Ultrastructural and Descriptive Study on the Adult Body Surface of Heortia vitessoides (Lepidoptera: Crambidae)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
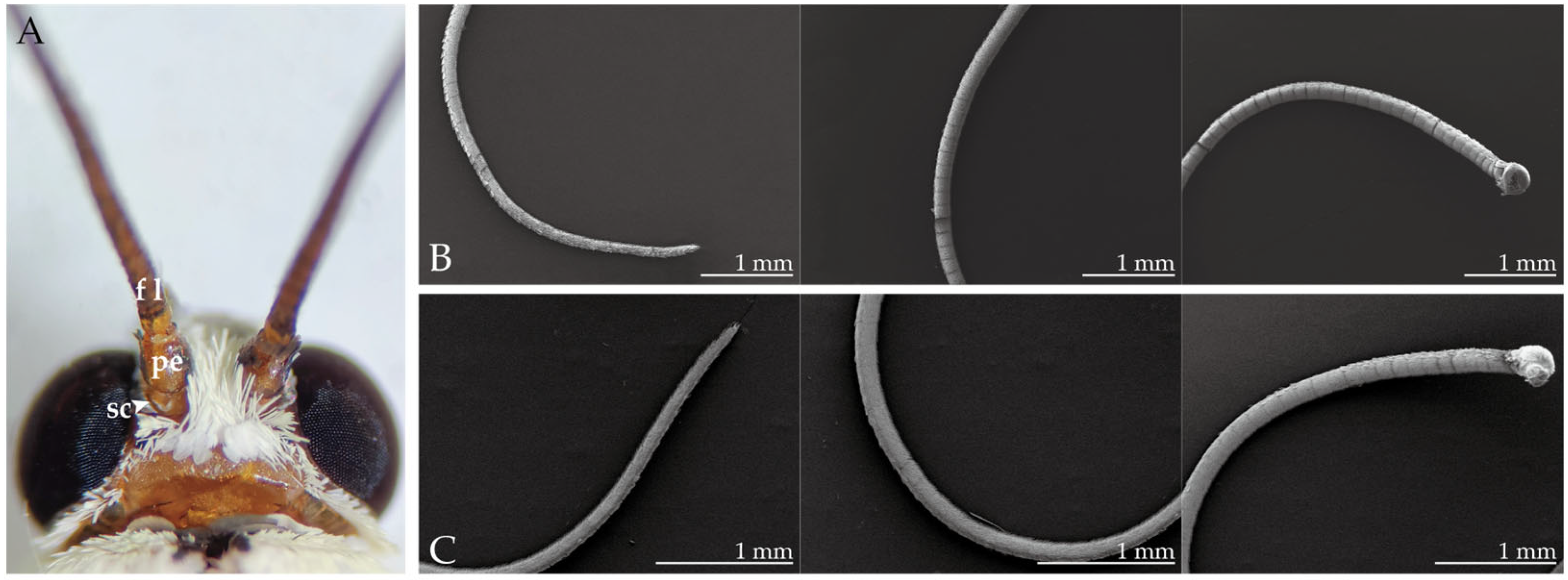
2.1. Morphological Structure of the Antennae
2.2. Antennal Sensilla in H. vitessoides
2.3. Morphological Structure of the Mouthparts
2.4. Proboscis and Labial Palps Sensilla in H. vitessoides
2.5. Morphological Structure of the Leg
2.6. Leg Sensilla in Adult H. vitessoides
3. Discussion
4. Materials and Methods
4.1. Insect
4.2. Scanning Electron Microscopy (SEM)
4.3. Terminology
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Field, L.; Pickett, J.; Wadhams, L. Molecular studies in insect olfaction. Insect Mol. Biol. 2000, 9, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatouros, N.E.; Dicke, M.; Mumm, R.; Meiners, T.; Hilker, M. Foraging behavior of egg parasitoids exploiting chemical information. Behav. Ecol. 2008, 19, 677–689. [Google Scholar] [CrossRef]
- Wilson, R.I.; Mainen, Z.F. Early events in olfactory processing. Annu. Rev. Neurosci. 2006, 29, 163–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, D. Insect Olfaction: Deciphering System for Chemical Messages. Science 1969, 163, 1031–1037. [Google Scholar] [CrossRef]
- Heppner, J.B. Butterflies and Moths (Lepidoptera). In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef] [Green Version]
- Guo, M.; Chen, Q.; Liu, Y.; Wang, G.; Han, Z. Chemoreception of mouthparts: Sensilla morphology and discovery of chemosensory genes in proboscis and labial palps of adult Helicoverpa armigera (Lepidoptera: Noctuidae). Front. Physiol. 2018, 9, 970. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiao, D.; Liu, F.F.; Yin, L.; Liu, T.X. Ultrastructure of the sensilla on antennae and mouthparts of larval and adult Plutella xylostella (Lepidoptera: Plutellidae). J. Integr. Agric. 2018, 17, 1409–1420. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Ghany, N.M.; Faucheux, M.J. The mouthparts and sensilla of the adult tomato leafminer moth, Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae). Arthropod Struct. Dev. 2022, 67, 101144. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, H.; Zhang, Y.; Zhang, X. Morphology and distribution of antennal, maxillary palp and labial palp sensilla of the adult bruchid beetles, Callosobruchus chinensis (L.) (Coleoptera: Bruchidae). Entomol. Res. 2018, 48, 466–479. [Google Scholar] [CrossRef]
- Gilbert, L.E. Pollen Feeding and Reproductive Biology of Heliconius Butterflies. Proc. Natl. Acad. Sci. USA 1972, 69, 1403–1407. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, X.; Cao, S.; Ma, B.; Guo, M.; Shen, J.; Wang, G. Fine structure and olfactory reception of the labial palps of Spodoptera frugiperda. Front. Physiol. 2021, 12, 880. [Google Scholar] [CrossRef]
- Ning, C.; Yang, K.; Xu, M.; Huang, L.-Q.; Wang, C.-Z. Functional validation of the carbon dioxide receptor in labial palps of Helicoverpa armigera moths. Insect Biochem. Mol. Biol. 2016, 73, 12–19. [Google Scholar] [CrossRef]
- Bogner, F.; Boppré, M.; Ernst, K.-D.; Boeckh, J. CO2 sensitive receptors on labial palps of Rhodogastria moths (Lepidoptera: Arctiidae): Physiology, fine structure and central projection. J. Comp. Physiol. A 1986, 158, 741–749. [Google Scholar] [CrossRef]
