang, H.T.; Yang, T.; Wang, G.R.; Yu, C.H. Gene cloning, prokaryotic expression, and biochemical characterization of a soluble trehalase in Helicoverpa armigera Hubner (Lepidoptera: Noctuidae). J. Insect Sci. 2018, 18, 22. [Google Scholar] [CrossRef]
- Arakane, Y.; Hogenkamp, D.G.; Zhu, Y.C.; Kramer, K.J.; Specht, C.A.; Beeman, R.W.; Kanost, M.R.; Muthukrishnan, S. Characterization of two chitin synthase genes of the red flour beetle, Tribolium castaneum, and alternate exon usage in one of the genes during development. Insect Biochem. Molec. 2004, 34, 291–304. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S.; Kramer, K.J.; Specht, C.A.; Tomoyasu, Y.; Lorenzen, M.D.; Kanost, M.; Beeman, R.W. The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 2005, 14, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Arakane, Y.; Specht, C.A.; Kramer, K.J.; Muthukrishnan, S.; Beeman, R.W. Chitin synthases are required for survival, fecundity and egg hatch in the red flour beetle, Tribolium castaneum. Insect. Biochem. Molec. 2008, 38, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, H.; Zou, L.; Tang, B.; Hu, J.; Zhang, W. Disruption of Spodoptera exigua larval development by silencing chitin synthase gene A with RNA interference. B Entomol. Res. 2008, 98, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.B.; Yang, Q. A novel alternative splicing site of class A chitin synthase from the insect Ostrinia furnacalis—Gene organization, expression pattern and physiological significance. Insect Biochem. Molec. 2011, 41, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Yu, L.H.; Jiang, X.Y.; Chen, Y.; Wang, S.T.; Chao, L.; Jiang, Z.Y.; He, B.E.; Xu, C.D.; Wang, S.G.; et al. Potential inhibitory effects of compounds ZK-PI-5 and ZK-PI-9 on trehalose and chitin metabolism in Spodoptera frugiperda (J. E. Smith). Front. Physiol. 2023, 14, 1178996. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Yang, L.; Jin, F.Q.; Yin, Y.L.; Xie, Z.Z.; Yang, L.F.; Zhao, S.F.; Zhang, G.Q.; Yang, D.S.; Han, X.Q. Untargeted UHPLC-MS metabolomics Reveals the metabolic perturbations of Helicoverpa armigera under the stress of novel insect growth regulator ZQ-8. Agronomy 2023, 13, 1315. [Google Scholar] [CrossRef]
- Ren, M.F.; Lu, J.J.; Li, D.Q.; Yang, J.; Zhang, Y.Y.; Dong, J.M.; Niu, Y.B.; Zhou, X.G.; Zhang, X.H. Identification and functional characterization of two chitin synthases in the black cutworm, Agrotis ipsilon (Hufnagel) (Lepidoptera: Noctuidae). J. Econ. Entomol. 2023, 116, 574–583. [Google Scholar] [CrossRef]
- Marten, A.D.; Stothard, A.I.; Karishma, K.; Swarts, B.M.; Conway, M.J. Validamycin A delays development and prevents flight in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1096–1103. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Chen, X.F.; Tang, B.; Tian, H.G.; Chen, J.; Yan, Q. Insect chitin biosynthesis and its regulation. Chin. J. Appl. Entomol. 2011, 48, 475–479. [Google Scholar]
- Gong, C.W.; Hasnain, A.; Wang, Q.L.; Liu, D.; Xu, Z.Z.; Zhan, X.X.; Liu, X.M.; Pu, J.; Sun, M.M.; Wang, X.G. Eco-friendly deacetylated chitosan base siRNA biological-nanopesticide loading cyromazine for efficiently controlling Spodoptera frugiperda. Int. J. Biol. Macromol. 2023, 241, 124575. [Google Scholar] [CrossRef]
