Using Gamma Irradiation to Predict Sperm Competition Mechanism in Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae): Insights for a Future Management Strategy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Rearing
2.2. Irradiation
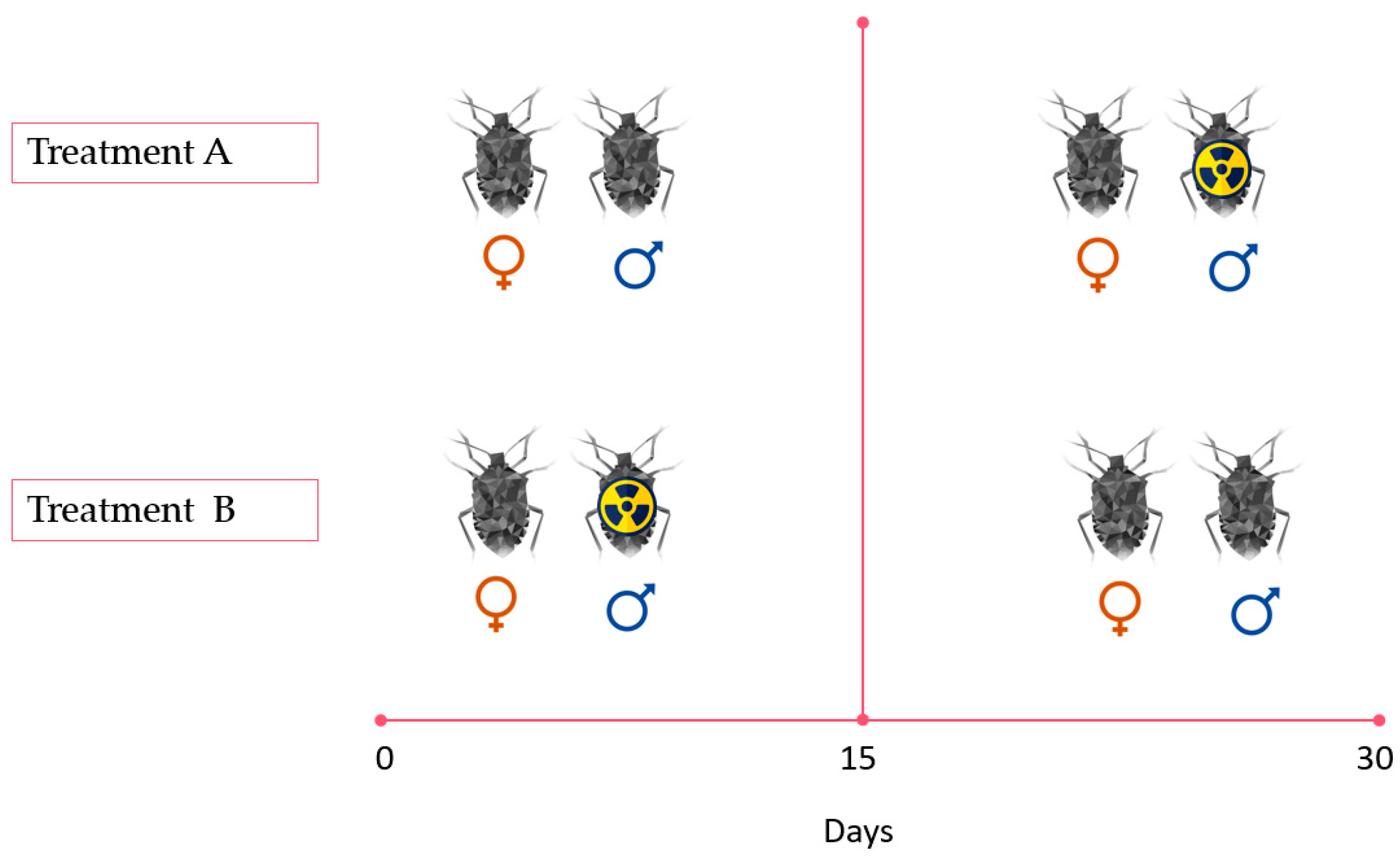
2.3. Experimental Set-Up
- -
- a non-irradiated–non-irradiated mating order, i.e., two non-irradiated males to verify the reproductive parameters (10 replicates) as positive control (Figure A2, Appendix A);
- -
- an irradiated–irradiated mating order (10 replicates) i.e., two irradiated males as negative control (Figure A2, Appendix A);
- -
- a female and a non-irradiated male, without male replacement (10 replicates) (Figure A2, Appendix A);
- -
- a single female virgin, to consider the number of eggs laid regardless of mating (10 replicates) (Figure A2, Appendix A).