- Calas, D.; Marion-Poll, F.; Steinbauer, M.J. Tarsal taste sensilla of the autumn gum moth, Mnesampela privata: Morphology and electrophysiological activity. Entomol. Exp. Appl. 2009, 133, 186–192. [Google Scholar] [CrossRef]
- Syazwan, S.A.; Lee, S.Y.; Ong, S.P.; Mohamed, R. Damaging insect pests and diseases and their threats to agarwood tree plantations. Sains Malays. 2019, 48, 497–507. [Google Scholar] [CrossRef]
- Hashim, Y.Z.H.-Y.; Kerr, P.G.; Abbas, P.; Salleh, H.M. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science and Technology Press: Beijing, China, 2010; Volume 1, pp. 192–193. [Google Scholar]
- Qiao, H.L.; Lu, P.F.; Chen, J.; Ma, W.S.; Qin, R.M.; Li, X.M. Antennal and behavioural responses of Heortia vitessoides females to host plant volatiles of Aquilaria sinensis. Entomol. Exp. Appl. 2012, 143, 269–279. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, J.; Liu, Y.; Qiu, Y.; Xie, Q.; Li, M.; Khan, I.; Wang, W. Advance in studies on chemical constituents, pharmacology and quality control of Aquilaria sinensis. Digit. Chin. Med. 2018, 1, 316–330. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Jin, Y.; Zhuo, Z.; Yang, H.; Hu, J.; Wang, R. Influence of climatic factors on the potential distribution of pest Heortia vitessoides Moore in China. Glob. Ecol. Conserv. 2020, 23, e01107. [Google Scholar] [CrossRef]
- Su, Y.-P. The biological characteristics of Heortia vitessoides Moore on Aquilaria sinensis. Chin. Herb. Med. 1994, 17, 7–9. [Google Scholar]
- Zhang, X.L.; Liu, Y.Y.; Wei, J.H.; Yang, Y.; Zhang, Z.; Huang, J.Q.; Chen, H.Q.; Liu, Y.J. Production of high-quality agarwood in Aquilaria sinensis trees via whole-tree agarwood-induction technology. Chin. Chem. Lett. 2012, 23, 727–730. [Google Scholar] [CrossRef]
- Liang, S.; Cai, J.; Chen, X.; Jin, Z.; Zhang, J.; Huang, Z.; Tang, L.; Sun, Z.; Wen, X.; Wang, C. Larval aggregation of Heortia vitessoides Moore (Lepidoptera: Crambidae) and evidence of horizontal transfer of avermectin. Forests 2019, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Wang, C.-Y.; Lyu, Z.-H.; Chen, J.-X.; Tang, L.-P.; Lin, T. Candidate olfactory genes identified in Heortia vitessoides (Lepidoptera: Crambidae) by antennal transcriptome analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.-L.; Lu, P.-F.; Liu, S.; Xu, C.-Q.; Guo, K.; Xu, R.; Chen, J. Ultrastructure observations on antennal sensilla of Heortia vitessoides the most serious pest of Aquilaria sinensis. China J. Chin. Mater. Medica 2019, 44, 2026–2031. [Google Scholar]
- Law, S.T.S.; Nong, W.; So, W.L.; Baril, T.; Swale, T.; Chan, C.B.; Tobe, S.S.; Kai, Z.-P.; Bendena, W.G.; Hayward, A.; et al. Chromosomal-level reference genome of the moth Heortia vitessoides (Lepidoptera: Crambidae), a major pest of agarwood-producing trees. Genomics 2022, 114, 110440. [Google Scholar] [CrossRef]
- Bawin, T.; Collard, F.; Backer, L.D.; Yarou, B.B.; Compère, P.; Francis, F.; Verheggen, F.J. Structure and distribution of the sensilla on the antennae of Tuta absoluta (Lepidoptera: Gelechiidae). Micron 2017, 96, 16–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Chao, X.; Dong, Z.; Lu, W. Morphological characterization and distribution of antennal sensilla of Diaphania angustalis Snellen (Lepidoptera: Crambidae). Microsc. Res. Tech. 2019, 82, 1632–1641. [Google Scholar] [CrossRef]