- An, S.; Liu, W.J.; Fu, J.W.; Zhang, Z.; Zhang, R.L. Molecular identification of the chitinase genes in Aedes albopictus and essential roles of AaCht10 in pupal-adult transition. Parasite Vector. 2023, 16, 120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, S.S.; Yu, Y.Y.; Cheng, Y.S.; Hu, C.X.; Zhou, M.; Li, C.; Tang, B.; Wu, Y. Effects of inhibiting the expression of chitin synthase gene SfCHSB on the metabolism of trehalose and chitin in Spodoptera frugiperda larvae. Agriculture 2022, 12, 2019. [Google Scholar] [CrossRef]
- Yu, H.Z.; Wen, D.F.; Wang, W.L.; Geng, L.; Zhang, Y.; Xu, J.P. Identification of genes putatively involved in chitin metabolism and insecticide detoxification in the rice leaf folder (Cnaphalocrocis medinalis) larvae through transcriptomic analysis. Int. J. Mol. Sci. 2015, 16, 21873–21896. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.T.; Fang, K.; Qi, L.Z.; Wang, X.; Pan, Y.; Li, Y.S.; Xi, J.H.; Zhang, J.H. Purification and functional characterization of a soluble trehalase in Lissorhoptrus oryzophilus (Coleoptera: Curculionidae). Insects 2022, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.Q.; Xue, Y.P.; Zheng, Y.G.; Shen, Y.C. Production of trehalase inhibitor validoxylamine A using acid-catalyzed hydrolysis of validamycin A. Catal. Commun. 2006, 7, 157–161. [Google Scholar] [CrossRef]
- Liebl, M.; Nelius, V.; Kamp, G.; Ando, O.; Wegener, G. Fate and effects of the trehalase inhibitor trehazolin in the migratory locust (Locusta migratoria). J. Insect Physiol. 2010, 56, 567–574. [Google Scholar] [CrossRef]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: A multifunctional molecule. Glycobiology 2003, 13, 17–27. [Google Scholar] [CrossRef]
- Bai, L.; Li, L.; Xu, H.; Minagawa, K.; Yu, Y.; Zhang, Y.R.; Zhou, X.F.; Fioss, H.G.; Mahmud, T.; Deng, Z.X. Functional analysis of the validamycin biosynthetic gene cluster and engineered production of validoxylamine A. Chem. Biol. 2006, 13, 387–397. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; My, T.T.A.; Hai, N.T.T.; Loan, H.T.P.; Hieu, L.T.; Hoa, T.T.; Bui, T.Q.; Tuong, H.N.; Thuy, N.T.T.; Dung, D.K.; et al. A molecular docking simulation study on potent inhibitors against Rhizoctonia solani and Magnaporthe oryzae in rice: Silver-tetrylene and bis-silver-tetrylene complexes vs. validamycin and tricyclazole pesticides. Struct. Chem. 2021, 32, 135–148. [Google Scholar]
- Yu, H.Z.; Huang, Y.L.; Lu, Z.J.; Zhang, Q.; Su, H.N.; Du, Y.M.; Yi, L.; Zhong, B.L.; Chen, C.X. Inhibition of trehalase affects the trehalose and chitin metabolism pathways in Diaphorina citri (Hemiptera: Psyllidae). Insect Sci. 2021, 28, 718–734. [Google Scholar] [CrossRef]
- Yu, H.Z.; Xie, Y.X.; Wang, J.; Wang, Y.; Du, Y.M.; Wang, H.G.; Zhong, B.L.; Zhu, B.; Yu, X.D.; Lu, Z.J. Integrated transcriptome sequencing and RNA interference reveals molecular changes in Diaphorina citri after exposure to validamycin. Insect Sci. 2021, 28, 1690–1707. [Google Scholar] [CrossRef]
- Allwood, A.J.; Chinajariyawong, A.; Kritsaneepaiboon, S.; Drew, R.A.I.; Hamacek, E.L.; Hancock, D.L.; Hengsawad, C.; Jipanin, J.C.; Jirasurat, M.; Krong, C.K.; et al. Host plant records for fruit flies (Diptera: Tephritidae) in southeast Asia. Raffles Bull. Zool. 1999, 7, 1–92. [Google Scholar]
- Hardy, D.E. Taxonomy and distribution of oriental fruit fly and related species (Tephritidae-Diptera). Proc. Hawaiian Entomol. Soc. 1969, 20, 395–428. [Google Scholar]