2.4. Data Analysis
3. Results
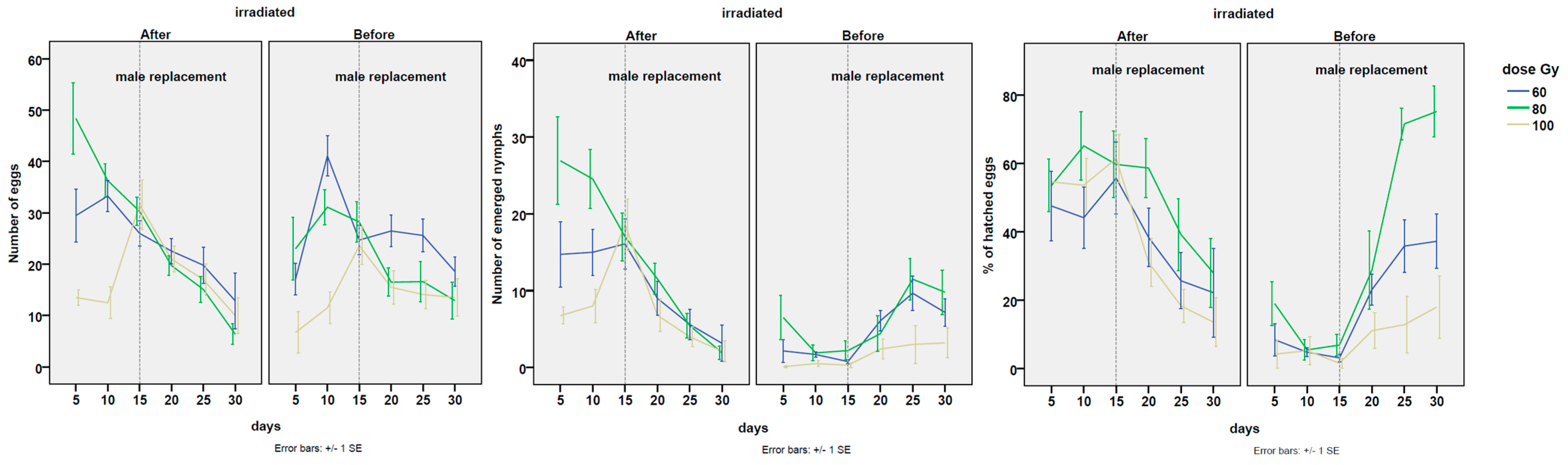
3.1. Effect of the Last Male on the Number of Oviposited Eggs
3.2. Effect of the Last Male on the Number of Newly Hatched Nymphs
3.3. Effect of the Last Male on the Percentage of Hatched Eggs
3.4. P2 estimates of the Sperm Transmission from the Last Male
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Order of Mating | Eggs (n.) | Emerged Nymphs (n.) | Hatched Eggs (%) | |||
|---|---|---|---|---|---|---|
| Gy | N−I | I | N−I | I | N−I | I |
| 0–60 | 29.58 | 19.14 | 15.27 | 6.32 | 49.12 | 30.87 |
| ±2.16 | ±2.13 | ±1.99 | ±1.30 | ±5.60 | ±5.41 | |
| 0–80 | 38.30 | 13.73 | 22.82 | 6.30 | 59.60 | 43.99 |
| ±2.93 | ±1.55 | ±2.55 | ±1.12 | ±5.27 | ±5.94 | |
| 0–100 | 20.63 | 16.18 | 12.21 | 4.39 | 57.38 | 21.60 |
| ±3.03 | ±1.88 | ±1.96 | ±1.00 | ±4.67 | ±3.86 | |
| I | N−I | I | N−I | I | N−I | |
| 60–0 | 27.62 | 23.62 | 1.54 | 7.59 | 5.31 | 31.74 |
| ±2.47 | ±1.80 | ±0.50 | ±1.04 | ±1.56 | ±3.96 | |
| 80–0 | 27.43 | 15.33 | 3.53 | 8.57 | 10.42 | 56.05 |
| ±2.65 | ±1.94 | ±1.14 | ±1.58 | ±2.73 | ±6.53 | |
| 100–0 | 13.93 | 14.37 | 0.30 | 2.87 | 3.39 | 13.93 |
| ±2.42 | ±1.81 | ±0.17 | ±1.09 | ±1.84 | ±4.31 | |
| N−I 1 | N−I 2 | N−I 1 | N−I 2 | N−I 1 | N−I 2 | |
| 0–0 | 19.48 | 13.76 | 10.71 | 6.57 | 55.02 | 46.46 |
| ±3.76 | ±1.85 | ±2.87 | ±1.47 | ±7.50 | ±7.82 | |
| I1 | I2 | I1 | I2 | I1 | I2 | |
| 60–60 | 19.60 | 8.93 | 0.73 | 0.00 | 3.42 | 0.00 |
| ±1.40 | ±0.83 | ±0.19 | ±0.00 | ±0.85 | ±0.00 | |
| 80–80 | 17.53 | 8.30 | 0.87 | 0.40 | 5.78 | 4.96 |
| ±2.21 | ±2.07 | ±0.28 | ±0.17 | ±2.06 | ±2.06 | |
| 100–100 | 9.83 | 10.97 | 0.17 | 0.17 | 1.76 | 1.55 |
| ±1.76 | ±1.47 | ±0.08 | ±0.08 | ±0.93 | ±0.97 | |
| Unchanged | Unchanged | Unchanged | ||||
| 0 | 12.45 | 4.27 | 32.53 | |||
| ±1.28 | ±0.73 | ±3.93 | ||||
| 60 | 12.38 | 0.68 | 3.86 | |||
| ±1.37 | ±0.16 | ±0.84 | ||||
| 80 | 11.12 | 0.40 | 4.87 | |||
| ±1.61 | ±0.10 | ±2.19 | ||||
| 100 | 11.17 | 0.05 | 0.32 | |||
| ±1.37 | ±0.03 | ±0.19 | ||||
| Unmated | Unmated | Unmated | ||||
| 0 | 5.68 | 0.00 | 0.00 | |||
| ±0.89 | ±0.00 | ±0.00 | ||||
References
- Howard, C.W. The Entomological Section: The Bagrada Bug (Bagrada hilaris). Transvaal Agric. J. 1906, 5, 168–176. [Google Scholar]
- Bundy, C.S.; Grasswitz, T.R.; Sutherland, C. First Report of the Invasive Stink Bug Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) from New Mexico, with Notes on Its Biology. Southwest. Entomol. 2012, 37, 411–414. [Google Scholar] [CrossRef]
- Matsunaga, J.N. Bagrada Bug Bagrada hilaris (Burmeister). State Hawaii Dep. Agric. New Pests Advis. 2014, 14, 2. [Google Scholar]
- Faúndez, E.I. From Agricultural to Household Pest: The Case of the Painted Bug Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) in Chile. J. Med. Entomol. 2018, 55, 1365–1368. [Google Scholar] [CrossRef]
- Carpintero, D.L.; Quiroga, V.N.; Celentano, E. Primer Registro De Bagrada hilaris (Burmeister, 1835) (Hemiptera: Pentatomidae) Para La República Argentina. Hist. Nat. 2021, 11, 179–184. [Google Scholar]
- Carapezza, A. Gli Eterotteri Dell’Isola Di Pantelleria (Insecta, Heteroptera). Il Nat. Sicil. 1981, 4, 73–91. [Google Scholar]
- Gunn, D. The Bagrada Bug (Bagrada hilaris). Union S. Afr. Dep. Agric. Pretoria Bull. 1918, 9, 1–19. [Google Scholar]
- Daiber, K.C. Insects and Nematodes That Attack Cole Crops in Southern Africa—A Research Review. J. Plant Dis. Prot. 1992, 99, 430–440. [Google Scholar]
- Reed, D.A.; Palumbo, J.C.; Perring, T.M.; May, C. Bagrada hilaris (Hemiptera: Pentatomidae), An Invasive Stink Bug Attacking Cole Crops in the Southwestern United States. J. Integr. Pest Manag. 2013, 4, C1–C7. [Google Scholar] [CrossRef]
- Bundy, C.S.; Perring, T.M.; Reed, D.A.; Palumbo, J.C.; Grasswitz, T.R.; Jones, W.A. Bagrada hilaris. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; CRC Press: Boca Raton, FL, USA, 2018; pp. 205–241. [Google Scholar]