- Yang, H.; Yan, S.C.; Liu, D. Ultrastructural observations on antennal sensilla of Coleophora obducta (Meyrick) (Lepidoptera: Coleophoridae). Micron 2009, 40, 231–238. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.Q.; Zhang, G. Ultrastructural observations on antennal sensilla of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Microsc. Res. Tech. 2011, 74, 113–121. [Google Scholar] [CrossRef]
- Chang, H.; Guo, M.; Wang, B.; Liu, Y.; Dong, S.; Wang, G. Sensillar expression and responses of olfactory receptors reveal different peripheral coding in two Helicoverpa species using the same pheromone components. Sci. Rep. 2016, 6, 18742. [Google Scholar] [CrossRef] [Green Version]
- Faucheux, M.J. Biodiversity and Unity of Sensory Organs in Lepidopteran Insects; Société des Sciences Naturelles de l’Ouest de la France: Nantes, France, 1999; Volume 296. [Google Scholar]
- Binyameen, M.; Anderson, P.; Ignell, R.; Seada, M.A.; Hansson, B.S.; Schlyter, F. Spatial Organization of Antennal Olfactory Sensory Neurons in the Female Spodoptera littoralis Moth: Differences in Sensitivity and Temporal Characteristics. Chem. Senses 2012, 37, 613–629. [Google Scholar] [CrossRef] [Green Version]
- Clyne, P.; Grant, A.; O’Connell, R.; Carlson, J.R. Odorant response of individual sensilla on the Drosophila antenna. Invertebr. Neurosci. 1997, 3, 127–135. [Google Scholar] [CrossRef]
- Shields, V.; Hildebrand, J. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J. Comp. Physiol. A 2001, 186, 1135–1151. [Google Scholar] [CrossRef]
- Faucheux, M.J. Morphology and distribution of sensilla on the cephalic appendages, tarsi and ovipositor of the European sunflower moth, Homoeosoma nebulella Den. & Schiff. (Lepidoptera: Pyralidae). Int. J. Insect Morphol. Embryol. 1991, 20, 291–307. [Google Scholar] [CrossRef]
- Hallberg, E.; Hansson, B.S.; Steinbrecht, R.A. Morphological characteristics of antennal sensilla in the European cornborer Ostrinia nubilalis (Lepidoptera: Pyralidae). Tissue Cell 1994, 26, 489. [Google Scholar] [CrossRef]
- Andersson, S. Antennal responses to floral scents in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonepteryx rhamni (Pieridae). Chemoecology 2003, 13, 13–20. [Google Scholar] [CrossRef]
- Larsson, M.C.; Hallberg, E.; Kozlov, M.V.; Francke, W.; Hansson, B.S.; Löfstedt, C. Specialized olfactory receptor neurons mediating intra-and interspecific chemical communication in leafminer moths Eriocrania spp.(Lepidoptera: Eriocraniidae). J. Exp. Biol. 2002, 205, 989–998. [Google Scholar] [CrossRef]
- Ansebo, L.; Ignell, R.; Löfqvist, J.; Hansson, B.S. Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensilla auricillica of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2005, 51, 1066–1074. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, L.; Ren, B. Fine structure and distribution of antennal sensilla of longicorn beetles Leptura arcuata and Leptura aethiops (Coleoptera: Cerambycidae). Ann. Entomol. Soc. Am. 2011, 104, 778–787. [Google Scholar] [CrossRef] [Green Version]