- Wang, L.; Wei, D.D.; Wang, G.Q.; Huang, H.Q.; Wang, J.J. High-sucrose diet exposure on larvae contributes to adult fecundity and insecticide tolerance in the oriental fruit fly, Bactrocera dorsalis (Hendel). Insects 2023, 14, 407. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Tipraqsa, P. Agricultural pesticides and land use intensification in high, middle and low income countries. Food Policy 2012, 37, 616–626. [Google Scholar] [CrossRef]
- Pan, H.P.; Preisser, E.L.; Chu, D.; Wang, S.L.; Wu, Q.J.; Carriere, Y.; Zhou, X.G.; Zhang, Y.J. Insecticides promote viral outbreaks by altering herbivore competition. Ecol. Appl. 2015, 25, 1585–1595. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Phys. 2015, 121, 122–128. [Google Scholar] [CrossRef]
- Wang, J.J.; Wei, D.; Dou, W.; Hu, F.; Liu, W.F.; Wang, J.J. Toxicities and synergistic effects of several insecticides against the oriental fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 2013, 106, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D.L. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.A.; Sternberg, M.J.E. Protein structure prediction on the Web: A case study using the Phyre server. Nat. Protoc. 2009, 4, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software news and update autodock vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Klutts, S.; Pastuszak, I.; Edavana, V.K.; Thampi, P.; Pan, Y.T.; Abraham, E.C.; Carroll, J.D.; Elbein, A.D. Purification, cloning, expression, and properties of mycobacterial trehalose-phosphate phosphatase. J. Biol. Chem. 2003, 278, 2093–2100. [Google Scholar] [CrossRef]
- Jin, L.Q.; Zheng, Y.G. Inhibitory effects of validamycin compounds on the termites trehalase. Pestic. Biochem. Phys. 2009, 95, 28–32. [Google Scholar] [CrossRef]
- Kyosseva, S.V.; Kyossev, Z.N.; Elbein, A.D. Inhibitors of pig kidney trehalase. Arch. Biochem. Biophys. 1995, 316, 821–826. [Google Scholar] [CrossRef]
- Ando, O.; Kifune, M.; Nakajima, M. Effects of trehazolin, a potent trehalase inhibitor, on Bombyx-mori and plant-pathogenic fungi. Biosci. Biotech. Bioch. 1995, 59, 711–712. [Google Scholar] [CrossRef]
- Yu, H.Z.; Zhang, Q.; Lu, Z.J.; Deng, M.J. Validamycin treatment significantly inhibits the glycometabolism and chitin synthesis in the common cutworm, Spodoptera litura. Insect Sci. 2022, 29, 840–854. [Google Scholar] [CrossRef]
- Luo, Y.J.; Chen, Y.; Wang, X.J.; Wang, S.T.; Yang, Y.Y.; Xu, H.X.; Qu, C.; Wu, Y.; Li, C.; Wang, S.G.; et al. Validamycin affects the development and chitin metabolism in Spodoptera frugiperda by inhibiting trehalase activity. Entomol. Gen. 2022, 42, 931–939. [Google Scholar] [CrossRef]
- Shao, Z.M.; Ding, J.H.; Jiang, D.L.; Liu, Z.X.; Li, Y.J.C.; Wang, J.; Wang, J.; Sheng, S.; Wu, F.A. Characterization and functional analysis of trehalase related to chitin Metabolism in glyphodes Pyloalis walker (Lepidoptera: Pyralidae). Insects 2021, 12, 370. [Google Scholar] [CrossRef]
- Tanaka, S.; Okuda, T.; Hasegawa, E.; Kono, Y. Suppression of oocyte development by a trehalase inhibitor, validoxylamine A, through inhibition of juvenile hormone biosynthesis and vitellogenesis in the migratory locust, Locusta migratoria L. Entomol. Sci. 1998, 1, 313–320. [Google Scholar]