- Hatting, J.L. Major Insect Pests and Their Natural Enemies Associated with Cultivation of Rooibos, Aspalathus linearis (Burm. f.) R. Dahlgren, in South Africa: A Review. S. Afr. J. Bot. 2017, 110, 118–123. [Google Scholar] [CrossRef]
- Colazza, S.; Guarino, S. Bagrada hilaris (Burmeister)(Heteroptera: Pentatomidae) a Pest of Capper in the Island of Pantelleria [Capparis spinosa L.; Sicily]. Inf. Fitopatol. Italy 2004, 12, 30–34. [Google Scholar]
- Ahuja, B.; Kalyan, R.K.; Ahuja, U.R.; Singh, S.K.; Sundria, M.M.; Dhandapani, A. Integrated Management Strategy for Painted Bug, Bagrada hilaris (Burm.) Inflicting Injury at Seedling Stage of Mustard (Brassica juncea) in Arid Western Rajasthan. Pestic. Res. J. 2008, 20, 48–51. [Google Scholar]
- Hill, D.S. Agricultural Insect Pests of the Tropics and Their Control, 2nd ed.; Cambridge University Press: Cambridge, UK, 1987; ISBN 978-0-521-24638-5. [Google Scholar]
- Huang, T.-I.; Reed, D.A.; Perring, T.M.; Palumbo, J.C. Feeding Damage by Bagrada hilaris (Hemiptera: Pentatomidae) and Impact on Growth and Chlorophyll Content of Brassicaceous Plant Species. Arthropod-Plant Interact. 2014, 8, 89–100. [Google Scholar] [CrossRef]
- Infantino, A.; Tomassoli, L.; Peri, E.; Colazza, S. Viruses, Fungi and Insect Pests Affecting Caper. Eur. J. Plant Sci. Biotechnol. 2007, 1, 170–179. [Google Scholar]
- Hinton, H.E. Biology of Insect Eggs, Volume 2; Pergamon Press: Oxford, UK, 1981; ISBN 0-08-021539-4. [Google Scholar]
- McPherson, J.E.; McPherson, R. Stink Bugs of Economic Importance in America North of Mexico, 1st ed.; CRC Press: Boca Raton, FL, USA, 2000; ISBN 978-0-429-11502-8. [Google Scholar]
- Taylor, M.E.; Bundy, C.S.; McPherson, J.E. Unusual Ovipositional Behavior of the Stink Bug Bagrada hilaris (Hemiptera: Heteroptera: Pentatomidae). Ann. Entomol. Soc. Am. 2014, 107, 872–877. [Google Scholar] [CrossRef]
- Azim, M.; Shafee, S. The Life Cycle of Bagrada picta (Fabricius). Articulata 1986, 11, 262–265. [Google Scholar]
- Palumbo, J.C.; Perring, T.M.; Millar, J.G.; Reed, D.A. Biology, Ecology, and Management of an Invasive Stink Bug, Bagrada hilaris, in North America. Annu. Rev. Entomol. 2016, 61, 453–473. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Dhillon, K.S.; Banga, S.S. Oilseed Brassicas. In Biofuel Crops: Production, Physiology and Genetics; Singh, B.P., Ed.; CABI International: Wallingford, UK, 2013; pp. 339–368. [Google Scholar]
- Mahmood, R.; Jones, W.A.; Bajwa, B.E.; Rashid, K. Egg Parasitoids from Pakistan as Possible Classical Biological Control Agents of the Invasive Pest Bagrada hilaris (Heteroptera: Pentatomidae). J. Entomol. Sci. 2015, 50, 147–149. [Google Scholar] [CrossRef]
- Ganjisaffar, F.; Talamas, E.J.; Bon, M.C.; Gonzalez, L.; Brown, B.V.; Perring, T.M. Trissolcus hyalinipennis Rajmohana & Narendran (Hymenoptera, Scelionidae), a Parasitoid of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae), Emerges in North America. J. Hymenopt. Res. 2018, 65, 111–130. [Google Scholar] [CrossRef]
- Power, N.; Ganjisaffar, F.; Xu, K.; Perring, T.M. Effects of Parasitoid Age, Host Egg Age, and Host Egg Freezing on Reproductive Success of Ooencyrtus mirus (Hymenoptera: Encyrtidae) on Bagrada hilaris (Hemiptera: Pentatomidae) Eggs. Environ. Entomol. 2021, 50, 58–68. [Google Scholar] [CrossRef]
- Martel, G.; Augé, M.; Talamas, E.; Roche, M.; Smith, L.; Sforza, R.F.H. First Laboratory Evaluation of Gryon gonikopalense (Hymenoptera: Scelionidae), as Potential Biological Control Agent of Bagrada hilaris (Hemiptera: Pentatomidae). Biol. Control 2019, 135, 48–56. [Google Scholar] [CrossRef]
- Hogg, B.N.; Hougardy, E.; Talamas, E. Adventive Gryon aetherium Talamas (Hymenoptera, Scelionidae) Associated with Eggs of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae) in the USA. J. Hymenopt. Res. 2021, 87, 481–492. [Google Scholar] [CrossRef]