- Merivee, E.; Rahi, M.; Luik, A. Antennal sensilla of the click beetle, Melanotus villosus (Geoffroy) (Coleoptera: Elateridae). Int. J. Insect Morphol. Embryol. 1999, 28, 41–51. [Google Scholar] [CrossRef]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y. Antennae and sensilla. In Comprehensive Insect Physiology, Biochemistry & Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; pp. 1–69. [Google Scholar]
- Altner, H.; Sass, H.; Altner, I. Relationship between structure and function of antennal chemo-, hygro-, and thermoreceptive sensilla in Periplaneta americana. Cell Tissue Res. 1977, 176, 389–405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-J.; Ning, C.; Guo, H.; Jia, Y.-Y.; Huang, L.-Q.; Qu, M.-J.; Wang, C.-Z. A gustatory receptor tuned to D-fructose in antennal sensilla chaetica of Helicoverpa armigera. Insect Biochem. Mol. Biol. 2015, 60, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Altner, H.; Routil, C.; Loftus, R. The structure of bimodal chemo-, thermo-, and hygroreceptive sensilla on the antenna of Locusta migratoria. Cell Tissue Res. 1981, 215, 289–308. [Google Scholar] [CrossRef]
- Kristoffersen, L.; Hallberg, E.; Wallén, R.; Anderbrant, O. Sparse sensillar array on Trioza apicalis (Homoptera, Triozidae) antennae—An adaptation to high stimulus levels? Arthropod Struct. Dev. 2006, 35, 85–92. [Google Scholar] [CrossRef]
- Chang, X.-Q.; Zhang, S.; Lv, L.; Wang, M.-Q. Insight into the ultrastructure of antennal sensilla of Mythimna separata (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 124. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, A.; Prabhakar, S.; Sudarsan, S.; Sane, S.P. The neural mechanisms of antennal positioning in flying moths. J. Exp. Biol. 2012, 215, 3096–3105. [Google Scholar] [CrossRef] [Green Version]
- Krenn, H.W. Feeding mechanisms of adult Lepidoptera: Structure, function, and evolution of the mouthparts. Annu. Rev. Entomol. 2010, 55, 307. [Google Scholar] [CrossRef] [Green Version]
- Guerenstein, P.G.; Hildebrand, J.G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol. 2008, 53, 161–178. [Google Scholar] [CrossRef]
- Faucheux, M.J. Sensillum types on the proboscis of the Lepidoptera: A review. Ann. Société Entomol. Fr. 2013, 49, 73–90. [Google Scholar] [CrossRef]
- Blaney, W.M.; Simmonds, M. Food selection in adults and larvae of three species of Lepidoptera: A behavioural and electrophysiological study. Entomol. Exp. Appl. 2011, 49, 111–121. [Google Scholar] [CrossRef]
- Inoue, T.A.; Asaoka, K.; Seta, K.; Imaeda, D.; Ozaki, M. Sugar receptor response of the food-canal taste sensilla in a nectar-feeding swallowtail butterfly, Papilio xuthus. Naturwissenschaften 2009, 96, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenn, H.W. Proboscis sensilla in Vanessa cardui (Nymphalidae, Lepidoptera): Functional morphology and significance in flower-probing. Zoomorphology 1998, 118, 23–30. [Google Scholar] [CrossRef]
- Dong, J.F.; Liu, H.; Tang, Q.B.; Liu, Y.; Zhao, X.C.; Wang, G.R. Morphology, type and distribution of the labial-palp pit organ and its sensilla in the oriental armyworm, Mythimna separata (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2014, 57, 681–687. [Google Scholar]