- Yang, H.J.; Cui, M.Y.; Zhao, X.H.; Zhang, C.Y.; Hu, Y.S.; Fan, D. Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata. Front. Physiol. 2023, 14, 1109661. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Shen, Q.D.; Wang, S.S.; Li, G.Y.; Wu, Y.; Xu, C.D.; Tang, B.; Li, C. Regulatory function of the trehalose-6-phosphate synthase gene TPS3 on chitin metabolism in brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2022, 31, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Xu, K.K.; Zhang, R.Y.; Dou, W.; Wang, J.J. Transcriptional regulation of a chitinase gene by 20-hydroxyecdysone and starvation in the oriental fruit fly, Bactrocera dorsalis. Int. J. Mol. Sci. 2013, 14, 20048–20063. [Google Scholar] [CrossRef]
- Liu, S.H.; Li, H.F.; Yang, Y.; Yang, R.L.; Yang, W.J.; Jiang, H.B.; Dou, W.; Smagghe, G.; Wang, J.J. Genome-wide identification of chitinase and chitin deacetylase gene families in the oriental fruit fly, Bactrocera dorsalis (Hendel). Comp. Biochem. Phys. D 2018, 27, 13–22. [Google Scholar] [CrossRef]
- Liu, S.H.; Xia, Y.D.; Zhang, Q.; Li, W.; Li, R.Y.; Liu, Y.; Chen, E.H.; Dou, W.; Stelinski, L.L.; Wang, J.J. Potential targets for controlling Bactrocera dorsalis using cuticle- and hormone-related genes revealed by a developmental transcriptome analysis. Pest. Manag. Sci. 2020, 76, 2127–2143. [Google Scholar] [CrossRef]
- Chen, E.H.; Hou, Q.L.; Dou, W.; Wei, D.D.; Yue, Y.; Yang, R.L.; Yu, S.F.; De Schutter, K.; Smagghe, G.; Wang, J.J. RNA-seq analysis of gene expression changes during pupariation in Bactrocera dorsalis (Hendel) (Diptera: Tephritidae). BMC Genom. 2018, 19, 693. [Google Scholar] [CrossRef]
- Gu, X.Y.; Li, Z.H.; Su, Y.; Zhao, Y.; Liu, L.J. Imaginal disc growth factor 4 regulates development and temperature adaptation in Bactrocera dorsalis. Sci. Rep. 2019, 9, 931. [Google Scholar] [CrossRef]
- Jiang, Z.X.; Hang, Q.; Wang, J.E.; Zhu, K.; Zhang, J.Y.; Deng, M.F.; Huang, J.X.; Duan, H.X. Advances in structures and inhibitors of trehalose synthetase. Chin. J. Pestic. Sci. 2021, 23, 209–225. [Google Scholar]
- Miao, Y.; Tenor, J.L.; Toffaletti, D.L.; Maskarinec, S.A.; Liu, J.Y.; Lee, R.E.; Perfect, J.R.; Brennan, R.G. Structural and in vivo studies on trehalose-6-Phosphate Synthase from pathogenic fungi provide insights into Its catalytic mechanism, biological necessity, and potential for novel antifungal drug design. MBio 2017, 8, e00643-17. [Google Scholar]
- Wang, J.; Fan, H.; Li, Y.; Zhang, T.F.; Liu, Y.H. Trehalose-6-phosphate phosphatases are involved in trehalose synthesis and metamorphosis in Bactrocera minax. Insect Sci. 2022, 29, 16. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, Y.; Wu, S.; Wang, B.; Li, Y.; Liu, Y.; Wang, J. Validamycin Inhibits the Synthesis and Metabolism of Trehalose and Chitin in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Insects 2023, 14, 671. https://doi.org/10.3390/insects14080671
Li Y, Xu Y, Wu S, Wang B, Li Y, Liu Y, Wang J. Validamycin Inhibits the Synthesis and Metabolism of Trehalose and Chitin in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Insects. 2023; 14(8):671. https://doi.org/10.3390/insects14080671
Chicago/Turabian StyleLi, Ying, Yonghong Xu, Shunjiao Wu, Baohe Wang, Yaying Li, Yinghong Liu, and Jia Wang. 2023. "Validamycin Inhibits the Synthesis and Metabolism of Trehalose and Chitin in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel)" Insects 14, no. 8: 671. https://doi.org/10.3390/insects14080671
APA StyleLi, Y., Xu, Y., Wu, S., Wang, B., Li, Y., Liu, Y., & Wang, J. (2023). Validamycin Inhibits the Synthesis and Metabolism of Trehalose and Chitin in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Insects, 14(8), 671. https://doi.org/10.3390/insects14080671