- Martel, G.; Sforza, R.F.H. Catch Me If You Can: Novel Foraging Behavior of an Egg Parasitoid, Gryon gonikopalense, against the Stinkbug Pest, Bagrada hilaris. J. Pest Sci. 2021, 94, 1161–1169. [Google Scholar] [CrossRef]
- Klassen, W. Area-Wide Integrated Pest Management and the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. ISBN 9781402040504. [Google Scholar]
- Cristofaro, M.; Sforza, R.F.H.; Roselli, G.; Paolini, A.; Cemmi, A.; Musmeci, S.; Anfora, G.; Mazzoni, V.; Grodowitz, M. Effects of Gamma Irradiation on the Fecundity, Fertility, and Longevity of the Invasive Stink Bug Pest Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae). Insects 2022, 13, 787. [Google Scholar] [CrossRef]
- Tillman, G.; Toews, M.; Blaauw, B.; Sial, A.; Cottrell, T.; Talamas, E.; Buntin, D.; Joseph, S.; Balusu, R.; Fadamiro, H.; et al. Parasitism and Predation of Sentinel Eggs of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae), in the Southeastern US. Biol. Control 2020, 145, 104247. [Google Scholar] [CrossRef]
- Bakri, A.; Mehta, K.; Lance, D.R. Sterilizing Insects with Ionizing Radiation. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 233–268. ISBN 9781402040504. [Google Scholar]
- Robinson, A. Genetic Basis of the Sterile Insect. In Sterile Insect Technique, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 144–146. ISBN 9781003035572. [Google Scholar]
- Parker, A.; Mehta, K. Sterile Insect Technique: A Model for Dose Optimization for Improved Sterile Insect Quality. Fla. Entomol. 2007, 90, 88–95. [Google Scholar] [CrossRef]
- Lance, D.R.; McInnis, D.O. Biological Basis of the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 69–94. ISBN 9781402040504. [Google Scholar]
- Klassen, W.; Curtis, C.F. History of the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 3–36. ISBN 9781402040504. [Google Scholar]
- Welsh, T.J.; Stringer, L.D.; Caldwell, R.; Carpenter, J.E.; Suckling, D.M. Irradiation Biology of Male Brown Marmorated Stink Bugs: Is There Scope for the Sterile Insect Technique? Int. J. Radiat. Biol. 2017, 93, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Suckling, D.; Cristofaro, M.; Roselli, G.; Levy, M.; Cemmi, A.; Mazzoni, V.; Stringer, L.; Zeni, V.; Ioriatti, C.; Anfora, G. The Competitive Mating of Irradiated Brown Marmorated Stink Bugs, Halyomorpha halys, for the Sterile Insect Technique. Insects 2019, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Roselli, G.; Anfora, G.; Suckling, D.M.; Mazzoni, V.; Vanoni, V.; Menegotti, L.; Fellin, L.; Rossi Stacconi, M.V.; Ioriatti, C.; Cristofaro, M. Effects of Irradiation on Biology and Mating Behaviour of Wild Males of Brown Marmorated Stink Bug Using a 6 MV Medical Linear Accelerator. Insects 2023, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Mau, R.; Mitchell, W.C.; Anwar, M. Preliminary Studies on the Effects of Gamma Irradiation of Eggs and Adults of the Southern Green Stink Bug, Nezara viridula (L.). Proc. Hawaiian Entomol. Soc. 1967, 19, 415–417. [Google Scholar]
- Dyby, S.D.; Sailer, R.I. Impact of Low-Level Radiation on Fertility and Fecundity of Nezara viridula (Hemiptera: Pentatomidae). J. Econ. Entomol. 1999, 92, 945–953. [Google Scholar] [CrossRef]
- Žunič, A.; Čokl, A.; Serša, G. Effects of 5-Gy Irradiation on Fertility and Mating Behaviour of Nezara viridula (Heteroptera: Pentatomidae). Radiol. Oncol. 2002, 36, 3. [Google Scholar]
- Horrocks, K.J.; Welsh, T.; Carpenter, J.E.; Suckling, D.M. Egg Sterilisation of Irradiated Nezara viridula (Hemiptera: Pentatomidae). Insects 2020, 11, 564. [Google Scholar] [CrossRef]
- Barclay, H.J. Mathematical Models for the Use of Sterile Insects. In Sterile Insect Technique; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 147–174. ISBN 9781003035572. [Google Scholar]
- Whitten, M.; Mahon, R.; Whitten, M.; Mahon, R. Misconception and Constraints. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 601–626. ISBN 9781402040504. [Google Scholar]