- Stange, G.; Monro, J.; Stowe, S.; Osmond, C.B. The CO2 sense of the moth Cactoblastis cactorum and its probable role in the biological control of the CAM plant Opuntia stricta. Oecologia 1995, 102, 341–352. [Google Scholar] [CrossRef]
- Amat, C.; Marion-Poll, F.; Navarro-Roldán, M.A.; Gemeno, C. Gustatory function of sensilla chaetica on the labial palps and antennae of three tortricid moths (Lepidoptera: Tortricidae). Sci. Rep. 2022, 12, 18882. [Google Scholar] [CrossRef]
- Keil, T.A. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 1997, 39, 506–531. [Google Scholar] [CrossRef]
- Stange, G.; Stowe, S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc. Res. Tech. 2015, 47, 416–427. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Loon, J.; Wang, C.Z. Tarsal taste neuron activity and proboscis extension reflex in response to sugars and amino acids in Helicoverpa armigera (Hübner). J. Exp. Biol. 2010, 213, 2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sensilla Type | Sex | Length (μm) | Basal Diameter (μm) | Width of Socket (μm) | Numbers of Spines |
|---|---|---|---|---|---|
| Sensilla trichodea I | Female | 31.09 ± 3.42 * | 2.18 ± 0.27 | 4.14 ± 0.39 | |
| Male | 28.76 ± 2.38 | 2.3 ± 0.25 | 3.99 ± 0.42 | ||
| Sensilla trichodea II | Female | 24.7 ± 3.75 * | 1.89 ± 0.38 * | 4.17 ± 0.47 * | |
| Male | 21.13 ± 2.45 | 1.69 ± 0.23 | 3.57 ± 0.36 | ||
| Sensilla coeloconica I | Female | 4.45 ± 0.67 | 8.6 ± 1.42 | 8–11 | |
| Male | 3.64 ± 0.63 | 8.23 ± 0.95 | 8–14 | ||
| Sensilla coeloconica II | Female | 5.06 ± 1.05 | 5.78 ± 1.19 | ||
| Male | 4.14 ± 0.54 | 7.61 ± 0.89 | |||
| Sensilla chaetica | Female | 49.21 ± 8.11 | 3.45 ± 0.51 | 6.79 ± 0.98 | |
| Male | 57.82 ± 7.5 * | 3.22 ± 0.41 | 7.21 ± 0.78 | ||
| Sensilla styloconica | Female | 21.54 ± 2.73 | 6.57 ± 1.22 | ||
| Male | 30.04 ± 7.44 * | 6.85 ± 1.6 | |||
| Sensilla basiconica I | Female | 6.03 ± 1.51 | 1.6 ± 0.25 | 3.96 ± 0.62 | |
| Male | 4.73 ± 1.52 | 1.59 ± 0.24 | 3.42 ± 0.7 | ||
| Sensilla basiconica II | Female | 19.63 ± 1.96 | 2.31 ± 0.23 * | 3.96 ± 0.35 | |
| Male | 20.74 ± 3.13 | 2.05 ± 0.41 | 3.96 ± 0.65 | ||
| Capitate sensilla basiconica | Female | 4.49 ± 0.48 | 1.95 ± 0.31 | 4.13 ± 0.52 | |
| Male | 4.58 ± 0.8 | 2.05 ± 0.37 | 4.46 ± 0.68 | ||
| Sensilla squamiformia | Female | 46.73 ± 3.05 | 1.65 ± 0.43 | 3.02 ± 0.57 | |
| Male | 50.24 ± 7.6 | 2.12 ± 0.45 | 5.49 ± 1.26 | ||
| Böhm bristles | Female | 23.46 ± 5.68 * | 2.11 ± 0.28 * | ||
| Male | 16.95 ± 3.83 | 1.7 ± 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Zhang, Y.; Yan, S.-C.; Yang, B.; Wang, G.-R. Ultrastructural and Descriptive Study on the Adult Body Surface of Heortia vitessoides (Lepidoptera: Crambidae). Insects 2023, 14, 687. https://doi.org/10.3390/insects14080687
Liu L, Zhang Y, Yan S-C, Yang B, Wang G-R. Ultrastructural and Descriptive Study on the Adult Body Surface of Heortia vitessoides (Lepidoptera: Crambidae). Insects. 2023; 14(8):687. https://doi.org/10.3390/insects14080687
Chicago/Turabian StyleLiu, Lei, Yan Zhang, Shan-Chun Yan, Bin Yang, and Gui-Rong Wang. 2023. "Ultrastructural and Descriptive Study on the Adult Body Surface of Heortia vitessoides (Lepidoptera: Crambidae)" Insects 14, no. 8: 687. https://doi.org/10.3390/insects14080687