- Musmeci, S.; Belvedere, S.; Sasso, R.; Arnone, S.; Cristofaro, M.; Nobili, P.; La Marca, A.; De Biase, A. Last-Male Sperm Precedence in Rhynchophorus ferrugineus (Olivier): Observations in Laboratory Mating Experiments with Irradiated Males. Bull. Entomol. Res. 2018, 108, 93–100. [Google Scholar] [CrossRef]
- Grodowitz, M.J.; Reed, D.A.; Elliott, B.; Perring, T.M. Female Reproductive System Morphology and the Development of a Physiological Age-Grading System for Bagrada hilaris (Hemiptera: Pentatomidae). J. Insect Sci. 2019, 19, 15. [Google Scholar] [CrossRef]
- Peccerillo, C.; Mainardi, C.E.; Nieri, R.; Fouani, J.M.; Cemmi, A.; Cristofaro, M.; Anfora, G.; Mazzoni, V. The Effect of the Sterile Insect Technique on Vibrational Communication: The Case of Bagrada hilaris (Hemiptera: Pentatomidae). Insects 2023, 14, 353. [Google Scholar] [CrossRef]
- Barclay, H. Mathematical Models for the Use of Sterile Insects. In Sterile Insect Technique Principles and Practice in Area-Wide Integrated Pest Management, 2nd ed.; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 147–174. ISBN 9781003035572. [Google Scholar]
- Harari, A.R. Prolonged Mate Guarding and Sperm Competition in the Weevil Diaprepes abbreviatus (L.). Behav. Ecol. 2003, 14, 89–96. [Google Scholar] [CrossRef]
- Simmons, L.W. Sperm Competition and Its Evolutionary Consequences in the Insects; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 978-0-691-20703-2. [Google Scholar]
- Lewis, S.M. Sperm Precedence and Sperm Storage in Multiply Mated Red Flour Beetles. Behav. Ecol. Sociobiol. 1998, 43, 365–369. Available online: http://www.jstor.org/stable/4601532 (accessed on 12 May 2023). [CrossRef]
- Singh, S.R.; Singh, B.N.; Hoenigsberg, H.F. Female Remating, Sperm Competition and Sexual Selection in Drosophila. Genet. Mol. Res. 2002, 3, 178–215. [Google Scholar]
- Schnakenberg, S.L.; Siegal, M.L.; Bloch Qazi, M.C. Oh, the Places They’ll Go: Female Sperm Storage and Sperm Precedence in Drosophila melanogaster. Spermatogenesis 2012, 2, 224–235. [Google Scholar] [CrossRef]
- Parker, G.A. Sperm Competition and the Evolution of Ejaculates: Towards a Theory Base. In Sperm Competition and Sexual Selection; Elsevier: Amsterdam, The Netherlands, 1998; pp. 3–54. ISBN 978-0-12-100543-6. [Google Scholar]
- Boorman, E.; Parker, G.A. Sperm (Ejaculate) Competition in Drosophila Melanogaster, and the Reproductive Value of Females to Males in Relation to Female Age and Mating Status. Ecol. Entomol. 1976, 1, 145–155. [Google Scholar] [CrossRef]
- Calkins, C.O.; Parker, A.G. Sterile Insect Quality. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. ISBN 9781402040504. [Google Scholar]
- Dhiman, A.; Gandhi, J.R. Aggregation, Arrival Responses and Growth of Bagrada hilaris (Heteroptera: Pentatomidae). Ann. Entomol. (Dehra Dun) 1988, 6, 19–26. [Google Scholar]
- Baccaro, S.; Cemmi, A.; Di Sarcina, I.; Ferrara, G. Gamma Irradiation Calliope Facility at ENEA—Casaccia Research Centre (Rome, Italy); ENEA Technical Report RT/2019/4/ENEA; ENEA: Rome, Italy, 2019. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1. [Google Scholar] [CrossRef]
- R Core Team. R. A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 31 May 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Crawley, M.J. The Gamma Distribution. In The R Book; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 293–295. [Google Scholar]
- Sillén-Tullberg, B. Prolonged Copulation: A Male “Postcopulatory” Strategy in a Promiscuous Species, Lygaeus equestris (Heteroptera: Lygaeidae). Behav. Ecol. Sociobiol. 1981, 9, 283–289. [Google Scholar] [CrossRef]
- Hunter, F.M.; Birkhead, T.R. Sperm Viability and Sperm Competition in Insects. Curr. Biol. 2002, 12, 121–123. [Google Scholar] [CrossRef]
- Stringer, L.D.; Harland, D.P.; Grant, J.E.; Laban, J.; Suckling, D.M. Effect of 40Gy Irradiation on the Ultrastructure, Biochemistry, Morphology and Cytology during Spermatogenesis in the Southern Green Stink Bug Nezara viridula (Hemiptera: Pentatomidae). bioRxiv 2017. [Google Scholar] [CrossRef]
- Ladle, R.J.; Foster, E. Are Giant Sperm Copulatory Plugs? Acta Oecologica 1992, 13, 635–638. [Google Scholar]
- Briskie, J.V.; Montgomerie, R.; Birkhead, T.R. The Evolution of Sperm Size in Birds. Evolution 1997, 51, 937–945. [Google Scholar] [CrossRef]
- Dybas, L.K.; Dybas, H.S. Coadaptation and Taxonomic Differentiation of Sperm and Spermathecae in Featherwing Beetles. Evolution 1981, 35, 168–174. [Google Scholar] [CrossRef]
- Sivinski, J.; Burk, T.; Webb, J.C. Acoustic Courtship Signals in the Caribbean Fruit Fly, Anastrepha suspensa (Loew). Anim. Behav. 1984, 32, 1011–1016. [Google Scholar] [CrossRef]
- Araújo, V.A.; Moreira, J.; Lino-Neto, J. Structure and Ultrastructure of the Spermatozoa of Trypoxylon (Trypargilum) albitarse Fabricius 1804 (Hymenoptera: Apoidea: Crabronidae). Micron 2009, 40, 719–723. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tanaka, T.; Miyata, T. Eupyrene and Apyrene Sperm and Their Numerical Fluctuations inside the Female Reproductive Tract of the Armyworm, Pseudaletia separata. J. Insect Physiol. 1995, 41, 689–694. [Google Scholar] [CrossRef]
- Itô, Y.; Yamamura, K.; Manoukis, N.C. Role of Population and Behavioural Ecology in the Sterile Insect Technique. In Sterile Insect Technique; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 269–296. ISBN 9781402040504. [Google Scholar]
- Cocroft, R.B.; Rodríguez, R.L. The Behavioral Ecology of Insect Vibrational Communication. BioScience 2005, 55, 323. [Google Scholar] [CrossRef]
- Scala, M.; Peccerillo, C.; Fouani, J.; Nieri, R.; Mazzoni, V.; Baser, N.; Verrastro, V.; Anfora, G. Vibrational Communication of Bagrada hilaris. In Proceedings of the III International Conference on Biotremology, Piran, Slovenia, 19–22 September 2022; p. 80737. [Google Scholar]
- Alcock, J. Postinsemination Associations Between Males and Females in Insects: The Mate-Guarding Hypothesis. Annu. Rev. Entomol. 1994, 39, 1–21. [Google Scholar] [CrossRef]
- Parker, G.A. The Reproductive Behaviour and the Nature of Sexual Selection in Scatophaga stercoraria L. (Diptera: Scatophagidae): II. The Fertilization Rate and the Spatial and Temporal Relationships of Each Sex around the Site of Mating and Oviposition. J. Anim. Ecol. 1970, 39, 205. [Google Scholar] [CrossRef]
- Mclain, D.K. Prolonged Copulation as a Post-Insemination Guarding Tactic in a Natural Population of the Ragwort Seed Bug. Anim. Behav. 1989, 38, 659–664. [Google Scholar] [CrossRef]
- Telford, S.R.; Dangerfield, J.M. Manipulation of the Sex Ratio and Duration of Copulation in the Tropical Millipede Alloporus uncinatus: A Test of the Copulatory Guarding Hypothesis. Anim. Behav. 1990, 40, 984–986. [Google Scholar] [CrossRef]
- Walker, W.F. Sperm Utilization Strategies in Nonsocial Insects. Am. Nat. 1980, 115, 780–799. [Google Scholar] [CrossRef]
- Parker, G.A. Sperm competition and the evolution of animal mating strategies. In Sperm Competition and the Evolution of Animal Mating Systems; Elsevier: Amsterdam, The Netherlands, 1984; pp. 1–60. [Google Scholar]
- Follett, P.A.; Calvert, F.; Golden, M. Genetic Studies Using the Orange Body Color Type of Nezara viridula (Hemiptera: Pentatomidae): Inheritance, Sperm Precedence, and Disassortative Mating. Ann. Entomol. Soc. Am. 2007, 100, 433–438. [Google Scholar] [CrossRef]
- Jang, E.B.; McInnis, D.O.; Kurashima, R.; Woods, B.; Suckling, D.M. Irradiation of Adult Epiphyas postvittana (Lepidoptera: Tortricidae): Egg Sterility in Parental and F1 Generations. J. Econ. Entomol. 2012, 105, 54–61. [Google Scholar] [CrossRef]
- Hight, S.D.; Carpenter, J.E.; Bloem, S.; Bloem, K.A. Developing a Sterile Insect Release Program for Cactoblastis cactorum (Berg) (Lepidoptera: Pyralidae): Effective Overflooding Ratios and Release-Recapture Field Studies. Environ. Entomol. 2005, 34, 850–856. [Google Scholar] [CrossRef]
- Bloem, K.A.; Bloem, S.; Carpenter, J.E. Impact of Moth Suppression/Eradication Programmes Using the Sterile Insect Technique or Inherited Sterility. In Sterile Insect Technique; Dyck, V., Hendrichs, J., Robinson, A.S., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 39–68. ISBN 9781402040504. [Google Scholar]
- Peccerillo, C.; Mainardi, C.E.; Musmeci, S.; Paolini, A.; Cemmi, A.; Anfora, G.; Cristofaro, M. Effects of Irradiation on Sexual Behavior and Feeding of Bagrada Hilaris (Burmeister) (Hemiptera: Pentatomidae). Insects, 2023; in press. [Google Scholar]
- Fortes, P.; Cônsoli, F.L. Are There Costs in the Repeated Mating Activities of Female Southern Stink Bugs Nezara viridula? Physiol. Entomol. 2011, 36, 215–219. [Google Scholar] [CrossRef]
- Knipling, E.F. The Basic Principles of Insect Population Suppression and Management; No. 512; US Department of Agriculture: Washington, DC, USA, 1979. [Google Scholar]
- Panizzi, A.R.; Vivan, L.M.; Corrêa-Ferreira, B.S.; Foerster, L.A. Performance of Southern Green Stink Bug (Heteroptera: Pentatomidae) Nymphs and Adults on a Novel Food Plant (Japanese privet) and Other Hosts. Ann. Entomol. Soc. Am. 1996, 89, 822–827. [Google Scholar] [CrossRef]
- Panizzi, A.R.; Parra, J.R.P.; Santos, C.H.; Carvalho, D.R. Rearing the Southern Green Stink Bug Using an Artificial Dry Diet and an Artificial Plant. Pesqui. Agropecuária Bras. 2000, 35, 1709–1715. [Google Scholar] [CrossRef]
- Medal, J.; Smith, T.; Fox, A.; Cruz, A.S.; Poplin, A.; Hodges, A. Rearing the Brown Marmorated Stink Bug Halyomorpha halys (Heteroptera: Pentatomidae). Fla. Entomol. 2012, 95, 800–802. [Google Scholar] [CrossRef]
- Knipling, E.F.; McGuire, J.U. Population Models to Appraise the Limitations and Potentialities of Trichogramma in Managing Host Insect Populations; Agricultural Research Service: Beltsville, MD, USA, 1968. [Google Scholar]
- Andress, E.; Walters, I.; Street, A. Release-Recapture of Sterile Male Mediterranean Fruit Flies (Diptera: Tephritidae) in Southern California. Environ. Sci. 2013, 45, 11–29. [Google Scholar]
- Dowell, R.V.; Worley, J.; Gomes, P.J.; Rendón, P.; Herrero, R.A. Supply, Emergence, and Release of Sterile Insects. In Sterile Insect Technique; Dyck, V.A., Hendrichs, J., Robinson, A.S., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 147–174. ISBN 9781003035572. [Google Scholar]
- Mastro, V.C.; Schwalbe, C.P. Status and Potential of F, Sterility for the Control of Noxious Lepidoptera. In Proceedings of the International Symposium on Modern Insect Control: Nuclear Techniques and Biotechnology, International Atomic Energy Agency and Food and Agriculture Organization of the United Nations, Vienna, Austria, 16–20 November 1987; STI/PUB/763. IAEA: Vienna, Austria, 1987; pp. 15–40. [Google Scholar]




| Experiment | Fixed Effects | Estimate | S.E. | z Value | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 3.33871 | 0.14388 | 23.204 | <2 × 10−16 *** | |
| Irradiated before | −0.04768 | 0.19401 | −0.246 | 0.80588 | |
| 60 Gy | Non-irradiated after | −0.24183 | 0.19680 | −1.229 | 0.21913 |
| Irradiated after | −0.45079 | 0.16758 | −2.690 | 0.00715 ** | |
| (Intercept) | 3.6455 | 0.1231 | 29.606 | <2 × 10−16 *** | |
| Irradiated before | −0.3338 | 0.1794 | −1.860 | 0.0628 | |
| 80 Gy | Non-irradiated after | −0.9155 | 0.1821 | −5.029 | 4.94 × 10−7 *** |
| Irradiated after | −1.0261 | 0.1782 | −5.760 | 8.43 × 10−9 *** | |
| (Intercept) | 3.0265 | 0.1966 | 15.395 | <2 × 10−16 *** | |
| Irradiated before | −0.3922 | 0.2652 | −1.479 | 0.139 | |
| 100 Gy | Non-irradiated after | −0.3616 | 0.2651 | −1.364 | 0.173 |
| Irradiated after | −0.2428 | 0.2688 | −0.903 | 0.366 | |
| (Intercept) | 2.8598 | 0.2770 | 10.32 | <2 × 10−16 *** | |
| Untreated control | 2nd non-irradiated | −0.3341 | 0.3930 | −0.850 | 0.395 |
| Experiment | Fixed Effects | Estimate | S.E. | z Value | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 2.2384 | 0.3443 | 6.501 | 8.00 × 10−11 *** | |
| Irradiated before | −2.2593 | 0.4855 | −4.653 | 3.26 × 10−6 *** | |
| 60 Gy | Non-irradiated after | −0.5793 | 0.4658 | −1.244 | 0.2136 |
| Irradiated after | −0.7673 | 0.3387 | −2.265 | 0.0235 * | |
| (Intercept) | 3.1276 | 0.2226 | 14.049 | < 2 × 10−16 *** | |
| Irradiated before | −1.8653 | 0.3347 | −5.573 | 2.51 × 10−8 *** | |
| 80 Gy | Non-irradiated after | −0.9797 | 0.3263 | −3.002 | 0.00268 ** |
| Irradiated after | −1.2865 | 0.3203 | −4.017 | 5.90 × 10−5 *** | |
| (Intercept) | 2.3465 | 0.3777 | 6.212 | 5.22 × 10−10 *** | |
| Irradiated before | −4.1015 | 0.6758 | −6.069 | 1.29 × 10−9 *** | |
| 100 Gy | Non-irradiated after | −2.1291 | 0.5757 | −3.698 | 0.000217 *** |
| Irradiated after | −1.1008 | 0.5290 | −2.081 | 0.037455 * | |
| (Intercept) | 1.8527 | 0.4317 | 4.291 | 1.78 × 10−5 *** | |
| Untreated control | 2nd non-irradiated | −0.3559 | 0.4428 | −0.804 | 0.422 |
| Experiment | Fixed Effects | Estimate | S.E. | z Value | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 3.8963 | 0.2227 | 17.495 | <2 × 10−16 *** | |
| Irradiated before | −2.2073 | 0.3061 | −7.211 | 5.54 × 10−13 *** | |
| 60 Gy | Non-irradiated after | −0.4355 | 0.3103 | −1.403 | 0.161 |
| Irradiated after | −0.4633 | 0.3402 | −1.362 | 0.173 | |
| (Intercept) | 4.0894 | 0.2339 | 17.485 | <2 × 10−16 *** | |
| Irradiated before | −1.7362 | 0.3362 | −5.164 | 2.42 × 10−7 *** | |
| 80 Gy | Non-irradiated after | −0.0613 | 0.3493 | −0.175 | 0.861 |
| Irradiated after | −0.30314 | 0.3424 | −0.885 | 0.376 | |
| (Intercept) | 4.0537 | 0.3755 | 10.794 | <2 × 10−16 *** | |
| Irradiated before | −3.4021 | 0.5906 | −5.760 | 8.39 × 10−9 *** | |
| 100 Gy | Non-irradiated after | −1.9794 | 0.7026 | −2.817 | 0.00484 ** |
| Irradiated after | −1.0899 | 0.3943 | −2.764 | 0.00571 ** | |
| (Intercept) | 0.02352 | 0.00745 | 3.158 | 0.00159 ** | |
| Untreated control | 2nd non-irradiated | 0.00094 | 0.00603 | 0.155 | 0.87648 |
| Estimate | S.E. | t Value | p-Value | |
|---|---|---|---|---|
| (Intercept) | 0.6492 | 0.1090 | 5.956 | 2.04 × 10−7 *** |
| 80 Gy | 0.2879 | 0.1637 | 1.759 | 0.08408 |
| 100 Gy | −0.4323 | 0.1629 | −2.654 | 0.01037 * |
| Dose 60: irradiated male | −0.2477 | 0.1636 | −1.514 | 0.13513 |
| Dose 80: irradiated male | −0.6857 | 0.1691 | −4.055 | 0.00015 *** |
| Dose 100: irradiated male | 0.4067 | 0.1711 | 2.377 | 0.02090 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainardi, C.E.; Peccerillo, C.; Paolini, A.; Cemmi, A.; Sforza, R.F.H.; Musmeci, S.; Porretta, D.; Cristofaro, M. Using Gamma Irradiation to Predict Sperm Competition Mechanism in Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae): Insights for a Future Management Strategy. Insects 2023, 14, 681. https://doi.org/10.3390/insects14080681
Mainardi CE, Peccerillo C, Paolini A, Cemmi A, Sforza RFH, Musmeci S, Porretta D, Cristofaro M. Using Gamma Irradiation to Predict Sperm Competition Mechanism in Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae): Insights for a Future Management Strategy. Insects. 2023; 14(8):681. https://doi.org/10.3390/insects14080681
Chicago/Turabian StyleMainardi, Chiara Elvira, Chiara Peccerillo, Alessandra Paolini, Alessia Cemmi, René F. H. Sforza, Sergio Musmeci, Daniele Porretta, and Massimo Cristofaro. 2023. "Using Gamma Irradiation to Predict Sperm Competition Mechanism in Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae): Insights for a Future Management Strategy" Insects 14, no. 8: 681. https://doi.org/10.3390/insects14080681
APA StyleMainardi, C. E., Peccerillo, C., Paolini, A., Cemmi, A., Sforza, R. F. H., Musmeci, S., Porretta, D., & Cristofaro, M. (2023). Using Gamma Irradiation to Predict Sperm Competition Mechanism in Bagrada hilaris (Burmeister) (Hemiptera: Pentatomidae): Insights for a Future Management Strategy. Insects, 14(8), 681. https://doi.org/10.3390/insects14080681








